Abstract
The kinetics of the initiated oxidation of 1,4-dioxane in the presence of azepanobetulin and methyl-3-(hydroxyimino)-lup-20(29)en-28-oate additives has been studied. It was found that the introduction of minor additives of these substances into 1,4-dioxane oxidized in the initiated mode leads to the appearance of induction periods on the kinetic curves of oxygen absorption. It was found that the stoichiometric coefficient of inhibition f, the value of which is proportional to the number of peroxyl radicals that interact with one inhibitor molecule, resulting in a oxidation chain break, is > > 2, which is explained by the reaction of regeneration of antioxidant molecules. The possibility of participation in this reaction of the 2-hydroxy-1,4-dioxane molecule, which is an intermediate product of 1,4-dioxane oxidation, is discussed. Oxidation of this product leads to the formation of hydroxyperoxyl radicals, which, according to a previously established mechanism, are capable of reducing the original antioxidant molecule from its radical in the act of chain termination. A reaction mechanism is formulated that satisfactorily describes the experimental results. A mathematical model of the reaction was formulated, the study of which, with the help of the «ChimKinOptima» software complex, made it possible to satisfactorily describe the experimental kinetic curves as well as to obtain the kinetic curve of the accumulation of hydroperoxide, the primary product of 1,4-dioxane oxidation, which was not observed in the experiment, and to determine the reaction rate constants included in the proposed mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triterpenoids, in particular betulin and betulinic acid, have various types of biological activity, including anti-inflammatory and antimalarial effects in vitro. Despite the fact that the biological activity of lupane triterpenoids has been known since the nineteenth century, the real explosion of interest in the pharmacological properties of lupane derivatives occurred in the last decade after the discovery that this group of substances is very promising as antitumor and antiviral agents [1,2,3,4]. As a consequence, the development of even more powerful biologically active substances based on betulin and its acid is becoming an urgent task. It should be noted that the objects of chemical research are mainly triterpenoids with pronounced biological activity [5,6,7,8,9]. In [10], it was shown that replacement of the native carbocyclic cycle with theazepanic cycle in the triterpenoid structure leads to the appearance of high antitumor, antituberculosis, and antidiabetic activity.Simultaneously with the manifestation of therapeutic activity, some representatives of this class of compounds exhibit antioxidant properties, i.e., they are able to reduce the rate of oxidative processes [11]. Note that over the past decade, the antioxidant properties of biologically active substances, including drugs, are considered to be one of the most important characteristics of therapeutic activity. A scientifically substantiated consideration of this property of potential drugs requires knowledge of the detailed mechanism of their action as antioxidants.In this regard, in this work, we investigated the kinetic patterns and mechanism of action as inhibitors of radical-chain oxidation of organic compounds of two representatives of triterpenoids of the lupane series, which contain polyfunctional fragments.
Experimental
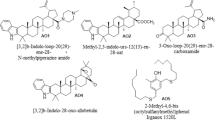
The antioxidant activity (AOA) of triterpenoids in the composition of 1,4-dioxane oxidized in the initiated mode was studied using a universal manometric setup [12] on the example of two compounds of the following formula:
Azepanobetulin, M = 441.74 g/mol (L1).

Methyl-3-(hydroxyimino)-lup-20(29)-ene-28-oate, M = 483.74 g/mol (L2).

In the experiments, the substances were synthesized according to the procedure described in [13, 14] and were not subjected to additional purification.
In the course of this work, the analysis of the transformation products of the triterpenoids under study was not carried out due to the low initial concentration of inhibitors (10–6–10–5 mol/L) and the shallow depth of the reaction. For these reasons, it is impossible to reliably specify the nature of the active center that is involved in the chain-breaking stage. Since it is known from the literature that the reactivity of peroxyl radicals with respect to the inhibitors depends on the strength of the X–H bond (where X is O, N, S, or Se) in most cases [15, 16], when analyzing the mechanism of antioxidant action, we assumed that the weak O–H or N–H bond of the antioxidant acts as the active center. When describing the analyzed reaction mechanism, we used the symbol In-H as an antioxidant molecule, indicating some weak bonding in the structure of the antioxidant molecule, and in this case, the resulting In* must not propagate the oxidative chain.
The oxidation substrate, 1,4-dioxane, was purified according to the known method [17]. The criterion of substrate purity was its oxidizability parameter. The initiator, 2,2'-azo-bis-isobutyronitrile (AIBN), was purified by double recrystallization from 96% ethyl alcohol.
Manometric method
Kinetic experiments were carried out using a universal manometric setup, which consists of two glass thermostatically controlled reactors of equal volume (27 cm3), one of which is working and the second is used for pressure equalization. The oxidation substrate, a solution of AIBN in 1,4-dioxane, was loaded into the working solution, and the inhibitor dissolved in 1,4-dioxane was added in the desired concentrations using a microsyringe. At the stage of design of the applied manometric setup, a calibration was carried out, according to which at a given volume of the reactor and 4 ml of solution injected into the preheated reactor, an isothermal mode is achieved in 5 min. Information on the temperature control time of the reaction mixture under similar conditions is also given in [18]. Thus, all necessary measurements were started after reaching a constant temperature of the reaction mixture. The kinetic curves of oxygen absorption are recorded on the computer screen as a continuous curve, and the points on the curve used in the simulation process are chosen arbitrarily according to the scale of the time axis.
The inhibitory effect of triterpenoids was studied by the effect of their additives on the rate of oxygen uptake in 1,4-dioxane oxidizing in the initiated mode at the initiation rate Vi = 3.2 × 10–7 mol/L s and temperature T = 348 K. For the rate constant of the decay reaction, we took the value lgkd = 15.8–E/2.303 × RT (E = 133 kJ/mol) (s–1) [19].
Mathematical modelling
To solve the inverse problem of chemical kinetics, we used the index method of conditional global optimization [20, 21] integrated into the software package «ChemKinOptima» [22, 23]. The concentration values of the observed substances were calculated by solving the direct kinetic problem based on an isothermal nonstationary model without volume changes in a closed system on the basis of the law of acting masses.
To solve the direct problem in ChemKinOptima, we chose the Michelsen method with automatic selection of the integration step. During mathematical modeling, we set the concentration of the observed substances in mol/L and time in hours. Note that the developed complex was previously successfully used by us to develop the mechanism of antioxidant action of aromatic amines [23], a number of uracil derivatives [24,25,26], fullerene C60 [27], and selenochromen [28].
Results and conclusions
Figs 1 and 2 show examples of typical kinetic curves of oxygen uptake in the reaction of initiated oxidation of 1,4-dioxane in the presence of additives L1 and L2.
Typical kinetic curves of oxygen uptake during oxidation of 1,4-dioxane in the presence of the inhibitor methyl-3-(hydroxyimino)lup-20(29)-en-28-oat; Vi = 3.2 × 10–7 mol/L s, 348 K. 1 - without additives, 2 - 5.0 × 10–6 mol/L, 3 - 6.5 × 10–6 mol/L, 4 - 9.6 × 10–6 mol/L, 5 - 2.0 × 10–5 mol/L, 6 - 1.5 × 10–5 mol/L
It turned out that the introduction of azepanobetulin and methyl-3-(hydroxyimino)lup-20(29)-en-28-oat into oxidizing 1,4-dioxane leads to the appearance of induction periods on the kinetic curves of oxygen absorption, which indicates the antioxidant effect of the studied compounds. The appearance of “hard” induction periods when oxygen uptake is not observed even at minor concentrations of added antioxidants indicates that the oxidation chain break occurs predominantly on peroxyl radicals of the oxidation substrate. The obtained experimental results can serve as a basis for using the known scheme of radical chain oxidation of organic compounds in the presence of inhibitors in the analysis of the reaction mechanism (Scheme 1) [29,30,31,32]**:

Here I is the initiator, RH is 1,4-dioxane, R* and RO2*are its alkyl and peroxyl radicals, respectively, and InH and In*are the studied antioxidant and its radical, respectively.
**—the numbering used in the literature for this type of reaction.
On the basis of Scheme 1, a mathematical modeling procedure was carried out; however, it was not possible to obtain a satisfactory agreement between the experimental and theoretical kinetic curves of oxygen absorption.
It is obvious that the given reaction mechanism in the form of Scheme 1 does not describe the experimental results. To obtain additional information on the mechanism of the reaction under study, we used experimental results, according to which the induction period increases linearlywith an increase in the initial concentration of antioxidants introduced into the oxidizing substrate (Fig. 3), which makes it possible to determine the stoichiometric coefficient of inhibition f (Eq. 1) [33] proportional to the number of peroxyl radicals that die on one inhibitor molecule.
Based on the processing of experimental results in the coordinates of Eq. 1, the values of the stoichiometric inhibition coefficient for azepanobetulin and methyl-3-(hydroxyimino)lup-20(29)-en-28-oat were determined, which were equal to f = (9.5 ± 0.1) (R = 0.98) and f = (13.0 ± 0.1) (R = 0.98) for L1 and L2, respectively. Note that such a phenomenon is not characteristic of such classical antioxidants as phenols (weak O–H bond) and aromatic amines (weak N–H bond), for which in the reactions of inhibited oxidation of individual compounds, f = 2 [34]. This means that for classical antioxidants, two radicals participating in the chain continuation reaction (reactions VII and VII′) die on one antioxidant molecule.
The fact that f > 2 was found in our experiments for the studied triterpenoids with similar active centers can be explained by the phenomenon of inhibitor regeneration in the event of chain termination when the molecule of the initial inhibitor is reduced from its radical (reactions XI, XII).
Note that this is known for the oxidation reaction of secondary alcohols inhibited by the addition of aromatic amines [33, 35]. This reaction is possible due to the fact that in the oxidation of secondary alcohols, the chain continuation reaction is carried out by oxyperoxyl radicals, which have a dual role: they can exhibit the properties of an oxidizing agent and continue chains, andas a reducing agent, they can reduce an inhibitor from its radical in oxidation reactions of organic compounds in the presence of an inhibitor.
However, in the classical mechanism of 1,4-dioxane oxidation reactions, the chain continuation reaction is carried out by peroxyl radicals of the substrate, while no hydroxyperoxyl radicals were found during its oxidation. We assume, however, that they can be formed during the oxidation of 2-hydroxy-1,4-dioxane, which was found among the products of the 1,4-dioxane oxidation reactionaccording to [36]. The authors of [36] did not discuss the mechanism of its oxidation, but we assumed that the formation of a hydroxy-derivative of 1,4-dioxane is a consequence of the degenerate chain branching reaction characteristic of radical-chain oxidation reactions:

In order to explain the high values of the stoichiometric inhibition coefficient, the reaction mechanism presented in Scheme 1 is supplemented with the stages that provide regeneration of the inhibitor, and thus, the reaction mechanism is formed (Scheme 2) on the basis of which the kinetic model used for mathematical modeling is constructed.
The result of this procedure is shown in Fig. 4, from which it follows that the calculated kinetic curves of dissolved oxygen absorption during oxidation of 1,4-dioxane in the presence of compounds L1 and L2 satisfactorily coincide with the experimental data. As a result of solving the inverse problem of chemical kinetics, the values of the rate constants of the stages describing the reaction mechanism were obtained (Table 1).
An important requirement for the solution was the compliance with the literature data in the order of magnitude of a part of the found constants. It should be borne in mind that in different literary sources the values of the constants differ within an order of magnitude. This assumption was laid down in the form of a search area for kinetic constants when solving inverse kinetic problems. Table 1 shows the rate constants of the stages found when solving the inverse problem of chemical kinetics, the value of which is consistent with the literature data [19, 31]. The satisfactory coincidence of the rate constants obtained as a result of mathematical modeling with the literature data indicates the adequacy of the proposed reaction mechanism to explain the observed experimental results. Thus, the error in the value of the fundamental chain continuation rate constant kII found on the basis of the calculation is within 15%. The value of the quadratic chain breaking rate constant kVI, the value of which lies within the range of values found by different authors, can also be considered satisfactory (Table 1). The rate constants kXI andkXII, which represent reactions between radicals, have values close to diffusion, which is typical for this type of reaction.
Using sets of constants from Table 1, direct problems of chemical kinetics were solved for each inhibitor and kinetic curves were obtained for all participants in a complex multistep reaction. The calculated kinetic curve of hydroperoxide accumulation (Fig. 5) agrees with the experimental kinetic curves of oxygen absorption and the appearance of induction periods on the kinetic curve of accumulation whose values are close to those found from the kinetic curves of oxygen absorption (Fig. 2).
Conclusions
In the present work, it has been experimentally established that the compounds under study have antioxidant properties and the observed stoichiometric inhibition coefficients for them are f > > 2. A similar effect is known from early studies for oxidation reactions of secondary alcohols in the presence of additives of aromatic amines and is explained by the regeneration of antioxidants from their radicals during the chain termination. We used this mechanism for the substances we studied, assuming that 2-hydroxy-1,4-dioxane known from [35] can serve as a source of hydroxyperoxyl radicals, which provide the regeneration of the initial inhibitor. The observed effect in this case is a high f value. On the basis of the proposed mechanism, a mathematical model was formed, which helped to solve the direct and inverse problems of chemical kinetics. As a result, the unobservable kinetic curve of the oxidizable substrate hydroperoxide as an intermediate product was restored, and the rate constants of the stages were found. It is noteworthy that the kinetic curve of hydroperoxide accumulation is in good agreement with the experimental oxygen absorption curve. These two curves are characterized by the presence of an induction period, the numerical values of which are close. Together with a satisfactory agreement between the experimental and calculated kinetic curves of oxygen absorption as well as the correspondence of the found rate constants to the literature data, this indicates the adequacy of the proposed mechanism to the observed results.
References
Liebscher G, Vanchangiri K, Mueller T, Feige K, Cavalleri JM, Paschke R (2016) Chem Biol Interact 246:20. https://doi.org/10.1016/j.cbi.2016.01.002
Periasamy G, Teketelew G, Gebrelibanos M, Sintayehu B, Gebrehiwot M, Karim A, Geremedhin G (2014) Arch Appl Sci Res 6(3):47
Król SK, Kiełbus M, Rivero-Müller A, Stepulak A (2015). Bio Med Res Int. https://doi.org/10.1155/2015/584189
Zhang DM, Xu HG, Wang L, Li YJ, Sun PH, Wu XM, Wang GJ, Chen WM, Ye WC (2015) Med Res Rev 35(6):1127
Baglin I, Poumaroux A, Nour M, Tan K, Mitaine-Offer AC, Lacaille-Dubois MA, Chauffert B, Cave CJ (2003) Enzyme Inhib Med Chem 18:111
Baltina LA, Flekhter OB, Nigmatullina LR, Boreko EI, Pavlova NI, Nikolaeve SN, Savinova OV, Tolstikov GA (2003) Bioorg Med Chem Lett 13:3549. https://doi.org/10.1016/s0960-894x(03)00714-5
Deng Y, Snyder JK (2002) J Org Chem 67:2864. https://doi.org/10.1021/jo010929h
Flekhter OB, Nigmatullina LR, Baltina LA, Karachurina LT, Galin FZ, Zarudii FS, Tolstikov GA, Boreko EI, Pavlova NI, Nikolaeva SN, Savinova OV (2002) J Pharm Chem 36:484. https://doi.org/10.1023/A:1021844705853
Mukherjee R, Jaggi M, Siddiqui MJA, Srivastava SK, Rajendran P, Vardhan A, Burman AC (2004) Bioorg Med Chem Lett 14:4087. https://doi.org/10.1016/j.bmcl.2004.05.034
Medvedeva NI, Kazakova OB, Lopatina TV, Smirnova IE, Giniyatullina GV, Baikova IP, Kataev VE (2018) Eur J Med Chem 143:464. https://doi.org/10.1016/j.ejmech.2017.11.035
Khairullina VR, Gerchikov AYA, Safarova AB, Khalitova RR, Spivak AYU, Shakurova ER, Odinokov VN (2011) Kinet Catal 52(2):186. https://doi.org/10.1134/S0023158411020091
Amorati R, Baschieri A, Valgimigli L (2017). J Chem. https://doi.org/10.1155/2017/6369358
Khusnutdinova EF, Petrova AV, Faskhutdinova LN, Kazakova OB (2018) Russian Biother J 17:78
Lopatina TV, Medvedeva NI, Baykova IP, Iskhakov AS, Kazakova OB (2019) Bioorg Chem 45(4):419. https://doi.org/10.1134/S0132342319040067)
Ingold KU, Pratt DA (2014) Chem Rev 114(18):9022. https://doi.org/10.1021/cr500226n
Grabovskiy SA, Grabovskaya YUS, Antipin AV, Kabalnova NN (2021) Kinet Catal 62(1):14. https://doi.org/10.1134/S002315842101002X
Gordon A, Ford R (1976) Sputnik Chemist. Mir, Moscow
Yakupova R, Nasibullina RA, Shamukaev VA, Sultanova RM, Safiullin RL (2020) Kinet Catal 61(2):232. https://doi.org/10.1134/S0023158420020123
Moroni AF (1967) Makromol Chem 105(6):43
Strongin RG, Sergeev YAD Dordrecht (2000) Kluver Academic Publishers. The Netherlands
Strongin RG, Gergel VP, Gorodetsky SYU, Grishagin VA, Markina MV (2002) Modern methods of making optimal decisions. N. Novgorod: Publishing house of NNSU
Tikhonova MV, Garifullina GG, Gerchikov AYA, Spivak SI (2014) Int J Chem Kinet 46(4):220. https://doi.org/10.1002/kin.20848
Tikhonova MV, Maskov DF, Spivak SI, Gubaidullin IM Program complex “KhimKinOptima” for mathematical modeling and optimization of chemical reactions based on kinetics using parallel computations and a database: evidence of registration of an electronic resource // INIPI RAO OFERNiO. No. 19247. Date of registration. 05/30/2013
Gerchikov AYA, Sharipova GM, Akhatova GR, Mustafin AG, Sakhibgareeva MV, Spivak SI (2015) Kinet Catal 56(5):563. https://doi.org/10.1134/S0023158415050067
Gerchikov AYA, Akhatova GR, Sharipova GM, Mustafin AG, Sakhibgareeva MV, Spivak SI (2015) Kinet Catal 56(3):300. https://doi.org/10.1134/S0023158415030052
Sharipova GM, Bulyakova RD, Safarova IV, Gerchikov AYA (2016) Bulletin of the Bashkir University 21(4):935
Gerchikov AYA, Sharipova GM, Safarova IV, Sakhibgareeva MV, Spivak SI (2017) J Phys Chem 96(6):957. https://doi.org/10.1134/s0036024417060103
Gerchikov AYA, Sharipova GM, Safarova IV, Kurmakaeva NV, Hairullina VR, Spivak SI (2020) Reac Kinet Mech Cat 131:89. https://doi.org/10.1007/s11144-020-01836-2
Cadenas E (1997) BioFactors 6:391
Nimse SB, Pal D (2015) RSC Adv 5(35):27986. https://doi.org/10.1039/C4RA13315C
Denisov ET, Azatian VV (2000) Inhibition of Chain Reactions. Gordon and Breach Sci. Publishers, London
Lente G (2015) Deterministic kinetics in chemistry and systems biology. Springer. 2015: 1–135
Denisov ET, Kharitonov VV (1963) Izv. Academy of Sciences of the USSR. Ser. Chem. 12: 2222
Emanuel NM, Denisov ET, Maizus EK (1965) Chain reactions of oxidation of hydrocarbons in the liquid phase // Moscow. Ed. The science
Vardanyan RL, Kharitonov VV, Denisov ET (1970) Izv. Academy of Sciences of the USSR. Ser. Chem. 7: 1536
Abdullaeva AS, Timashova EA, Bukin EYU, Oshanina IV, Brook LG, Temkin ON (2008) Bull MITHT 3(4):63
Acknowledgements
The work is supported by RSF, Project No. 19-73-20073. The authors express their gratitude to the Laboratory of Insect Bioregulators, UfICh UFIC RAS, represented by Dr. Kazakova O.B., for kindly providing the substances for this study.
Funding
This study was funded by Russian Science Foundation (19-73-20073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharipova, G.M., Gerchikov, A.Y., Safarova, I.V. et al. Kinetics and mechanism of antioxidant action of triterpenoids in the liquid-phase oxidation reaction of 1,4-dioxane. Reac Kinet Mech Cat 134, 629–640 (2021). https://doi.org/10.1007/s11144-021-02103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02103-8