Penetration of sodium into carbon-graphite material (CGM) specimens previously modified with lithium is studied. Sodium diffusion coefficients are calculated after treating CGM with lithium vapor and values are determined for activation energy of diffusion under different conditions. The kinetic dependences obtained make it possible to determine the sodium diffusion mechanism in modified CGM. The efficiency is demonstrated of preliminary treatment with lithium vapor that makes it possible to prevent aluminum electrolyzer cathode lining surface layer breakdown during operation. The tests on CGM specimens performed make it possible to create prerequisites for developing technology for hearth surface protection from sodium penetration during electrolysis in molten cryolite-alumina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the main operating indices of contemporary aluminum electrolyzers is the service life of the cathode unit, i.e., the main assembly of an aluminum electrolyzer. An increase in cathode assembly life is determined by the capacity of the carbon-graphite hearth, and also the side silicon carbide lining, to retain their maximum properties during a prescribed period of time (planned indices >2000 days).
The industrial method of preparing aluminum by electrolysis of cryolite-alumina melts (CAM) has a marked disadvantage, i.e., the short service life of electrolyzers. One of the main reasons for a reduction in cathode assembly operating life of an aluminum electrolyzer is breakdown of its carbon part as a result of introduction of sodium into the crystal lattice of carbon-graphite material (CGM) that leads to proliferation of internal stresses, swelling, disintegration and formation of breaks with microcracks that increases conditions for subsequent absorption of electrolyte [1,2,3,4].
Patented technical solutions exist [5, 6] aimed at increasing the life of a carbon-graphite lining and its protection from sodium diffusion with application of various coatings, wetted by molten aluminum on the basis of high temperature additions of titanium diboride and aluminum oxide. Non-carbon inert cathode materials have been developed, whose use makes it possible to reduce considerably expenditure and increase in lining service life [7. 8]. However, in spite of all the achievements of these solutions they are not used extensively in industry in view of expensiveness, susceptibility to abrasive wear, and difficulties of introduction into existing aluminum production.
Research has been conducted previously pointing to a significant reduction on cathode block deformation due to lithium intercalation. Even in 1955 in the work of VAMI “Improvement of the cathode block life of aluminum electrolyzers” [9], M. B. Rapoport studied in detail the process of intercalation and concluded that lithium atoms may without hindrance and unfavorable consequences be introduced into the interlayer of CGM crystal lattice structural spaces with formation of lamellar LixCy compounds. In 1967 in the well-known work [4] M. B. Rapoport presented results of research which showed that during electrolysis in lithium cryolite and with complete absence of sodium in a melt there may be reactions of forming an interlayer interstitial product and interaction of carbon with lithium with a marked reduction in CGM deformation by a factor of 9 – 10 compared with deformation CGM subjected to sodium cryolite. However, in this work there was no structural analysis of the specimens obtained before treatment and after action of CAM.
Independent of this research D. S. Newman, H. Justness, and H. Oye [10] in 1986 established that the rate of sodium penetration into cathode material not impregnated with lithium is several orders of magnitude faster than into previously impregnated material. The authors proposed that lithium atoms during intercalation occupy almost all areas stopping penetration of sodium in CAM and sodium reaction with carbon. It was determined that local expansion in the lining is prevented and the level of fluorine layer impregnation is reduced [10], although there was no indication of kinetic conditions of lithium atom penetration into the surface layers of CCM.
In fundamental work Sorlie and Oye [3] also pointed to the favorable effect of adding lithium salt LiF both on the properties of the electrolyte itself (CAM) and also on the life of an aluminum electrolyzer carbon-graphite hearth. According to their research compared with other alkali metal additions lithium with its least atomic radius is capable of occupying vacant positions between layers of carbon and graphite, without disrupting the overall structure of a cathode block, whereas both sodium and potassium cause much greater breakdown, which with respect to volume of failure as a whole exceeds the action of sodium on a lining, breaking in this way the layered structure of the graphitized part. In the most important work [11] for studying the effect of lithium additions on CAM a favorable effect has been demonstrated of adding lithium to CAM on the properties of a carbon-graphite hearth lining. An active effect of lithium on the physical and operating properties of a lining as a result of intercalation has been established. Improvement of electrolyzer carbon-graphite lining properties has been confirmed by a series of experimental works carried out with lithium electrolytes.
It should be noted that in spite of the proof of the favorable effect of lithium on the life of a carbon-graphite lining, these technical solutions have been developed for the technology of protecting the surface of a carbon hearth, making it possible to increase the service life of an aluminum electrolyzer. Currently addition of lithium is used exclusively for modifying electrolyte with the aim of increasing current efficiency. Use of lithium electrolytes is economically suitable in electrolyzer baths of the old type, if the problem is set of increasing their productivity. In contemporary electrolyzers high indices are achieved without using lithium-containing electrolytes, and therefore the main aluminum producers do not use lithium. Therefore, in existing conditions the effect of introducing lithium should be considered as the most preferable solution for improving operating and physical properties of cathode blocks, based on creating a barrier anti-friction layer due to lithium atoms, intercalated into cathode block surface layers
In spite or previous study of the questions connected with introducing sodium and lithium into a carbon-graphite lining [3, 12,13,14], currently there are no intentional engineering and production solutions for use of lithium and its additives as modifying additions for changing the structural characteristics of the CCM surface layers, and correspondingly for increasing the service life of an aluminum electrolyzer above planned values. In order to implement this aim in the present work it is proposed to use preliminary treatment of a CCM surface with lithium vapor, and therefore the scientific and practical interest of the work includes studying the process of penetration of sodium into a CCM surface previously treated with lithium vapor.
Research presented in the present work is based on conclusions and results of published work obtained previously [14], which may be considered as a continuation of the study of use of lithium additions in order to improve CCM properties.
Experimental Section
The object studied for the process of sodium penetration into CCM and also the effect of surface treatment with lithium vapor on this process is a GK Énergoprom standard carbon-graphite block. In order to treat the surface of a carbon-graphite specimen with lithium vapor a shaft furnace 1 was used, within the complex of an experimental unit (Fig. 1). Cylindrical specimens 240 mm in diameter, prepared from industrial carbon-graphite cathode blocks, were placed in a suspended condition in sealed steel vessel 3. The temperature was monitored by means of a thermocouple 9 pressed the lid of the vessel. The calculated lithium vapor pressure was established by controlling the shutter. The starting lithium-containing raw material used was lithium carbonate. It is well known [15] that during heating in a shaft furnace Li2CO3 up to 750°C there is formation of lithium oxide, which partly volatilizes above 1000°C by a reaction corresponding to the thermodynamic calculation:
Diagram of laboratory unit: 1) shaft electric resistance furnace SShOL-35/11; 2) carbon-graphite specimen; 3) steel vessel; 4) lithium carbonate; 5) silicon; 6) control panel; 7) vacuum pump; 8) vacuum gauge; 9) platinum platinum-rhodium thermocouple; 10) sealing cover; 11) steel vessel sealing cover; 12) nichrome winding.
In order to obtain lithium vapor the lithium oxide formed was reduced with finely dispersed crystalline silicon:
This solution has been used in some works and it is more profitable and economically expedient [11, 14]. Then on the bottom of the vessel a layer of lithium carbonate was poured uniformly, the surface of which was uniformly covered with a layer of finely dispersed crystalline silicon. During subsequent heating above 1000°C in the furnace there was formation of silicon dioxide and lithium, whose vapor gradually ascended above the specimen surface saturating it to a certain value. For complete reaction and covering of the specimen surface during an experiment the vessel was under an excess pressure of argon (0.6 – 0.8 Pa). After the end of treatment with lithium vapor the reaction vessel was removed from the furnace and the cylindrical specimen obtained was extracted. Presence of lithium inclusions in pores and within the structure of carbon-graphite specimen, and also the depth of its penetration into the structure were determined by x-ray photo-electron spectroscopy. It was revealed that during a certain time there was induced action of argon ions on a CCM graphite structure surface. Results of studying the introduction of lithium into CCM, presented in work published previously [14], showed quite deep penetration of lithium after 12 h exposure of CCM during treatment with vapor.
In order to study sodium penetration into a carbon-graphite specimen, and also the effect of surface treatment with lithium vapor, an experimental unit was used in the form of an electrolytic cell with variable electrode polarity (Fig. 2). The cathode 4 used was standard carbon-graphite specimens without lithium vapor treatment, prepared previously in the experimental unit. The anode was a standard carbon crucible 7. Electrolyte of prescribed composition and molten aluminum were poured into the carbon crucible. In order to simulate melt movement in an electrolyzer a test specimen was connected with a stirrer 8 with the possibility of controlling rotation frequency. Temperature was monitored by means of a contact thermocouple, fastened in a support. In order to study the effect of temperature and contact time for CAM with a carbon-graphite specimen the electrolysis process was carried out at 950, 980, and 1000°C for 6, 9, and 12 h respectively. After the end of a test the cylindrical specimen was extracted, the surface was cleaned from electrolyte and aluminum, and then it was sent for studying the depth of sodium penetration by drilling a specimen 40 mm in diameter with a drill bit with a pitch of 6 mm (method of layer removal) by the scheme shown in Fig. 3.
The concentration of sodium introduced was determined by titration of alkaline solution, obtained after boiling a carbon-graphite sample in distilled water, with sulfuric acid solution with a methyl orange indicator. Results of analysis are presented in Table 1 and shown in Fig. 4.
Results and Discussion
Results of analysis (see Table 1, Fig. 4) show that with an increase in specimen exposure in electrolyte and with an increase in melt temperature the depth of sodium penetration and its concentration increase. A reduction is also observed in sodium concentration for treated specimens in test temperature and time ranges. This points to a favorable effect of intercalation of lithium atoms, which is accompanied by structural changes of the specimen surface during a prescribed time interval. From the nature of the dependences obtained (see Fig. 4) it may be concluded that it is possible to make an assumption about aggressive action of sodium on a standard carbon-graphite specimen. After treatment in CAM the depth of sodium penetration 0.3 – 1.0 cm for standard specimens (see Fig. 4a – c) corresponds to a linear relationship, which is confirmed by a determination coefficient in the range from 0.90 to 0.96; in this case in treated specimens sodium is distributed over the depth uniformly by an exponential curve. A study of impregnation with sodium was conducted in order to obtain comparison specimens and the level of impregnation with a prescribed degree of their graphitization in the original condition of a standard cathode block.
It has been proposed [3, 4, 9] that sodium penetration into CCM is diffusion in nature, obeying Fick’s second law
where C is sodium concentration, wt.%; t is specimen exposure in melt, sec; D is sodium diffusion coefficient, cm2/sec; x is sodium penetration depth, cm.
In accordance with this assumption as a criterion for determining the activity and rate of sodium introduction into the structure of a carbon-graphite specimen a sodium diffusion coefficient D was selected, which points to a kinetic dependence of introduction, i.e., how the amount of sodium passes through a section of an area in unit time. In order to determine D it is effective to use an equation of Fick’s second law. In this case the process of sodium introduction may be considered as transient diffusion into a semi-bounded body from a constant supply source with the following boundary conditions: C = C0 = const (x = 0, t > 0) and C = 0 (x > 0, t = 0). Then solution of Eq. (3) with prescribed boundary conditions takes the following form
where C0 is sodium surface concentration, wt.%; erf is Gauss error function.
Solution of the Gauss error function in this equation takes the form
Knowing values of starting data, presented in Table 1, and with agreement of the solution of gauss error functions sodium diffusion coefficients were determined in all of the test points with different temperatures and exposure in CAM. Calculated values of diffusion coefficients are provided in Table 2.
The D obtained as a result of calculations with different experimental conditions show that with an increase in temperature and exposure their values increase. These data coincide with results [16], in which it was shown that the rate of sodium introduction into CCM slowly decreases with a reduction in temperature. It this case it should be noted that the process of sodium lamellar compound formation with carbon NaxCy has a dynamic character, which is more significant than the thermodynamic character.
Thermodynamic calculations show that the flow of particles in a chemical field is determined not by their concentration gradient, but by the change in chemical potential. In the test system carbon atoms form a densely packed and highly ordered crystal lattice, and therefore this structural state does not facilitate active sodium diffusion. In spite of this, sodium atoms are introduced into the lattice internodes, forming a strongly disordered system that is a factor of forming high diffusion mobility. Therefore, as a result of local shifts conditions are created for fast diffusion flows that penetrate through a rigid framework of the sub-lattice of CMM basic substance, i.e., graphite.
Graphical dependences of D on exposure in melt are shown in Fig. 5. It is seen that for a standard specimen in the initial instant of time of 6 and 9 h melt temperature is a governing factor for penetration and diffusion of sodium into a layer. Under experimental conditions it was shown that at 960°C for 6 h D = 1.0972 × 10–5 cm2/sec, whereas at 1000°C it comprises 1.8182 × 10–5 cm2/sec, i.e., almost twice as fast. This again confirms that the rate of sodium penetration into CCM increases with an increase in temperature and depends on the degree of CMM graphitization.
According [17] it is known that D in solids depends considerably in crystal lattice defects, arising during heating, under action of stresses, deformation, or other mechanical action. An increase in the number of defects mainly leads to an increase in the number of vacancies, which facilitates movement of atoms in a solid and increases the value of D. With a further increase in exposure for standard specimens in melt stabilization of D is observed. After 12 h exposure in melt its value varies in the range from 1.8182 × 10–5 to 2.3031 × 10–5 cm2/sec.
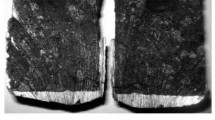
An inverse connection is observed for specimens treated with lithium. In the initial instant of time with exposure of specimens in melt for 6 h a change in melt temperature has almost no effect on the change in D, which varies from 0.4356 × 10–5 to 0,5534 × 10–5 cm2/sec. During subsequent exposure for 9 and 12 h it is seen that the melt temperature has a more marked effect on activity and sodium introduction rate. An increase in D with an increase in exposure duration for specimens in melt at a different temperature points to the irreversible nature of the process of sodium introduction. This is explained by the fact that the intercalated surface layer formed with thickness of 5 – 7 mm due to prior treatment with lithium vapor has more effective active protection in the initial instant of time of specimen exposure in electrolyte. With an increase CAM contact duration at a treated specimen surface there is slipping through of individual sodium atoms, accompanied by formation of lamellar compounds. This is caused by presence within an original specimen of microcracks and pores, which are not subject to the action surface treatment with lithium vapor. In a reacted section of a carbon-graphite specimen cracks and breakdown with inclusions of electrolyte are seen (Fig. 6) containing sodium, and therefore it may be suggested that in order to increase the activity and degree of activity and introduction of lithium atoms it is necessary to increase the lithium vapor partial pressure in the reaction vessel due to an increase in furnace temperature.
There are reasons [4] that lamellar compounds of alkali metals with graphite decompose at high temperature. The authors of publication [13] held carbon materials in an atmosphere of sodium vapor at different temperature and showed that with an increase in temperature there is a reduction in sodium content. On the other hand, it is seen from these results that with an increase in temperature the diffusion coefficient for sodium contained in CCM increases by almost a factor of two. This is explained by the fact that the small size interstitial atoms differ significantly from the lattice parameters and sizes of atoms of element-solvent, which are held in their positions and the bonds are less stable than nodal atoms of the CCM crystal lattice. Therefore, under action of an electric current and with exposure within the test temperature limits in the system there are two parallel reversible reactions:
With an increase in temperature equilibrium of reaction (6) moves to the right, and reaction (7) moves to the left, i.e., an increase in temperature increases the two contradictory processes. At high temperature the equilibrium activity of sodium metal by reaction (6) is higher than its equilibrium activity by reaction (7). Consequently, reaction (7) will shift to the right with formation of NaCx. Therefore, an increase in temperature and exposure of carbon-graphite specimens in CAM increases the sodium content in graphite. In spite of these phenomena, which proceed during contact of cathode block specimens with CAM, the sodium diffusion coefficient with reaction of melt with treated carbon-graphite specimens is reduced by almost a factor 2 – 3 compared with the standard. Therefore, as a result of tests a favorable effect has been demonstrated of prior treatment of a carbon-graphite specimen surface with lithium vapor.
Processes of sodium penetration into the graphite crystal lattice according to work in [3, 4, 9] are described by diffusion rules for an interstitial mechanism, which includes mass transfer of substances by intermodal atoms, located in cavities of the original material crystal lattice, i.e., graphite. Movement of diffusing lithium atoms by an interstitial principle and filling of vacancies occurs in the case when their relationship is affected by such factors as volumetric compression of a diffusing atom, chemical reaction with other atoms, present in the crystal lattice, and presence of local defects in the original crystal lattice. All of these indices determine the level of energy action, and in fact the activation energy, which it is necessary to apply to a diffusing atom within a given volume of a crystal lattice in order that it is intercalated from an original position into the next connecting nodes. In view of the above mentioned in order to move to determining the field for occurrence of the process it is necessary to consider that mechanism of occurrence of sodium reaction with the surface of a carbon-graphite specimen at prescribed temperatures and time intervals in in melt. A known equation was used for determining values of activation energy for heterogeneous processes in the CCM – CAM system.
where D0 is pre-exponential multiple; Ea is process activation energy, kJ/mole; R is universal gas constant, J/(mole·K); T is melt temperature, K.
Determination of values of activation energy was accomplished by plotting a graphical dependence on coordinates lnD – 1/T for which the slope equalled –Ea/R. Curves for the dependence lnD – 1/T are shown in Fig. 7, and after their treatment values of sodium diffusion process activation energy were determined in a standard carbon-graphite specimen: Ea = 108 kJ/mole, and also for the diffusion process of sodium in a carbon-graphite specimen treated with lithium: Ea = 166 kJ/mole.
The values of activation energy obtained according to [18] exceed the values of activation energy for an ideal diffusion process, for which the value of activation energy comprises 10 – 40 kJ/mole. This is explained by the fact that during diffusion of sodium into the graphite part of CCM apart from mas transfer there is also chemical diffusion, since sodium atoms need a reserve of energy for this, not only to overcome the potential energy barrier for introduction into a specimen surface, but also to increase the reaction energy with other CAM components.
Since the system studied is multicomponent, and apart from sodium diffusion there are processes of introduction accompanying impregnation of a surface with electrolyte, such as formation of aluminum carbide in the presence of impurities due to current-conducting and current-supplying elements, and mechanical breakdown of the surface of carbon-graphite specimen as a result of movement and melt high temperature. It has been determined that sodium diffusion process activation energy in a treated carbon-graphite specimen is greater by a factor of 1.5. This indicates that apart from the processes described above a sodium atom needs to overcome an energy barrier of the of intercalation bonds formed of type LiCx during treatment with lithium vapor. In this case it should be noted that only free atoms participate in diffusion, and therefore sodium atoms exhibiting sufficient free energy manage to break the lithium bond and its temporary compounds with carbon in one place or accomplish transfer into a vacant position in the direction of chemical potential movement (perpendicular to the specimens surface), and then again combine with carbon in a new place. According to data [18, 19] activation energy with strong reactions comprises more 150 kJ/mole and is chemical. With a similar order between two colliding elements there is stronger reaction with subsequent orbital mutual mixing of charges and creation of a new orbital condition in a system of atoms and molecules, and in fact formation of a complex of lamellar compounds. It may also be proposed that a high diffusion coefficient for a specimen treated with lithium is explained by the fact that the protected surface is not entirely wetted with electrolyte, and as a result of this there is a reduction in sodium diffusion flow.
As confirmation of the results obtained according to [20] diffusion coefficient and activation energy data are only described for different regions and an area of occurrence of diffusion processes. Therefore in order to explain the sodium diffusion process in a standard carbon-graphite specimen normal grain boundary diffusion is adopted, which is realized over grain boundaries and is specified by high values of diffusion coefficient and low process activation energy. Then sodium diffusion in a carbon-graphite specimen treated with lithium vapor may be determined as a volumetric diffusion process, with which it proceeds within the volume of grains and is characterized by higher values of activation energy and a low value of diffusion coefficient.
Conclusion
The results presented for a study of introduction of sodium into CCM treated with lithium vapor during electrolysis of CAM demonstrate the favorable effect of prior treatment of the surface of carbon-graphite specimens. A reduction is detected in sodium concentration at an identical distance from the surface of treated specimens in the test temperature and time ranges.
Results of experiments have made it possible to calculate diffusion coefficients for the processes that occur, on whose basis it is possible to suggest that the diffusion stage determines the rate of the process of forming lamellar compounds within the CCM structure. Comparison of diffusion coefficients has shown that formation of an intercalation layer 5 – 7 mm thick due to prior treatment with lithium vapor has more effective active protection in the initial instant of specimen exposure in electrolyte during 8 – 9 h, which entirely satisfies the industrial requirement since according to industrial practice the greatest degree of carbon-graphite lining breakdown occurs at the instant of start-up of electrolyzers. Consequently, the maximum protection of a cathode lining surface in the first hours of electrolyzer operation is most significant and governs the whole operating life cycle of an electrolyzer.
Calculated values of diffusion process activation energy for sodium indicate that sodium diffusion into CCM apart from mass transfer is of chemical diffusion in nature. Activation energy of the sodium diffusion process in a treated carbon-graphite specimen is greater by a factor of 1.5 than in standard material, which proposes the hypothesis of presence of intercalation compounds of the LiCx type after treatment and a requirement for greater sodium atom potential energy for overcoming these bonds during diffusion.
The effect obtained of introducing lithium may be considered as the most preferred solution for improving the operating characteristics and increasing operating efficiency of carbon-graphite cathode blocks, based on creating a barrier anti-diffusion layer due to introduction of lithium atoms followed by formation of intercalation compounds in the CCM surface layers.
References
V. M. Sizyakov, V. Yu. Bazhin, R. K. Patrin, et al., “Features of high-amperage electrolyzer hearth breakdown,” Refract. Indust. Ceram., 54(3), 151 – 154 (2013).
A. V. Saitov, V. Yu. Bazhin, and R. Yu. Feshchenko, “Operational problems of a graphitized cathodic block lining in contemporary aluminum electrolyzers,” Refract. Indust. Ceram., 58(2), 126 – 129 (2017).
M. Sorlie and H. Oye, Cathodes in Aluminium Electrolysis, Aluminium-Verlag, Düsseldorf (2013).
M. B. Rapoport, Carbon-Graphite Interlayer Compounds and their Value in Aluminum Metallurgy [in Russian], TsNIItsvetmetinformatsiya, Moscow (1967).
E. S. Gorlanov, V. Yu. Bazhin, and S. N. Fedorov, “Carbide formation at a carbon-graphite lining cathode surface wettable with aluminum,” Refract. Indust. Ceram., 57(5), 292 – 296 (2016).
S. Bao, K. Tang, A. Kvithyld, T. Engh, and M. Tangstad, “Wetting of pure aluminium on graphite, SiC and Al2O3 in aluminium filtration,” Trans. Nonferrous Metals Society of China, 22(8), 1930 – 1938 (2012).
V. De Nora and T. Nguyen, “Inert anode: Challenges from fundamental research to industrial application,” Light Metals, 417 – 421 (2009).
Han-bing He, Y. Wang, Jia-ju Long, and Zhao-hui Chen, “Corrosion of NiFe2O4–10NiO–based cermet inert anodes for aluminium electrolysis,” Trans. Nonferrous Metals Society of China., 23(12), 3816 – 3821 (2013).
M. B. Rapoport, “Increase in the life of cathode blocks of aluminum electrolyzers,” Trudy VAMI, 38 (1955).
D. S. Newman, H. Justnes, and H. A. Oye, “The effect of Li on graphitic cathodes used in aluminum electrolysis,” Metall., 6, 582 – 584 (1986).
V. Yu. Bazhin and A. V. Saitov, “Improvement of the physical and operating properties of carbon-graphite lining with lithium additions,” Novye Ogneupory, No. 1, 49 – 54 (2018).
G. V. Galevskii, N. M. Kulagin, M. Ya. Mintsis, and G. A. Sirazutdinov, Aluminum Metallurgy. Technology, Electrical Supply, Automation: Hgih School Texbook [in Russian], Nauka, Moscow (2008).
A. R. Ubbelohde and F. A. Lewis, Graphite and its Crystal Compounds Oxford University Press (1965).
V. Yu. Bazhin, R. Yu. Feshchenko, A. V. Saitov, and E. A. Kuznetsova, “Protection of an aluminum electrolyzer carbon-graphite lining by a lithium intercalation layer,” Refract. Indust. Ceram., 55(2), 81 – 83 (2014).
V. A. Emel’kin, M. G. Ktakherman and B. A. Pozdnyakov, “Preparation of lithium oxide with decomposition of lithium carbonate in a stream of heat carrier,” Teoret. Osnovy. Khim. Tekhnol., 43(1), 93 – 98 (2009).
E. W. Dewing, “The reaction of sodium with nongraphitic carbon: Reactions occurring in the linings of aluminium reduction cells,” Trans. Metall Soc. AIME., 227(12), 1328 – 1334 (1963).
P. Sh’yumon, Diffusion in Solids [in Russian], Metallurgiya. Moscow (1966).
B. V. Romanovskii, Bases of Chemical technology: High School Textbook [in Russian], Ekzamen, Moscow (2006).
N. K. Kondrasheva, F. D. Baitalov, and A. A. Boitsova, “Comparative assessment of structural-mechanical properties of heavy oils of Timano-Pechorskaya province,” Zapiski Gornogo Instituta, 225, 320 – 329 (2017).
O. Yu. Elagin, Production Methods for Increasing the Wear Resistance of Machine Components: Textbook [in Russian], Univer. Kniga, Logos (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 5, pp. 56 – 65, May, 2018.
Rights and permissions
About this article
Cite this article
Saitov, A.V., Bazhin, V.Y. Features of Using Modified Carbon-Graphite Lining Materials in Aluminum Electrolyzers. Refract Ind Ceram 59, 278–286 (2018). https://doi.org/10.1007/s11148-018-0221-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-018-0221-5