The influence of synthesis conditions on the development, thermal behavior, and sintering characteristics was investigated for nano- and macro-crystalline Bi5FeTi3O15-based ceramic materials with a perovskite-like four-layered Aurivillius phase structure. It was shown that the start of grains sintering corresponds with the start of surface phase melting, given that the surface phase composition can be controlled by changing the chemical composition of the initial mixture. The temperatures of crystallization, phase transition, decomposition, sintering activation of the produced materials were determined along with the thermal coefficient of their linear expansion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The search for effective methods for obtaining high-temperature multiferroics is an urgent problem of materials science in the field of creating new magnetic materials. Materials based on perovskite-like oxides are increasingly used in such rapidly developing areas of technology as nanoelectronics, spintronics, and photovoltaics [1, 2]. The functional properties and performance characteristics of such substances depend on their morphology, grain-size composition, structural and thermal stability.
Layered perovskite-like oxides that are Aurivillius phases [3] with the general formula Am–1Bi2BmO3m+3 (A —Bi; B — Ti, Fe) are promising magnetic materials, including high-temperature multiferroics [4,5,6]. The Bi5FeTi3O15 compound (number of layers m = 4) has a four-layer perovskite-like structure and a rhombic unit cell (space group Fmmm) at room temperature [7], in which four perovskite-like blocks {(Bi3FeTi3O13)2–} alternate with fluorite-like layers {(Bi2O2)2+}.
As shown in [8, 9], thermal stability of macrocrystalline ceramic materials based on the Aurivillius phases decreases with increasing iron content and the number of layers in the perovskite-like block. The composition of the perovskite-like layer of Aurivillius multilayer phases (m > 7) approaches the BiFeO3 composition, which is metastable [10,11,12] and has a limited temperature range of stability. Therefore, the compound Bi5FeTi3O15, as the extreme iron-containing component of the Am–1Bi2BmO3m+3 series of homologues, shows greater perspective of being used as a high-temperature multiferroic, since it has the highest thermal stability in the series of compounds under consideration and a high Curie temperature (TC = 750°C [8]). The study of physicochemical and technological factors that allow controlling the functional properties of materials based on this compound is an urgent task.
The purpose of the present work is a comparative study of the effect of synthesis conditions on the formation and thermal properties of nano- and macrocrystalline ceramic materials based on the Aurivillius phase Bi5FeTi3O15.
Experimental Procedure
Two methods were used to synthesize the compound Bi5FeTi3O15: low-temperature – coprecipitation from a solution of salts, and high-temperature – ceramic. In the first case, the material was synthesized by heat treatment of the precipitate obtained by coprecipitation from a solution of pure (at least 98% pure) bismuth nitrate Bi(NO3)3·5H2O and iron nitrate Fe(NO3)3·9H2O salts and titanium isopropoxide C12H28O4Ti (97%) in a 25% aqueous solution of NH4OH. The nitrates were pre-dissolved in dilute nitric acid at a concentration of 0.1 M (pH < 2). Titanium isopropoxide was dissolved in ethyl alcohol to prevent premature hydrolysis and added to the nitrate solution. The prepared clear solution was poured into an aqueous ammonia solution with a pH > 8. The resulting precipitate was washed on the filter with distilled water and dried. The sample was then calcined for 1 hr at each temperature in the range of 350 – 800°C.
In the second case, as the starting reagents, the following were used: bismuth oxides (>98% pure), iron (III) (>99% pure), titanium (IV) (>99.9% pure). After the initial mixture was homogenized, the sample was sequentially calcined for 1 hr at each temperature in the range of 500 – 900°C.
For calcining, aWiseTherm furnace (Germany) was used in the “heating – isothermal hold – cooling” regime. The microstructure and elemental composition of the samples were determined by scanning electron microscopy and energy dispersive microanalysis (FEI Quanta 200 Scanning Electron Microscope with EDAX attachment). The phase state of the samples was determined from x-ray diffraction data (XRF, Shimadzu XRD-7000 diffractometer, Cu Kα radiation, λ = 15.401 nm). The parameters of the elementary cells were calculated using the software package PDWin 4.0. The dimensions of the crystallites were determined by the Scherrer equation, which takes into account the width of the x-ray peak. The pycnometric density of powders was determined by helium pycnometry (Ultra Pycnometer 1000, Quanta Chrome).
Thermal behavior was investigated by differential scanning calorimetry (DSC) together with thermogravimetry (TG) in the range of 25–1250°C in air with a heating rate of 10°C/min (NETZSCH STA 429 analyzer). The change in the linear size of the sample was determined by dilatometry in air at a heating rate of 10°C/min (using a NETZSCH DIL 402 E dilatometer). Samples were made in the form of a tablet with a diameter of 5 and a thickness of 3 mm.
Results and Discussion
By the method of coprecipitation from a salt solution and by ceramic technology, nano- and macrocrystalline powders were obtained, in which the ratio of elements Bi:Fe:Ti, according to elemental analysis, corresponds to the stoichiometry of Bi5FeTi3O15.
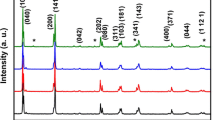
The results of x-ray phase analysis of samples at different stages of heat treatment are shown in Fig. 1. The main reflections of the target products obtained at the final stages of heat treatment are indicated and the parameters of the elementary cells are given in Table 1. The experimental data are consistent with the literature data [13], and the resulting compounds correspond to the four-layer Aurivillius phase Bi5FeTi3O15.
In x-ray diffraction patterns of the sample obtained by the coprecipitation method, up to 400°C only the amorphous phase is present (see Fig. 1a ). At 400°C, the fraction of this phase decreases, and, presumably, reflections of bismuth hydroxy-carbonate and hydroxy-nitrate appear. When the temperature is raised to 450°C, the Aurivillius phase crystallizes with an average crystal size d = 30 ± 5 nm. This temperature is consistent with the results of [14] and corresponds to the onset of formation of Aurivillius phases in “soft chemistry” conditions (450 ± 50°C), which is activated when the surface of particles is transformed into a liquid-like state and, correspondingly, mass transfer increases in the system. At 600°C, along with appreciable broadening, a splitting of the reflexes of the main phase and their shift begin to take place with a further increase in temperature. This suggests that the four-layer Bi5FeTi3O15 compound is formed sequentially and at the initial stages the system also contains the Aurivillius phase with the number of layers m < 4 or with an unordered alternation of layers in the perovskite-like block. In publications [9, 15] it was shown that the Aurivillius phases can have a complex alternation of perovskite-like layers in the block and for compounds obtained by ceramic technology, a similar formation mechanism was observed.
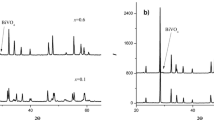
The described transformations correspond to the DSC/TG data of the coprecipitated sample (Fig. 2, curves 1, 2). The TG curve (curve 1) shows a two-step mass loss by the sample, accompanied by a complex shape peak on the DSC curve (curve 2) in the 400 – 600°C interval. Considering the x-ray phase analysis data, several processes occur simultaneously in this temperature range – the decomposition of residual amounts of hydroxycarbonates and hydroxynitrates and the onset of crystallization of Aurivillius phases with different values of m at 460 ± 5 and 560 ± 5°C. With a further increase in temperature, nanocrystals grow as the amorphous phase crystallizes. After isothermal holding for 1 hr at 700°C (see Fig. 1a ), the amorphous phase almost completely disappears. In this case, the absence of other crystalline phases in the reaction system shows that it is completely spent on the formation of nanocrystals of the main product. Heat treatment of the sample for 1 hr at 800°C leads to the formation of the Aurivillius phase of a given composition with an average crystallite size d = 80 ± 2 nm.
At temperatures above 900°C, a series of peaks associated with the decomposition of the material are visible on the DSC curve. A small endothermic peak at 970 ± 5°C can be associated with the decomposition of Bi2Fe4O9 [10] that is usually formed in the reaction system in the synthesis of Aurivillius phases above 800°C. The start temperature of the peritectic decomposition of the target product on the DSC curve corresponds to the beginning of the intense peak at 1120 ± 5°C. At a temperature of 1180 ± 5°C, corresponding to the end peak maximum on the DSC curve, complete melting of the material is achieved. These peaks are repeated on the DSC curve when the sample is cooled (see Fig. 2a, curve 2-1), and their shift to some extent is related to kinetic effects.
According to the x-ray phase analysis of the sample obtained by the ceramic preparation method (see Fig. 1b ), at 500 – 600°C, i.e., after the melting of the surface phase based on bismuth oxide, α-Bi2O3 interacts with titanium and iron oxides to form γ-Bi2O3 as the main phase. A small amount of the Aurivillius phase and BiFeO3 appears in the reaction system only at a temperature of 700°C, which is substantially higher than in the case of coprecipitation. The compounds γ-Bi2O3 (sillenite structure) and BiFeO3 (perovskite structure) are intermediate products in the synthesis of macrocrystalline Aurivillius phases by ceramic technology. Their formation in the reaction system promotes the formation of a layered perovskite-like structure of a given composition due to a gradual rearrangement in the first coordination sphere of bismuth [15], but the presence of these by-products slows down the synthesis kinetics. At temperatures above the onset of the decomposition of γ-Bi2O3 (>700°C), only reflections of the Aurivillius phases and BiFeO3 are observed on the diffractograms, the change in the intensity ratio of which indicates a gradual formation of a layered perovskite-like structure. The formation of a single phase target product occurs after heat treatment of the sample for 1 hr at 900°C, i.e., above the decomposition temperature of BiFeO3 (855 ± 5°C [10]). Thermal analysis data show that peritectic decomposition of macrocrystalline material, as well as of nanocrystalline material, starts at 1100 ± 5°C, and at 1200 ± 5°C it is completely melted (see Fig. 2b, curves 1, 2). A high-temperature phase transformation at 750°C corresponding to the Curie temperature is observed on the DSC curve (curve 2) [7].
Thermal expansion curves of the obtained materials (Fig. 3) illustrate the nature of grain sintering. The ΔL/L0 (T) curves of both samples have a maximum corresponding to the sintering temperature TS (Table 2). In the material obtained from the coprecipitated mixture, TS = 500 ± 5°C, i.e., the process of active sintering begins immediately after the crystallization of the main phase. As shown by electron microscopy data (Fig. 4a ) of the sample after the last stage of heat treatment at 800°C (see Fig. 1a ), a well-sintered nanocrystalline ceramic material with a low porosity was obtained (see Table 2).
The value of TS for the macrocrystalline material is higher than that for the nanocrystalline material and is about 790 ± 5°C, which also correlates with the temperature of formation of the main product (see Fig. 1b ) and with the surface phase composition of the grains. After the final heat treatment, the powder consists of grains of various sizes (see Fig. 4b ) and its porosity is higher than that of the nanocrystalline material (see Table 2). Thermal coefficients of linear expansion (TCLE) αt were determined (see Table 2) in the temperature range of 350–450°C, in which no appreciable phase transformations occur and materials can be used as bulk ceramics. Thus, the grain-size composition, the morphology of the material, the sintering conditions of the starting material and the value of αt can be controlled by the chemical composition of the starting mixture. The choice of the described technological regimes of synthesis of ceramic materials based on the Aurivillius phase Bi5FeTi3O15 affects the efficiency of their functional application, including at temperatures above ambient.
Conclusion
The processes of formation and thermal behavior of nano- and macrocrystalline ceramic materials based on Bi5FeTi3O15 are described. It is shown that in the synthesis by coprecipitation, the formation of Bi5FeTi3O15 nanocrystals occurs at 450°C. At 800°C, a single-phase nanocrystalline material with an average crystallite size of 80 ± 2 nmwas obtained. In the synthesis by ceramic technology, the beginning of the formation of the Aurivillius phase occurs at 700°C, and the target product in the form of a macrocrystalline powder is obtained at 900°C. In both cases, the start temperature of the sintering of grains correlates with the melting of their surface phase, the composition of which can be controlled by changing the chemical composition of the initial mixture. In this case, synthesis by coprecipitation results in a significant improvement in the sintering of the material.
References
Jiagang Wu, Zhen Fan, Dingquan Xiao, Jianguo Zhu, and John Wang, “Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures”, Progr. Mater. Sci., 84, 335 – 340 (2016). DOI: https://doi.org/10.1016/j.pmatsci.2016.09.001.
M. I. H. Ansari, A. Qurashi, and M. K. Nazeeruddin, “Frontiers, opportunities, and challenges in perovskite solar cells: A critical review”, J. Photochem. Photobiol. C: Photochem. Rev., 35, 1 – 24 (2018). DOI: https://doi.org/10.1016/j.jphotochemrev.2017.11.002.
B. Aurrivillius, “Mixed bismuth oxides with layer lattices I”, Ark. Kemi., Bd. 1, No. 1, 463 – 471 (1949).
L. Keeney, T. Maity, M. Schmidt, et al., “Magnetic field – induced ferroelectric switching in multiferroic aurivillius phase thin films at room temperature”, J. Am. Ceram. Soc., 96, 2339 – 2357 (2013). DOI: https://doi.org/10.1111/jace.12467.
T. Pikula, J. Dzik, P. Guzdek, et al., “Magnetic properties and magnetoelectric coupling enhancement in Bi5Ti3FeO15 ceramics”, Ceram. Int., 43(14), 11442 – 11449 (2017). DOI: https://doi.org/10.1016/j.ceramint.2017.06.008.
A. Y. Birenbaum and C. Ederer, “The potentially multiferroic Aurivillius phase Bi5Ti3FeO15: cation site preference, electric polarization, and magnetic coupling from first principles”, Phys. Rev. B, 90(21), 214109 – 214112 (2014). DOI: https://doi.org/10.1103/PhysRevB.90.214109.
G. A. Smolensky, V. A. Isupov, and A. I. Agranovskaya, “A new group of ferroelectrics (with a layered structure)” [in Russian], Fiz. Tverd. Tela, 1, 169 – 170 (1959).
N. A. Lomanova, V. L. Ugolkov, and V. V. Gusarov, “Thermal behavior of layered perovskite-like compounds in the Bi4Ti3O12–BiFeO3 system” [in Russian], Fizika i Khimiya Stekla, 33(6), 608 – 612 (2007). DOI: https://doi.org/10.1134/S1087659607060120.
N. A. Lomanova and V. V. Gusarov, “Phase states in the Bi4Ti3O12–BiFeO3 cross-section of the Bi2O3–TiO2–Fe2O3 system” [in Russian], Zh. Neorg. Khim., 56(4), 661 – 665 (2011). DOI: https://doi.org/10.1134/S0036023611040188.
M. I. Morozov, N. A. Lomanova, and V. V. Gusarov, “Specific Features of BiFeO3 Formation in a Mixture of Bismuth (III) and Iron (III) Oxides” [in Russian], Zh. Obshch. Khim., 73(11), 1772 – 1776 (2003). DOI: https://doi.org/10.1023/B: RUGC.0000018640.30953.70.
M. Valant, A.-K. Axelsson, and N. Alford, “Peculiarities of a solid-state synthesis of multiferroic polycrystalline BiFeO3”, Chem. Mater., 19, 5431 – 5436 (2007). DOI: https://doi.org/10.1021/cm071730+.
T. Rojac, A. Bencan, B. Malic, et al., “BiFeO3 ñeramics: processing, electrical, and electromechanical properties”, J. Am. Ceram. Soc., 97, 1993 – 2011 (2014). DOI: https://doi.org/10.1111/jace.12982.
Ch. H. Hervoches, A. Snedden, R. Riggs, et al., “Structural behavior of the four-layer Aurivillius-phase ferroelectrics SrBi4Ti4O15 and Bi5Ti3FeO15”, J. Sol. St. Chem., 164, 280 – 291 (2002). DOI: https://doi.org/10.1006/jssc.2001.9473.
N. A. Lomanova, M. V. Tomkovich, V. L. Ugolkov, and V. V. Gusarov, “Formation and Thermal Properties of Nanocrystalline Bi4Ti3O12” [in Russian], Zh. Prikl. Khim., 90(6), 673 – 679 (2017). DOI: https://doi.org/10.1134/S1070427217060015.
M. I. Morozov and V. V. Gusarov, “Synthesis of Am–1Bi2MmO3m+3 compounds in the Bi4Ti3O12–BiFeO3 system” [in Russian], Zh. Neorg. Khim., 38(7), 867 – 874 (2002). DOI: https://doi.org/10.1023/A:1016252727831.
The work was financially supported by the Russian Foundation for Basic Research (grant no. 16-03-01056).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 6, pp. 29 – 33, June 2018.
Rights and permissions
About this article
Cite this article
Lomanova, N.A. Synthesis and Thermal Properties of Nano- and Macro-Crystalline Ceramic Materials Based on Bi5FeTi3O15. Refract Ind Ceram 59, 301–305 (2018). https://doi.org/10.1007/s11148-018-0225-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-018-0225-1