Abstract
Solid solutions Bi4V2-x Fe x/2Nb x/2O11-δ (х = 0.05–1.0) and Bi4V2-х-y Fe x Nb y O11–δ (with fixed x or y = 0.2 and variable х or y = 0.2–0.5 with step 0.1) were synthesized by the standard ceramic technology in the temperature range 773–1113 K and by mechanochemical activation method using Bi2O3, V2O5 Fe2O3, and Nb2O5 oxides as initial compounds. The formation of solid solutions was studied. Ranges of stability and temperature values of phase transitions for different polymorphous modifications were defined using dylatometric and thermo gravimetric studies. The morphology and the local chemical composition of the ceramic samples were studied. Samples with concentration of dopants x > 0.3 contain two phases; both major and impurity phases are solid solutions of the BIFENBVOX type although the dopants atoms distribution between them is random. The thermal expansion coefficients (TEC) were measured. The electrical conductivity of ceramic samples was investigated in a wide range of temperatures. The highest conductivity values among the studied solid solutions are observed for the sample with a small amount of dopants x = 0.25.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bi2O3-based complex oxides form a wide range of materials which drive attention of fundamental and applied science. Among the practically important physical-chemical properties of these compounds, there are oxygen-ionic and mixed electrical conductivity at the intermediate temperature range (500–900 K), ferroelectric and magnetic effects. The trend of the modern scientific investigations has become a complication of composition and structure of bismuth-containing compounds in order to find materials which are characterized by not only high electrical conductivity but also by sufficient stability in reducing atmospheres, absence of polymorphic transitions with varying temperature. Bi4V2O11-based solid solutions where vanadium is partly substituted by another cation Me form the BIMEVOX family. There are two different notations of these solid solutions which are used in scientific literature: Bi4V2-х Ме х O11-δ [1–3] or Bi2V1-z Ме z O5.5-δ [4], where z = x/2. The highest conductivity of the family members is usually characteristic for high-temperature tetragonal γ-modification. Its stabilization at room temperature can be achieved by some methods including doping of vanadium by several (more than one) cations. A number of publications concern double substitution of vanadium by metal cations resulting in the formation of BIMEME'VOX solid solutions, where МE is bivalent cation, МE' is tetravalent or pentavalent metal cation due to possible enhance of ionic conductivity. Authors [5] performed double substitution of vanadium by copper and titanium to form a solid solution BICUTIVOX (Bi2V0.9Cu0.05Ti0.05O5.4). All calculated X-ray profiles, cell parameters, and theoretical density values corresponded to the γ-BICUVOX.10 (Bi2V0.90Cu0.1O5.35) sample. The conductivity value of Bi2V0.9Cu0.05Ti0.05O5.4 at 573 K was equal to 10−2 Ω−1 cm−1 and was comparable to that of BICUVOX.10. The paper [6] concerns composition synthesized by the standard solid state technology as well as by hot compression method. The latter sample was characterized by a slightly higher relative density (97 %) comparing to the solid state sample (95 %). Electrical conductivity was estimated by means of impedance spectroscopy method. For the sample synthesized by hot compression method, the conductivity was equal to 7 × 10−2 Ω−1 cm−1 at 773 K. Authors [7–10] showed that solid solution BICUTIVOX (Bi4V2–x Cu x/2Ti x/2O11–δ) is formed by simultaneous doping of vanadium sublattice by copper and titanium and is crystallized in tetragonal γ-modification only at 0.25 ≤ х ≤ 0.30 (Sp.gr. I4/mmm). But at the same time, inserting of titanium atoms into the solid solution crystal lattice is random and is accompanied by formation of non-controlled micro-impurities of phases enriched by titanium. Moreover, prolong stability of BICUTIVOX γ-modification is impossible as it was shown by long-term annealing at heating-cooling cycles [9].
Authors [11] synthesized Bi2V0.9Co0.1-x Ti x O5.35+x (0.02 ≤ x ≤0.08) through standard solid state reaction method, and it was found that the tetragonal γ-phase was stabilized and γ/↔γ phase transition was observed for all compositions studied. The thermal dependence of total ionic conductivity revealed maximum (2.64 × 10−4 Ω−1 cm−1) for x = 0.04 at 573 K.
The article [2] concerns double substitution of vanadium by niobium and copper with the summary amount of dopants 10 %. The Bi4V1.8Cu0.2-x Nb x O10.7-3x/2 solid solution crystallizes at 0 < х < 0.05 in tetragonal γ-phase and at 0.1 < х < 0.15 forms orthorhombic β-modification. Electrical conductivity values of Bi4V1.8Cu0.15Nb0.05O10.625 are higher than that of the doped by copper solely Bi4V1.8Cu0.2O10.7. The authors note that increasing of the final temperature and time of synthesis results in increasing of grain sizes (from 8–12 μm at 1073 K to 15–20 μm at 1103 K) and appearance of cracks on the surface of the sintered pellets of Bi4V1.8Cu0.2-x Nb x O10.7-δ.
Samples of BICUMGVOX (Bi2Cu0.10−x Mg x V0.9O5.35) were synthesized by citrate sol–gel method [12]. It was found that the highly conductive γ–phase was effectively stabilized at room temperature for compositions with x ≥ 0.06, and their thermal stability increases with Mg content. The highest value of conductivity 1.5 × 10−3 Ω−1 cm−1 at 573 K is obtained fщк the composition x = 0.06.
The efficiency of iron and niobium atoms as dopants for formation of stable and high-conducting phases of BIMEVOX solid solutions was shown in the articles [13–17]. The present paper concerns synthesis and properties of solid solutions denoted as BIFENBVOX with variation of iron and niobium atoms ratio namely Bi4V2-x Fe x/2Nb x/2O11-δ and Bi4V2-х-y Fe x Nb y O11–δ.
Single-doped solid solutions BINBVOX and BIFEVOX were studied previously, and it was shown that niobium and iron are the most prospective dopants from the stability and conductivity values point of view. In the present work, we tried to combine these systems.
Experimental
Synthesis procedures
The objects of the present paper are two series of solid solutions of the following compositions: Bi4V2-x Fe x/2Nb x/2O11-δ (х = 0.05–1.0) and Bi4V2-х-y Fe x Nb y O11-δ (with fixed x or y = 0.2 and variable х or y = 0.2–0.5 with step 0.1). Synthesis was made using Bi2O3, V2O5 Fe2O3, and Nb2O5 oxides as initial compounds and by two methods. The standard ceramic technology was used for synthesis in the temperature range 773–1113 K with the step 50 K and stepwise regrindings in ethanol. Mechanochemical activation method involved the AGO-2 balling mill (maximum centrifugal factor g = 60) treatment by steps and the maximum treatment time t = 23 min.
Characterization of powders and ceramics
The phase composition of the products was controlled by X-ray method (DRON-3 diffractometer, CuK α -radiation, pyrolitical graphite monochromator, and reflected beam). Calculation of the unit cell parameters was made using TOPAS [18] and LMGP [19] software packages. Particle sizes were estimated by means of SALD-7101 disperse analyzer. The obtained powders were pressed into pellets followed by annealing at 1093 K during 2 h. Microstructure analysis of the samples was carried out using scanning electron microscope JEOL JSM 6390LA, equipped with EDX micro-analyzer JED 2300. Frequency vibration characteristics of metal-oxygen bonds were determined by means of IR Fourier method (Thermo Scientific Nicolett 6700 spectrometer, diffuse reflection method). Dilatometric analysis of the sintered pellets was carried out by means of DIL 402 C Netzsch dilatometer (heating rate 2 deg/min, temperature range 293–923 K). Differential thermal analysis was carried out in the temperature range 293–973 K using STA 409 PC Luxx Netzsch thermoanalyzer (Al2O3 as a reference standard). Electrical conductivity was measured by impedance spectroscopy method (impedance meters Elins Z-2000, Elins Z-350 M) within the temperature range 1073–473 K and the frequency range 1 Hz–2000 KHz.
Results and discussion
BIFENBVOX powders and ceramics characterization
According to the X-ray diffraction analysis data, the homogeneity region of the Bi4V2-x Fe x/2Nb x/2O11-δ solid solutions with the equivalent dopants concentration in the studied series was determined to be equal to x ≤ 0.575. Increasing summary concentration of the dopants higher than that of x ≥ 0.575 results in formation of BiVO4 impurity phase. It was determined that at x = 0.1–0.15 the monoclinic α-modification of the solid solutions is formed (Sp.gr. C2/m). Samples with 0.2 < x < 0.575 crystallize at room temperature as γ-modification (Sp.gr. I4/mmm).
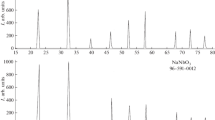
All Bi4V2-х-y Fe x Nb y O11-δ samples had tetragonal structure and were attributed to γ-modification (Sp.gr. I4/mmm). However, the excess of niobium (х = 0.2, y = 0.5) results in appearance of BiVO4 bismuth vanadate impurity. Thus, compositions with the fixed concentration of niobium y = 0.2 and variable concentration of iron up to х = 0.5 are included into the homogeneity region of the solid solutions, and the limit in this case is х > 0.5. To the contrary, the solid solutions series with х = 0.2 (fixed amount of iron) have a limit of homogeneity region y < 0.5. Figure 1a, b shows the examples of X-ray plots of single- and multi-phase samples with different dopants concentration.
Figure 2 shows an example of unit cell parameters plots vs. dopant concentration for Bi4V2-x Fe x/2Nb x/2O11-δ where regions of different structural modifications are marked. Inessential change of parameters corresponds to the closeness of radii of dopant ions with the appropriate coordination (VI)—iron, niobium, and vanadium ions (rV 5+ = 0.54 Å, rFe 3+ = 0.55 Å, and rNb 5+ = 0.64 Å).
In order to determine the mechanism of the mechanochemical synthesis of Bi4V2-x Fe x/2Nb x/2O11-δ (х = 0.3), the diffraction spectra of the mixtures treated during different periods of time were analyzed. Compositions of the mixtures are shown in Table 1. The mixture treated during 0.5 min contains the highest amount of different intermediate phases. The initial Bi2O3 oxide is present in the mixture as both α- and β-modifications. It reacts totally with other components forming complex oxides after 2 min of mechanical activation. The final product Bi4V2-x Fe x/2Nb x/2O11-δ starts to appear also after 2 min of mechanical activation. Solid solutions formation goes through formation of BiVO4 vanadate which is characteristic for BIMEVOX solid solutions synthesis [9, 15–17]. The γ-phase of BIFENBVOX is formed after 8 min of mechanical treatment and is totally crystallized after additional 3-h annealing at 873 K. Figure 3 shows the evolution of the X-ray patterns of mechanochemical synthesis of Bi4V1.7Fe0.15Nb0.15O11-δ in steps for different times of treatment. This corresponds to the general way of mechanochemical synthesis of BIMEVOX where the dopants are solely iron or niobium [15, 16]. The average grain size of the synthesized powders does not significantly depend on dopants concentration and lies within the 1–50 μm range with the maximum at 10–30 μm. Applying mechanochemical method of synthesis allows obtaining powders with larger part of small particles of about 1–5 μm size.
The frequency characteristics of metal-oxygen groups were determined by means of IR-spectroscopy. Major characteristic lines for some compositions are given in Table 2. The IR-spectra of the pure bismuth vanadate α-Bi4V2O11 and BIFENBVOX solid solutions are characterized by a series of bands corresponding to valent and deformational oscillations of V–O bonds in VO4 polyhedra, valent, and deformational oscillations of O–V–O bonds in the (VO3.5◻0.5)2− layer and Bi-O oscillations in the Bi2O2 layer (Table 2). These results correspond to those in the paper [20] indicating mainly tetrahedral coordination of vanadium atoms surrounded by oxygen atoms. Bands in the low-frequency region 70–200 cm−1 according to the paper [21] also correspond to the oscillations of the bismuth-oxygen Bi2O2 layer. Asymmetric oscillations indicate possible distortions of V–O polyhedra. Oscillations of the FeO4 group were observed in the 600–800 cm−1 region [22]. Increasing of dopant concentration results in decreasing of intensity and number of asymmetric valent V–O oscillations, as well as of frequency of symmetrical valent oscillations. As it was previously mentioned with respect to other BIMEVOX systems (ME = Ca, Hf) such a change of IR-spectrum may indicate the insertion of a dopant (in our case Nb and Fe) into vanadium positions in the VO4 polyhedra [23, 24].
In order to establish possible polymorphism of BIFENBVOX, the dilatometric and thermal methods were used. Samples which crystallize at room temperature in α-modification, e.g., Bi4V2-xFex/2Nbx/2O11-δ (х = 0.1) (Fig. 4a), are characterized by significant changes of the linear dimensions and TEC values due to α↔β↔γ phase transitions during heating and cooling. These changes are accompanied by evident temperature hysteresis. Compositions that crystallize at room temperature in γ-modification show practically linear behavior of the plots (Fig. 4b). Increasing of dopants concentration results in diminishing hysteresis between heating and cooling plots which indicates increasing of structure stability. LTEC values for BIFENBVOX are equal to (14–18) × 10−6 K−1 and correspond to those of the compositions doped with iron solely [15].
The DSC/TG plot recorded for the Bi4V1.6Fe0.2Nb0.2O11-δ sample is presented in Fig. 5 as an example for the system. The absence of any peak in the DSC plot and gassing during heating-cooling cycle of the γ-phase samples confirms its tetragonal symmetry and is in a good agreement with the above mentioned X-ray results.
In order to study the electrical conductivity, the BIFENBVOX powders were pressed into pellets with the sintering density of 75–80 % which is less than for BIFEVOX or BINBVOX (more than 90 %). Microstructure of the sintered pellets on the surface and in the bulk was studied by means of scanning electron microscopy method (SEM). As for example, Fig. 6 shows the photomicrographies of the surface and cross-section of Bi4V1.4Nb0.2Fe0.4O11-δ briquette where pores with approximate diameter 1–2 μm are seen. The surface contains impurities which are characterized by enlarged concentration of iron comparing to the major phase. This was also proved by EDX method. Similar impurities are detected also in the cross-section of the briquettes but in a significantly smaller amount. The second phase cannot be seen by XRD method. The similar situation was observed for BICUTIVOX samples [9] where the impurity phase was described as BIMEVOX composition with only one dopant. Its structure was described as tetragonal unit cell (Sp.gr. I4/mmm) like in the major phase, and its unit cell parameters were close to those of the major phase. In our case, the iron concentration in the impurity phase is approximately 2.5 times larger than in the major phase, and the ratio of the major components (Bi:V) is close to the theoretical (4:1.4). Therefore, both major and impurity phases are solid solutions of the BIFENBVOX type although the dopants atoms distribution between them is random.
Electrical characterization of BIFENBVOX
Electrical conductivity of BIFENBVOX was studied by means of impedance spectroscopy method using platinum electrodes. Typical experimental Nyquist plots of BIFENBVOX at different temperatures and modeling results using equivalent circuits approach are shown in Figs. 7 and 8. Modeling of the diagrams was carried out using Zview software package (Version 2.6b, Scribner Associates, Inc.). Examples of equivalent circuits modeling in two temperature ranges are presented in Table 3. According to the CPE1-T and CPE2-T values equal to 10−12 and 10−10 F accordingly, the high-frequency semicircle at low temperature is attributed to the bulk conductivity of the sample and the low-frequency one describes the blocking effect of the grain boundaries [25]. Thus, the total conductivity of the sample without taking into consideration the electrode processes is calculated as R = Rb + Rgb.
Increasing temperature results in the change of the shape of the diagram and at the same time the sum of bulk and grain boundary resistances corresponds to R1 resistance of the appropriate equivalent circuit.
According to the impedance measurements data, the temperature dependencies of the BIFENBVOX total conductivity were plotted (Fig. 9). The general shape of the temperature conductivity plots of the solid solutions studied is characteristic for the BIMEVOX family. For the samples with low dopant concentration (up to x + y ≤ 0.2), the changes of slope are observed in the temperature range 823–743 K. They correspond to consecutive phase transitions of γ → β → α polymorphs and are characterized by change of conductivity activation energy. While increasing dopants concentration, the plots smooth out and become almost linear. Insignificant change of slope of the lg σ–103/T plot accompanied by increasing of activation energy from 0.4–0.6 eV to 0.7–0.9 eV while cooling corresponds to transition into the ordered γ-modification of the solid solutions [26]. There is a very good convergence of both conductivity values and general shape of conductivity plots for the samples synthesized by conventional solid state route and by mechanical activation method. As for example, Fig. 10 shows the temperature conductivity dependencies for the х = 0.3 sample. The highest conductivity values among the studied solid solutions are observed for the sample with a small amount of dopants x = 0.25 which is typical for BIMEVOX family. As a whole conductivity, values of BIFENBVOX are comparable to that of the BIFEVOX and BINBVOX (Table 4). However, the conductivity values of these compounds are slightly lower than those observed for Bi4V2O11 doubly substituted samples. But ceramics are different concerning the relative density, for example, Bi4V1.8Cu0.15Nb0.05O10.625 with relative density 84 % had conductivity 10−1 Ω−1 cm−1 at 873 K [2]; Bi2V0.9Cu0.05Ti0.05O5.4 at 573 K with relative density 97 % had the conductivity value equal to 6.9 × 10−2 Ω−1 cm−1 [6]. For Bi2V0.9Co0.1-xTixO5.35+x [11] and Bi2Cu0.10−xMgxV0.9O5.35 [12], values of relative density are unknown.
In the whole, the wide region of structural stability of the solid solutions BIFENBVOX and high enough conductivity value makes the studied compositions prospective for further investigation and testing as components of electrochemical devices.
Conclusions
Solid solutions Bi4V2-x Fe x/2Nb x/2O11-δ (х = 0.05–1.0) and Bi4V2-х-y Fe x Nb y O11-δ (with fixed x or y = 0.2 and variable х or y = 0.2–0.5 with step 0.1) were synthesized. Samples with concentration of dopants x > 0.3 contain two phases; both major and impurity phases are solid solutions of the BIFENBVOX type although the dopants atoms distribution between them is random. The homogeneity region of the Bi4V2-x Fe x/2Nb x/2O11-δ solid solutions with the equivalent dopants concentration in the studied series was determined to be equal to x ≤ 0.575: at x = 0.1–0.15, the monoclinic α-modification of the solid solutions is formed (Sp.gr. C2/m). Samples with 0.2 < x < 0.575 crystallize at room temperature as γ-modification (Sp.gr. I4/mmm). However, the excess of niobium (х = 0.2, y = 0.5) results in appearance of BiVO4 bismuth vanadate impurity. Thus, compositions with the fixed concentration of niobium y = 0.2 and variable concentration of iron up to х = 0.5 are included into the homogeneity region of the solid solutions and the limit in this case is х > 0.5. To the contrary, the solid solutions series with х = 0.2 (fixed amount of iron) have a limit of homogeneity region y < 0.5. The mechanochemical synthesis of BIFENBVOX corresponds to the general way of the process of BIMEVOX where the dopants are solely iron or niobium, forming single-phase solid solution after 8–13 min of treatment. Linear TEC values for BIFENBVOX are equal to (14–18) × 10−6 K−1 and correspond to those of the compositions doped with iron solely. The highest electrical conductivity values among the studied solid solutions are observed for the sample with a small amount of dopants x = 0.25 (around 10−2 Ω−1 cm−1 at 1073 K). Activation energy of total conductivity is 0.4–0.6 eV at high temperatures and 0.7–0.9 eV at low temperatures.
References
Morozova MV, Buyanova ES, Emelyanova JV, Zhukovskiy VM, Petrova SA (2011) Highconducting oxide ceramics BIMEVOX: Synthesis, structure, and properties. Solid State Ionics 192:153–157. doi:10.1016/j.ssi.2010.04.020
Alga M, Ammar A, Tanouti B, Outzourhit A, Mauvy F, Decourt R (2005) Effect of niobium doping on structural, thermal, sintering and electrical properties of Bi4V1.8Cu0.2O10.7. J Solid State Chem 178:2873–2879. doi:10.1016/j.jssc.2005.06.030
Guillodo M, Bassat JM, Fouletier J, Dessemond L, Del Gallo P (2003) Oxygen diffusion coefficient and oxygen exchange coefficient of BIMEVOX.10 (ME=Cu, Co) ceramic membranes. Solid State Ionics 164:87–96. doi:10.1016/S0167-2738(03)00306-0
Krok F, Abrahams I, Malis M, Bogusz W, Nelstrop JAG (1997) Structural and electrical characterisation of BICOCUVOX. Ionics 3:235–238
Paydar MH, Hadian AM, Fafilek G (2001) Studies on preparation, characterization and ion conductivity of Ti – Cu double substituted Bi4V2O11. J Eur Ceram Soc 21:1821–1824
Fuierer P, Maier R, Röder-Roith U, Moos R (2011) Processing issues related to the bi-dimensional ionic conductivity of BIMEVOX ceramics. J Mater Sci 46:5447–5453. doi:10.1007/s10853-011-5486-8
Emel’yanova YV, Tsygankova EN, Petrova SA, Buyanova ES, Zhukovskii VM (2007) Synthesis, structure, and conduction of solid solutions BIMEVOX (Me = Cu, Ti) R. J Electrochem 43(6):737–741. doi:10.1134/S1023193507060183
Buyanova ES, Petrova SA, Emel’yanova YV, Borodina NA, Zakharov RG, Zhukovskii VM (2009) Crystal structure and conduction of BICUTIVOX R. J Inorg Chem 54(6):864–872. doi:10.1134/S0036023609060084
Buyanova ES, Morozova MV, Emel’yanova YV, Mikhailovskaya ZA, Petrova SA, Zakharov RG, Tarakina NV, Zhukovskii VM (2011) Some features of the preparation, structure, and properties of BICUTIVOX R. J Inorg Chem 56(12):1853–1857. doi:10.1134/S0036023611120291
Morozova MV, Buyanova ES, Emelyanova YV, Zhukovskiy VM, Petrova SA, Zakharov RG, Tarakina NV (2011) Specific features in the synthesis, crystal structure and electrical conductivity of BICUTIVOX. Solid State Ionics 201:27–34. doi:10.1016/j.ssi.2011.07.010
Beg S, Salami NS (2014) Study on the electrical properties of Co–Ti double substituted Bi4V2O11. J Alloys Compd 586:302–307
Beg S, Hafeez S, Al-Areqi NAS (2014) Study of phase stabilization and oxide–ion conductivity in BICUMGVOX solid electrolyte. Solid State Ionics 261:125–130
Joubert O, Jouanneaux A, Ganne M, Vannier RN, Mairesse G (1994) Solid phase synthesis and characterization of new BIMEVOX series: Bi4V2−xMxO11 (M = Sbv, Nbv). Solid State Ionics 73(3–4):309–318. doi:10.1016/0167-2738(94)90049-3
Steil MC, Ratajczak F, Capoen E, Pirovano C, Vannier RN, Mairesse G (2005) Thermal stability and preparation of dense membrane ceramics of BIMEVOX. Solid State Ionics 176:2305–2312. doi:10.1016/j.ssi.2005.06.017
Buyanova ES, Petrova SA, Emel’yanova YV, Blinova AL, Morozova MV, Zhukovskii VM, Zhuravlev VD (2009) Preparation, structure, and charge transport characteristics of BIFEVOX ultrafine powders R. J Inorg Chem 54(8):1193–1204. doi:10.1134/S0036023609080051
Buyanova ES, Morozova MV, Emel’yanova YV, Petrova SA, Zakharov RG, Zhukovskii VM (2013) Synthesis, structure, and conductivity of BINBVOX ceramics R. J Inorg Chem 58(3):259–264. doi:10.1134/S0036023613030030
Buyanova ES, Morozova MV, Emelyanova JV, Petrova SA, Zakharov RG, Tarakina NVC, Zhukovskiy VM (2013) Structure, thermal stability and electrical conductivity of BINBVOX. Solid State Ionics 243:8–17. doi:10.1016/j.ssi.2013.04.009
Diffrac Plus: Topas Bruker AXS GmbH, Ostliche. Rheinbruckenstraße 50, D-76187, Karlsruhe, Germany. 2006
Laugier J, Bochu B (2003) // LMGP-suite of programs for the interpretation of X-ray experiments. ENSP. Grenoble, Lab. Materiaux Genie Phys
Alga M, Ammar A, Essalim R, Tanouti B, Mauvy F, Decourt R (2005) Synthesis, sintering and electrical properties of P-doped Bi4V2O11 ceramics. Solid State Sci 7:1173–1179. doi:10.1016/j.solidstatesciences.2005.06.011
Sannes NM, Tompsett G, Cartner AM (1995) Characterization of bismuth lead oxide by vibrational spectroscopy. J Mater Sci 30(17):4299–4308
Wu Y, Wang X (2011) Preparation and characterization of single-phase α-Fe2O3 nano-powders by pechini sol–gel method. Mater Lett 65:2062–2065. doi:10.1016/j.matlet.2011.04.004
Beg S, Al-Areqi NAS, Al-Alas A (2009) Composition dependence of the phase transition and ionic conductivity in BIHFVOX system. J Alloys Compd 479:107–112
Beg S, Hafeez S, Al-Areqi NAS (2010) Influence of calcium substitution on the phase transition and ionic conductivity in BICAVOX oxide ion conductor. Phase Transit 83:169–181. doi:10.1080/01411591003607263
Irvine JTS, Sinclair DC, West AR (1990) Electroceramics: characterization by impedance spectroscopy. Adv Mater 2(3):132–138
Abrahams I, Krok F (2002) Defect chemistry of the BIMEVOXes. J Mater Chem 12:3351–3362. doi:10.1039/B203992N
Acknowledgments
This research project has been supported by UrFU under the Framework Program of development of UrFU through the “Young scientists UrFU” competition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buyanova, E.S., Morozova, M.V., Emelyanova, Y.V. et al. Synthesis, structure, and properties of BIFENBVOX. Ionics 21, 2815–2823 (2015). https://doi.org/10.1007/s11581-015-1483-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1483-2