Abstract
Growing evidence reports that obesity might play a role in erectile dysfunction (ED), but limited knowledge is available. We conducted a meta-analysis to estimate the prevalence of ED in overweight men and men with obesity. We performed a systematic review up to 01/04/2019 to investigate the associations between obesity and ED. Applying a random-effect model, we calculated the prevalence of ED, the odds ratio (OR) for the presence of ED by Body Mass Index (BMI) categories and the mean differences between ED and controls in BMI and Waist Circumference (WC). Among 3409 studies, we included 45 articles with 42,489 men (mean age = 55 years). Taking normal weight men as reference, the prevalence of ED was significantly higher in overweight (OR = 1.31; 95%CI: 1.13–1.51; I2 = 72%) and in men with obesity (OR = 1.60; 95%CI: 1.29–1.98; I2 = 79%). Adjusting our analyses for potential confounders, the results were confirmed in men with obesity (OR = 1.46; 95%CI: 1.24–1.72; I2 = 72%). ED was associated with significant higher values of BMI (MD = 0.769; 95%CI: 0.565–0.973 Kg/m2; I2 = 78%) and WC (MD = 5.251 cm; 95%CI: 1.295–9.208; I2 = 96%). Considering the high prevalence of ED among men with obesity, clinicians should screen for this clinical condition in this population. Findings from the present study suggest that reducing adiposity is a crucial approach in patients with ED who are affected by obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Overweight and obesity are defined by the World Health Organization (WHO) as abnormal or excessive fat accumulation that may impair health [1]. It is estimated that the worldwide prevalence of obesity nearly tripled between 1975 and 2016 [1]. In 2016, more than 1.9 billion adults aged 18 years and older were overweight and of these over 650 million were affected by obesity [1].
Obesity is a major public health issue related to several unwanted conditions and diseases [2,3,4,5,6,7,8]. Growing evidence is reporting that obesity might play a key role in male sexual function, including fertility and erectile dysfunction (ED) [9, 10]. ED, defined as the inability to achieve and/or maintain a sufficient erection to permit satisfactory sexual intercourse, is the most common sexual health issue in men and is associated with a substantial range of physical and psychological adverse effects, including anxiety, low mood and reductions in personal and couple’s quality of life [11].
Although ED is considered an age-related disease, affecting 20% of men aged >40 years, it can be present across the lifespan from adolescence, especially when risk factors such as diabetes, metabolic syndrome, cardiovascular diseases, physical inactivity and hypertension coexist [11]. Interestingly, it has been demonstrated not only that obesity is an independent risk factor for ED, but also that the incidence rate of ED is directly proportional to the level of obesity [12]. ED in patients affected by severe obesity has also been linked to a reduced responsiveness to phosphodiesterase 5 (PDE5) inhibitors. Moreover, obesity appears to be a driving factor in decreasing testosterone level since adipose cells secrete leptin, which results in a decrease in testosterone level [12, 13]. On the contrary, a reduction in adiposity among men with obesity has been shown to improve erectile function [9].
Although these studies provide important information, to date there is no meta-analysis of the synthesized data which would help provide the most reliable estimates of ED prevalence in men with obesity compared with controls. Given this, the aim of this study was to conduct a meta-analysis of existing data to estimate the prevalence of ED in men affected by obesity, compared to other measurements of adiposity.
2 Methods
This systematic review and meta-analysis is adherent to the PRISMA [14] and MOOSE [15] statements. The work followed a predetermined, but unpublished protocol.
2.1 Data sources and literature search strategy
Two investigators (NV and DP) independently conducted a literature search using PubMed, EMBASE, SCOPUS, Cochrane Central Register of Controlled Trials and Clinicaltrials.gov without language restriction, from database inception until 01st April 2019 for studies investigating the association between BMI and/or waist circumference (WC) and presence of ED. Any inconsistencies were resolved by consensus with a third author (LS).
In PubMed, the following search strategy was used: “(“erectile dysfunction“ OR “erectile function” OR “sexual dysfunction” OR “sexual function” OR “impotence“) AND (“Body Mass Index“[Mesh] OR “Obesity”[Mesh] OR “Overweight”[Mesh] OR “Body Mass Index“ OR “Quetelet Index” OR “Quetelet’s Index” OR “Quetelets Index” OR “BMI” OR “Obesity” OR “Obesities” OR “obese” OR “adipose tissue hyperplasia” OR “adipositas” OR “adiposity” OR “excess body weight” OR “obesitas” OR “overweight” NOT (“Controlled Clinical Trial”[Publication Type] OR “Randomized Controlled Trial” [Publication Type] “Clinical Trial” [Publication Type] OR “Clinical Trial, Phase IV” [Publication Type] OR “Clinical Trial, Phase III” [Publication Type] OR “Clinical Trial, Phase II” [Publication Type] OR “Clinical Trial, Phase I” [Publication Type])”. Reference lists of included articles were hand-searched to identify any potential additional relevant articles, whilst conference abstracts were excluded.
2.2 Study selection
Inclusion criteria for this meta-analysis were: i) observational studies (case-control, cross-sectional, prospective) reporting the prevalence of ED across standardized BMI categories [16] or reporting data regarding mean BMI values in ED vs. healthy controls or reporting other evaluations of adiposity (e.g. WC) in ED vs. healthy controls; and ii) used a validated tool for the detection of ED (e.g. the International Index of Erectile Function, IIEF-5 [17]);
2.3 Data extraction
Two independent investigators (NV and DP) extracted key data from the included articles in a standardized Excel sheet. A third independent investigator (LS) checked the extracted data. For each article, data were extracted on authors, year of publication, country, number of participants, demographics (mean age), methods of assessment of adiposity (BMI; WC) and of ED; number and type of adjustments.
2.4 Outcomes
The primary outcome was the prevalence of ED by standardized BMI category (<18.5, 18.5–24.9, 25–29.9, > 30 Kg/m2) and for degree of severity of obesity (30–34.9 and > 35 Kg/m2). In all analyses, normal weight men (18.5–24.9) were taken as the reference. In secondary analyses, odds ratios (ORs) adjusted for potential confounders for the association between BMI categories and ED, where these data were available, were included. Finally, means (with their standard deviations, SDs) for adiposity parameters (BMI, WC) in men with ED vs. healthy controls were reported.
2.5 Assessment of study quality
Study quality was assessed by two investigators (DP, LS) using the Newcastle-Ottawa Scale (NOS) [18, 19]. A third reviewer was available for mediation (NV). The NOS assigns a maximum of 9 points based on three quality parameters: selection, comparability, and outcome.
2.6 Data synthesis and statistical analysis
All analyses were performed using Comprehensive Meta-Analysis (CMA) 2. The primary analysis reported the prevalence of ED as absolute estimates (in %) with their 95% confidence intervals (CIs) and across standardized BMI categories taking normal weight men as reference. Next, ORs and 95% confidence intervals (CIs) were calculated, applying a random-effect model [20]. Moreover, the mean differences (MDs) with their 95% CI in men with ED vs. healthy controls were reported.
Heterogeneity across studies was assessed by the I2 metric and taking as measure of high heterogeneity an I2 > 50% and/or p < 0.05 [21]. The mean age of the total sample size as a potential moderator in case of high heterogeneity of the findings was investigated.
Publication bias was assessed by a visual inspection of funnel plots and calculating the Egger bias test [22]. In case of publication bias (p < 0.10), the trim and fill-analysis [23] was applied. For all analyses, a p value <0.05 was considered statistically significant.
3 Results
3.1 Search results
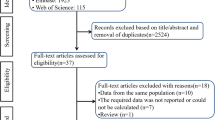
As shown in Supplementary Fig. 1, the searches produced 3409 non-duplicated articles. After excluding 3263 articles based on title/abstract review, 146 articles were retrieved for full text review and 45 articles were included in the qualitative/quantitative synthesis (the general data of selected studies are reported in Supplementary Table 1) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
3.2 Study and patient characteristics
The 45 studies included a total of 42,489 participants. The majority of the studies (n = 18) were conducted in Europe, 8 in Middle East and 8 in North America, 7 in Asia, 3 in Africa and 1 in Oceania. The mean age was 55 years (SD = 10.2). The median quality of the studies was 4.8 (range: 3–7), indicating an overall good quality of the studies, according to the NOS (Supplementary Table 2).
3.3 Prevalence and odds of ED by BMI categories
Table 1 shows the prevalence of ED in normal weight men, overweight and men with obesity. Overall, it was observed that the prevalence of ED in normal weight men was 34.8% (95%CI: 26.4–44.2; n = 9428), in overweight was 40.7% (95%CI: 32.5–49.4; n = 12,173) and in men with obesity 37.4% (95%CI: 27.3–43.0; n = 5540). In three studies reporting specific data for categories characterized by obesity, the prevalence of ED in those with a BMI between 30 and 34.9 was 71.5% and in those with a BMI > 35 Kg/m2 was 73.6% (Table 1).
Table 2 reports the prevalence of ED in overweight and in men with obesity, compared to normal weight participants. In 19 studies involving 21,601 participants, the prevalence of ED was significantly higher in overweight vs. normal weight men (OR = 1.31; 95%CI: 1.13–1.51; p < 0.0001; I2 = 72%). The mean age of the sample did not significantly moderate the results (beta = 0.81; p = 0.24). Similarly, in 15 studies reporting data regarding men with obesity, it was observed that the prevalence of ED was significantly higher in men affected by obesity than in the normal weight reference group (OR = 1.60; 95%CI: 1.29–1.98; p < 0.0001; I2 = 79%). Again, the mean age of the sample did not significantly moderate these results (beta = 0.25; p = 0.74). In three studies, a significantly higher presence of ED was found in men affected by obesity with a BMI between 30 and 34.9 Kg/m2 and in those with a BMI > 35 Kg/m2.
Figure 1 reports the association between overweight and ED (vs. normal weight men) after taking into consideration potential confounders. After adjusting for a mean of 5 (range: 1–14) confounders, no significant association between overweight status and ED in 11 studies was found (OR = 1.08; 95%CI: 0.93–1.26; p = 0.31; I2 = 82%). No evidence of publication bias was present for this outcome. After removing an outlier study in a sensitivity analysis (Štulhofer et al., 2006) the re-calculated OR was not significant (OR = 1.06; 95%CI:0.91–1.23; p = 0.45). Fig. 2 shows the association between obesity and ED, in multivariable analyses. Again, in 11 studies and after adjusting for a mean of 5 potential confounders, the OR was 1.46 (95%CI: 1.24–1.72; p < 0.0001; I2 = 72%). For this outcome the presence of publication bias was observed (Egger’s test: 1.70; p = 0.02). After trimming 3 studies at the left of the mean the re-calculated OR was 1.36 (95%CI: 1.15–1.61).
3.4 Mean differences in adiposity parameters between ED and controls
Figure 3 reports the mean difference between ED and controls. Overall, 3304 participants with ED were compared to 4450 without in 32 studies. The MD was 0.769 (95%CI: 0.565–0.973 Kg/m2; p < 0.0001; I2 = 78%). The presence of publication bias was observed (Egger’s test: 1.22; p = 0.003), but the trim and fill analysis did not change results. Mean age did not moderate results (beta = 0.21; p = 0.84). Similarly, in Fig. 4, it was found that in 16 studies comparing ED (1623 participants) vs. controls (2971 men), ED was significantly associated with higher WC values (MD = 5.251 cm; 95%CI: 1.295–9.208; p < 0.0001; I2 = 96%). No evidence for publication bias was present for this outcome and mean age of the sample did not explain any heterogeneity (beta = 0.21; p = 0.84).
4 Discussion
To our knowledge, this is the first meta-analysis to assess the prevalence of ED among men with obesity, synthesizing a large body of international literature, including 45 studies and 42,489 participants. The main results confirm the very high prevalence of ED in the studies included, ranging from 34.8% in normal weight men to almost 3/4 of participants with grade II-III obesity. Overall, taking normal weight men as reference, the prevalence of ED was significantly higher in overweight (31%) and in men with obesity (60%). After taking into consideration potential confounders, obesity was still independently associated with higher odds of having ED. Finally, men with ED reported significantly higher values of BMI and WC, further confirming that (central) obesity is associated with ED.
Considering that sexual function and penile erection is a bio-psychosocial process that involves the coordination of psychological, endocrine, vascular, and neurological systems [69], we can hypothesize that in men affected by obesity at least two mechanisms are probably involved, i.e. vascular and endocrinological pathways. From a vascular point of view, erection is the result of relaxation of the cavernosal smooth muscles, driven by the nitric oxide (NO) released by the endothelium, leading to compression of the subtunical small veins and occluding local venous return [70]. Indeed, endothelial dysfunction is the common etiologic factor for several vascular risk factors that can lead to arteriogenic ED. [71, 72] Thus, considering the well-known detrimental effects of adipose tissue dysfunction and of the associated metabolic abnormalities (including low grade chronic inflammation, high blood pressure, dyslipidemia and oxidative stress) in promoting impaired endothelial function [6], we can hypothesize obesity as major risk factor for ED.
From the endocrinological point of view, the role played by androgens (foremost testosterone) in enhancing sexual desire and maintaining adequate sleep-related erections is well established [73]. Hence, obesity directly impacts ED due to its action on testosterone. Importantly, adipocytes have high expression of aromatase that enzymatically converts testosterone to estradiol lowering circulating androgens. At the same time, estrogens causes a negative feedback mechanism upon the hypothalamo-pituitary axis suppressing gonadotropin-releasing hormone and, thus, luteinizing hormone, contributing to a reduction in gonadal testosterone release [74]. Moreover, testosterone is a key regulator for the expression of NO synthase inside the penis, exacerbating the previous described mechanism [75]. Reduction in plasma testosterone contributes to increased arterial stiffness, which in turn has been associated with increased cardiovascular risk [76]. The positive interaction between androgens and the vessel wall has been demonstrated both in animal models, where testosterone supplementation inhibits atheroma formation, both in men, where androgen withdrawal has been associated with decreased central arterial compliance and testosterone has been suggested to act as a protective factor against atherosclerosis because of its immunomodulating effects on plaque development and stability [76].
Finally, although the role of estradiol is not entirely clear, it is likely that a cycle exists by which the increased estradiol levels in men with obesity, further contribute to the progression of obesity [77]. Estradiol is secreted in the testis and adipose tissue trough the irreversible conversion of testosterone by aromatase. Its main negative effect on male gonadal function is due to the suppression of hypothalamic-pituitary-testicular axis, limiting testosterone secretion in the testis [77].
Importantly, growing evidence has shown the importance of targeting lifestyle factors commonly associated with ED. [78] In particular, exercise and caloric restriction could improve ED by acting both on risk factors of ED and on ED itself [79]. Indeed, physical activity has been shown to be inversely associated with ED. [80,81,82] Interestingly, authors showed that frequent vigorous exercise was associated with lower risk for ED. [80] Moreover, a small body of literature suggests that men with ED have higher calorie intake due to frequent eating and a Western diet pattern as opposed to a Mediterranean-style dietary pattern [83]. This aspect is crucial considering that currently available pharmacological treatments for ED are ineffective in a significant portion of men and only temporarily restore sexual activity with no long-term impact on the underlying vascular dysfunction or risk factors of ED. [84] Thus, the implementation of lifestyle changes, alone or in combination with pharmacotherapy, may represent a milestone in the treatment of ED.
Male infertility is a crucial aspect related both with obesity and ED. In fact, in addition to the aforementioned risks for hypogonadism and ED, increasing evidence highlights the negative effects of obesity on semen parameters [85]. The possible mechanisms include epigenetic (DNA methylation, histone modification and RNA alteration), hormonal and other factors such as ED, stress, inflammation and sleep apnea [85]. Obesity may also play a negative role in assisted reproductive technologies (ART). In fact, although these data are to be confirm by more and larger studies, men affected by obesity undergoing ART have a statistically significant decrease in live birth rate compared with normal-weight and present a higher risk of nonviable pregnancy [85].
The present study offers novel insights into the extent of ED among men with obesity. However, some limitations need to be considered. First, due to the small number of primary studies that provide complete clinical and biological (e.g. serum testosterone or estradiol levels) features of the participants, it was not possible to run meta-regression analyses using well-known independent risk factors for ED (such as dyslipidemia, hypertension, diabetes mellitus and depression) as moderators of findings. Importantly, after adjusting for confounders no significant association between overweight BMI category and ED was observed. In a sensitivity analyses, after removing a highly significant study, the present results became not statistically significant and in adjusted analyses no association was present between that BMI category and ED. Second, the data regarding abdominal obesity are limited only to a comparison of means between ED and controls, not taking in consideration potential confounders [12]. However, thedata shows that the ED group had, on average, 5 cm more abdominal girth than healthy controls suggesting an important role of abdominal adiposity for ED. Finally, data were mainly derived from cross-sectional and case-control studies that have inherent limitations, such as reverse causation.
In conclusion, the present study provides new insight on the prevalence of ED in men with obesity. Future prospective and longitudinal studies in populations with overweight and obesity are needed to characterize others risk factors such as diabetes, smoking and depression which are involved in the pathogenesis of ED. The findings suggest that clinicians should consider screening for ED in men affected by obesity as part of their routine cardiovascular disease risk assessment. Moreover, because of the common metabolic substrate of these two clinical conditions, managing obesity is a crucial approach in patients with ED who are affected by obesity. Physicians should also promote and stress the importance of lifestyle modifications when providing recommendations to patients regarding effective treatments for ED.
References
WHO. World Health Organization obesity and overweight fact sheet. In:2016.https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed August 2019).
Graham C, Mullen A, Whelan K. Obesity and the gastrointestinal microbiota: a review of associations and mechanisms. Nutr Rev. 2015;73(6):376–85.
Mazon JN, de Mello AH, Ferreira GK, Rezin GT. The impact of obesity on neurodegenerative diseases. Life Sci. 2017;182:22–8.
Jung SY, Park DC, Kim SH, Yeo SG. Role of obesity in Otorhinolaryngologic diseases. Curr Allergy Asthma Rep. 2019;19(7):34.
Poddar M, Chetty Y, Chetty V. How does obesity affect the endocrine system? A narrative review. Clinical obesity. 2017;7(3):136–44.
Vecchié A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. European journal of internal medicine. 2018;48:6–17.
Tomlinson D, Erskine R, Morse C, Winwood K, Onambélé-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17(3):467–83.
Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017;6(2):195–203.
Moon KH, Park SY, Kim YW. Obesity and erectile dysfunction: from bench to clinical implication. The world journal of men's health. 2019;37(2):138–47.
Škurla M, Rybář R. Obesity and reduced fertility of men. Ceska gynekologie. 2018;83(3):212–7.
Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34(9):1185–92.
Fillo J, Levcikova M, Ondrusova M, Breza J, Labas P. Importance of different grades of abdominal obesity on testosterone level, erectile dysfunction, and clinical coincidence. Am J Mens Health. 2017;11(2):240–5.
Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. ISA, ISSAM, and EAU recommendations. Eur Urol. 2005;48(1):1–4.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100–0.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA : the journal of the American Medical Association. 2000;283:2008–12.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:i-xii, 1–253.
Rosen RC, Cappelleri J, Smith M, Lipsky J, Pena B. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–26.
Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. (Available from: URL: http://wwwohrica/programs/clinical_epidemiology/oxfordasp). 2012:2012–2012.
Luchini C, Brendon S, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5:80–4.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of. Vol Version 5.2008.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(September):629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Adebusoye LA, Olapade-Olaopa OE, Ladipo MM, Owoaje ET. Prevalence and correlates of erectile dysfunction among primary care clinic attendees in Nigeria. Glob J Health Sci. 2012 Jun 8;4(4):107–17.
Ahmed A, Alnaama A, Shams K, Salem M. Prevalence and risk factors of erectile dysfunction among patients attending primary health care centres in Qatar. East Mediterr Health J. 2011 Jul;17(7):587–92.
Al-Hunayan A, Al-Mutar M, Kehinde EO, Thalib L, Al-Ghorory M. The prevalence and predictors of erectile dysfunction in men with newly diagnosed with type 2 diabetes mellitus. BJU Int. 2007 Jan;99(1):130–4.
Al-Turki YA. Erectile dysfunction among diabetic patients in Saudi Arabia: a hospital-based primary care study. J Family Community Med. 2007 Jan;14(1):19–23.
Andersen I, Heitmann BL, Wagner G. Obesity and sexual dysfunction in younger Danish men. J Sex Med. 2008 Sep;5(9):2053–60.
Bhojani N, Perrotte P, Hutterer G, Suardi N, Jeldres C, Shariat SF, et al. Body mass index and its association with genitourinary disorders in men undergoing prostate cancer screening. J Sex Med. 2008 Sep;5(9):2141–51.
Egan KB, Burnett AL, McVary KT, Ni X, Suh M, Wong DG, et al. The co-occurring syndrome-coexisting erectile dysfunction and benign prostatic hyperplasia and their clinical correlates in aging men: results from the National Health and nutrition examination survey. Urology. 2015 Sep;86(3):570–80.
Garimella PS, Paudel ML, Ensrud KE, Marshall LM, Taylor BC. Fink HA; osteoporotic fractures in men (MrOS) research group. Association between body size and composition and erectile dysfunction in older men: osteoporotic fractures in men study. J Am Geriatr Soc. 2013 Jan;61(1):46–54.
Ghalayini IF, Al-Ghazo MA, Al-Azab R, Bani-Hani I, Matani YS, Barham AE, et al. Erectile dysfunction in a Mediterranean country: results of an epidemiological survey of a representative sample of men. Int J Impot Res. 2010 May-Jun;22(3):196–203.
Goyal A, Singh P, Ahuja A. Prevalence and severity of erectile dysfunction as assessed by IIEF-5 in north Indian type 2 diabetic males and its correlation with variables. J Clin Diagn Res. 2013 Dec;7(12):2936–8.
Gündüz MI, Gümüs BH, Sekuri C. Relationship between metabolic syndrome and erectile dysfunction. Asian J Androl. 2004 Dec;6(4):355–8.
Han TS, Tajar A, O'Neill TW, Jiang M, Bartfai G, Boonen S, et al. Impaired quality of life and sexual function in overweight and obese men: the European male ageing study. Eur J Endocrinol. 2011 Jun;164(6):1003–11.
Holden CA, McLachlan RI, Pitts M, Cumming R, Wittert G, Ehsani JP, et al. Determinants of male reproductive health disorders: the men in Australia telephone survey (MATeS). BMC Public Health. 2010 Feb 24;10:96.
Janiszewski PM, Janssen I, Ross R. Abdominal obesity and physical inactivity are associated with erectile dysfunction independent of body mass index. J Sex Med. 2009 Jul;6(7):1990–8.
Karadag H, Oner O, Karaoglan A, Orsel S, Demir AU, Firat H, et al. Body mass index and sexual dysfunction in males and females in a population study. Klinik Psikofarmakoloji Bülteni-Bulletin of Clinical Psychopharmacology. 2014;24(1):76–83.
Palmer MR, Holt SK, Sarma AV, Dunn RL, Hotaling JM, Cleary PA, et al. Longitudinal patterns of occurrence and remission of erectile dysfunction in men with type 1 diabetes. J Sex Med. 2017 Oct;14(10):1187–94.
Seid A, Gerensea H, Tarko S, Zenebe Y, Mezemir R. Prevalence and determinants of erectile dysfunction among diabetic patients attending in hospitals of central and northwestern zone of Tigray, northern Ethiopia: a cross-sectional study. BMC Endocr Disord. 2017 Mar 15;17(1):16.
Stulhofer A, Bajić Z. Prevalence of erectile and ejaculatory difficulties among men in Croatia. Croat Med J. 2006 Feb;47(1):114–24.
Uffort EE, Jensen JC. Impact of obesity on early erectile function recovery after robotic radical prostatectomy. JSLS. 2011 Jan-Mar;15(1):32–7.
Zhang X, Yang B, Li N, Li H. Prevalence and risk factors for erectile dysfunction in Chinese adult males. J Sex Med. 2017 Oct;14(10):1201–8.
Aleid M, Muneer A, Renshaw S, George J, Jenkinson AD, Adamo M, et al. Early effect of bariatric surgery on urogenital function in morbidly obese men. J Sex Med. 2017 Feb;14(2):205–14.
Chao JK, Ma MC, Lin YC, Chiang HS, Hwang TI. Study on alcohol dependence and factors related to erectile dysfunction among aborigines in Taiwan. Am J Mens Health. 2015 May;9(3):247–56.
Chao J-K, Ma M-C, Chao I-HC. Erectile Dysfunction, Hormone Levels, Inflammation in Male Patients with Metabolic Syndrome. J Mens Health. 2018;14(4):e25–37.
Chao JK, Kuo WH, Chiang HS, Hwang TI, Chao IC, Chiang SK. Association of metabolic syndrome, atherosclerosis risk factors, sex hormones in ED in aboriginal Taiwanese. Int J Impot Res. 2012;24(4):141–6.
Dan A, Chakraborty K, Mondal M, Neogi R, Chatterjee S, Makhal M. Erectile dysfunction in patients with diabetes mellitus: its magnitude, predictors and their bio-psycho-social interaction: a study from a developing country. Asian J Psychiatr. 2014 Feb;7(1):58–65.
Derosa G, Romano D, Tinelli C, D'Angelo A, Maffioli P. Prevalence and associations of erectile dysfunction in a sample of Italian males with type 2 diabetes. Diabetes Res Clin Pract. 2015 May;108(2):329–35.
Dursun M, Besiroglu H, Cakir SS, Otunctemur A, Ozbek E. Increased visceral adiposity index associated with sexual dysfunction in men. Aging Male. 2018;21(3):187–92.
El Saghier EO, Shebl SE, Fawzy OA, Eltayeb IM, Bekhet LM, Gharib A. Androgen deficiency and erectile dysfunction in patients with type 2 diabetes. Clin Med Insights Endocrinol Diabetes. 2015;8:55–62.
Elzanaty S, Rezanezhad B, Willenheimer R, Borgquist R. Association between erectile function and biomarkers of subclinical atherosclerosis: a study based on middle-aged healthy men from the general population. Curr Urol. 2016;9(3):119–23.
Ettala OO, Syvänen KT, Korhonen PE, Kaipia AJ, Vahlberg TJ, Boström PJ, et al. High-intensity physical activity, stable relationship, and high education level associate with decreasing risk of erectile dysfunction in 1,000 apparently healthy cardiovascular risk subjects. J Sex Med. 2014 Sep;11(9):2277–84.
Fung MM, Bettencourt R, Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: the rancho Bernardo study. J Am Coll Cardiol. 2004 Apr 21;43(8):1405–11.
Gatti A, Mandosi E, Fallarino M, Radicioni A, Morini E, Maiani F, et al. Metabolic syndrome and erectile dysfunction among obese non-diabetic subjects. J Endocrinol Investig. 2009 Jun;32(6):542–5.
Giugliano F, Esposito K, Di Palo C, Ciotola M, Giugliano G, Marfella R, et al. Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men. J Endocrinol Investig. 2004 Jul-Aug;27(7):665–9.
Gonçalves MA, Guilleminault C, Ramos E, Palha A, Paiva T. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med. 2005 Jul;6(4):333–9.
Hermans MP, Ahn SA, Rousseau MF. Erectile dysfunction, microangiopathy and UKPDS risk in type 2 diabetes. Diabetes Metab. 2009 Dec;35(6):484–9.
Meluzín J, Vasků A, Kincl V, Panovský R, Srámková T. Association of coronary artery disease, erectile dysfunction, and endothelial nitric oxide synthase polymorphisms. Heart Vessel. 2009 May;24(3):157–63.
Min SK, Choi K, Kim SK, Lee GI, Cho IC. Phosphorus as predictive factor for erectile dysfunction in middle aged men: a cross sectional study in Korea. Investig Clin Urol. 2016 Nov;57(6):442–8.
Moore RH, Sarwer DB, Lavenberg JA, Lane IB, Evans JL, Volger S, et al. Relationship between sexual function and quality of life in obese persons seeking weight reduction. Obesity (Silver Spring). 2013 Oct;21(10):1966–74.
Al Naimi A, Majzoub AA, Talib RA, Canguven O, Al AA. Erectile dysfunction in Qatar: prevalence and risk factors in 1,052 participants-a pilot study. Sex Med. 2014 Jun;2(2):91–5.
Pohjantähti-Maaroos H, Palomäki A, Hartikainen J. Erectile dysfunction, physical activity and metabolic syndrome: differences in markers of atherosclerosis. BMC Cardiovasc Disord. 2011 Jun 27;11:36.
Pohjantähti-Maaroos H, Palomäki A. Comparison of metabolic syndrome subjects with and without erectile dysfunction - levels of circulating oxidised LDL and arterial elasticity. Int J Clin Pract. 2011 Mar;65(3):274–80.
Ramírez R, Pedro-Botet J, García M, Corbella E, Merino J, Zambón D, et al. Erectile dysfunction and cardiovascular risk factors in a Mediterranean diet cohort. Intern Med J. 2016 Jan;46(1):52–6.
Schipilliti M, Caretta N, Palego P, Selice R, Ferlin A, Foresta C. Metabolic syndrome and erectile dysfunction: the ultrasound evaluation of cavernosal atherosclerosis. Diabetes Care. 2011 Aug;34(8):1875–7.
Stolic RV, Bukumiric ZM. Intima-media thickness of carotid arteries and erectile dysfunction in hemodialysis patients. Hemodial Int. 2010 Oct;14(4):510–4.
Tsao CW, Hsu CY, Chou YC, Wu ST, Sun GH, Yu DS, et al. Is obesity correlated with sexual function in young men? J Androl. 2009 May-Jun;30(3):275–9.
Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20(1):17–29.
Lue TF. Erectile dysfunction. N Engl J Med. 2000;342(24):1802–13.
Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder?: a study of arterial risk factors in 440 impotent men. Lancet. 1985;325(8422):181–4.
La Vignera S, Vicari E, Condorelli RA, Di Pino L, Calogero AE. Arterial erectile dysfunction: reliability of penile Doppler evaluation integrated with serum concentrations of late endothelial progenitor cells and endothelial microparticles. J Androl. 2012;33(3):412–9.
Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2010;95(6):2536–59.
Yassin A, Nettleship JE, Talib RA, Almehmadi Y, Doros G. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. The Aging Male. 2016;19(1):64–9.
Traish AM, Munarriz R, O'Connell L, et al. Effects of medical or surgical castration on erectile function in an animal model. J Androl. 2003;24(3):381–7.
Foresta C, Caretta N, Lana A, et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006 Nov;91(11):4599–602.
Carrageta DF, Oliveira PF, Alves MG, Monteiro MP. Obesity and male hypogonadism: Tales of a vicious cycle. Obes Rev. 2019 Aug;20(8):1148–58.
Matsui H. A Sopko N, L Hannan J, J Bivalacqua T. pathophysiology of erectile dysfunction. Curr Drug Targets. 2015;16(5):411–9.
Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med. 2009;6:254–61.
La Vignera S, Condorelli RA, Cannarella R, Giacone F, Calogero AE. Arterial erectile dysfunction is an early sign of vascular damage: the importance for the prevention of cardiovascular health. Ann Transl Med. 2019;7(Suppl 3):S124.
Seo DY, Lee SR, Kwak HB, Park H, Seo KW, Noh YH, et al. Exercise training causes a partial improvement through increasing testosterone and eNOS for erectile function in middle-aged rats. Exp Gerontol. 2018;108:131–8.
Duca Y, Calogero AE, Cannarella R, Giacone F, Mongioi LM, Condorelli RA, et al. Erectile dysfunction, physical activity and physical exercise: recommendations for clinical practice. Andrologia. 2019;51(5):e13264.
Esposito K, Ciotola M, Giugliano F, de Sio M, Giugliano G, D'armiento M, et al. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res. 2006;18(4):405–10.
Sato Y, Tanda H, Kato S, Onishi S, Nitta T, Koroku M. How long do patients with erectile dysfunction continue to use sildenafil citrate? Dropout rate from treatment course as outcome in real life. Int J Urol. 2007;14(4):339–42.
Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril. 2017 Apr;107(4):848–59.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
(PNG 837 kb)
Supplementary Table 1
(DOCX 489 kb)
Supplementary Table 2
(DOCX 11 kb)
Supplementary Table 3
(DOCX 483 kb)
Rights and permissions
About this article
Cite this article
Pizzol, D., Smith, L., Fontana, L. et al. Associations between body mass index, waist circumference and erectile dysfunction: a systematic review and META-analysis. Rev Endocr Metab Disord 21, 657–666 (2020). https://doi.org/10.1007/s11154-020-09541-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09541-0