Abstract
The kinetics of the antioxidant action of a number of triterpenoids—derivatives of betulinic acid have been studied. The rate constants of the oxidation chain termination reaction were found within the framework of the model reaction of the initiated oxidation of ethylbenzene. It has been found that when a mixture of antioxidants comprising a triterpenoid molecule and a spatially hindered phenol is used, a synergistic effect is observed, which significantly increases the antioxidant efficiency of the mixture. Disclosed is a mechanism which enhances the inhibitory effect of a mixture of antioxidants. According to this mechanism, the reduction of the original structure of the terpenoid from its radical occurs due to the transfer of the hydrogen atom from the weak O–H phenol bond to the terpenoid radical. Thus, the synergistic effect reflects the effect of the antioxidant mixture on the rate of oxidation reaction. Based on the comparison of the results of this work with those obtained earlier, it was concluded that it is possible to regenerate the terpenoid molecule by two fundamentally different mechanisms: (1) by using a mixture of antioxidants with different bond strengths in active sites that determine the inhibition effect, which manifests itself in a significant decrease in the oxidation rate—a synergistic effect; (2) due to the reaction in solvents, the peroxyl radicals of which have dual reactivity: the property of an oxidizing agent in the reaction of chain continuation by reaction with a substrate molecule and a reducing agent in its reaction with a terpenoid radical. In this case, the inhibitor is reconstituted in the cleavage act, which results in an increase in the effective chain break rate constant by increasing the stoichiometric inhibition coefficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, specialists in the field of pharmacology and related majors have paid close attention to the special properties of biologically active substances—potential drugs. Hereby the ability of these substances to inhibit the oxidation of organic substances by oxygen is meant. This refers, in particular, to the well-known process of lipid peroxidation of cell membranes of living organisms, which in the scientific literature is designated by the abbreviation LPO [1, 2]. This reaction proceeds by the mechanism of radical chain oxidation and is accompanied by the formation of various forms of active oxygen—radicals and labile intermediates. This process accompanies the course of any pathology and leads to the destruction of the cell membrane and the cell death. In this regard, when searching for new biologically active substances, researchers seek to find those in which, among the target characteristics, there was also the ability of these substances to inhibit the LPO process, that is, to act as an antioxidant. Note that the combination of the target therapeutic effect of drugs with the property of an antioxidant has long been noted for many drugs [3,4,5].
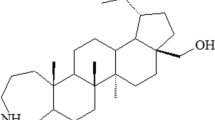
In particular, triterpenoids of the lupane and ursane series have this property of multifunctionality. Previously [6], we studied the kinetics of the antioxidant action of new triterpenoids of the lupane series and identified their mechanism of action, including the reaction of regeneration of the original antioxidant. At the same time, it is known that triterpenoids and derivatives based on them exhibit antiviral and antitumor activity [7,8,9,10]. Among the native triterpenoids of the lupane series, betulinic acid has the highest activity [11], in connection with which, in this work, the antioxidant properties of betulinic acid derivatives AO1–AO4 have been studied (Fig. 1).
The description of the synthesis of these compounds and their identification are given in references [10, 12, 13].
Experimental
The study of the properties of AO1–AO4 compounds as antioxidants has been carried out on the example of a model reaction of initiated oxidation of ethylbenzene. Note that the mechanism and quantitative characteristics of the oxidation substrate are well studied [14], which makes it possible to use these data in the analysis of a complex reaction in the presence of the studied triterpenoids. The reaction was carried out at 343 K in the initiated mode (the initiator was azodiisobutyronitrile AIBN), providing the initiation rate Vi = 3.8 × 10−7 mol/l s. The reaction mixture containing the required amount of the initiator and the test substances was placed in a thermostatically controlled reactor of a differential m universal manometric setup (UMS) with a high sensitivity pressure sensor.
Manometric method
The manometric method is based on the study of the mechanism and kinetics of the processes of liquid-phase oxidation of organic compounds and the principle of operation and the device of this installation are given in references [15, 16].
UMS consists of two glass thermostatically controlled reactors of equal volume, one of which is working and the second is used for pressure equalization. Through capillary tubes, the reactors are connected to a differential pressure sensor, as well as to a system of gas valves designed to equalize the pressure between the reactors, as well as to fill the gas medium in the reactors. The sensor and valve system are integrated into the main unit of the installation. Reaction mixtures are loaded into the working reactor, which may differ from each other in concentrations, the presence of a catalyst or an inhibitor. Then the valves are closed, and the pressure in the working reactor begins to change in accordance with the volume of gas absorbed during the chemical reaction. The pressure difference is measured by a highly sensitive differential pressure sensor based on a silicon membrane element and recorded in time dependence by a recording device. By differentiating the obtained graph, the rate of pressure change can be used to determine the rate of gas absorption, which corresponds to the rate of chemical reactions in reactors.
During the experiment, the oxidation substrate, a solution of AIBN in oxidation substrate, was loaded into the working solution, and the inhibitor dissolved in oxidation substrate was added in the desired concentrations using a microsyringe. At the stage of design of the applied manometric setup, a calibration was carried out, according to which at a given volume of the reactor and 4 ml of solution injected into the preheated reactor, an isothermal mode is achieved in 5 min. Information on the temperature control time of the reaction mixture under similar conditions is also given in [16]. Thus, all necessary measurements were started after reaching a constant temperature of the reaction mixture. The sensor response (in volts-V) is converted to oxygen consumption (M) for further calculations using a conversion factor that is determined in separate calibration experiments.
Initial substances and their purification
The oxidation substrate was subjected to purification according to known methods [17].
Purification of ethylbenzene was started by addition of sulfuric acid under prolonged stirring (2–3 h) until a clear black precipitate was formed. Then the excess black layer was removed (H2SO4) through the extractor, repeating this step several times until the black precipitate was no longer formed. This was followed again by re-stirring 3–4 times. The residual sulfuric acid was removed by washing with 0.1n sodium hydroxide (NaOH) solution. Residues of sodium hydroxide are removed by extraction and also by washing with distilled water. Then the solution is left for 24 h with a desiccant (CaCl2, MgSO4, Na2SO4). The final stage of purification was a simple distillation with a chlorocalcium tube.
The initiator of oxidation was azobisisobutyronitrile (AIBN; pure grade), which was recrystallised using ethanol. The homolytic decomposition of AIBN proceeds at constant rate at a given temperature, thereby providing a constant rate of initiation [18]. The initiation rate was calculated by the equation: Ri = ki × [AIBN], where ki is the initiator decay rate constant (s−1), which is equal ki = 2ekp, where kp is the rate constant of AIBN decomposition in solvent and e is the probability of the radical appearance in the reaction volume. For the decomposition reaction rate constant (kd) of ethylbenzene, we took the value given for cyclohexanol in the literature [19], as lgkd = 17.7 − E/2.303 × RT (E = 146.3 kJ/mol) (s−1), e = 0.5 [20].
Results and discussions
Additives of AO1–AO4 compounds lead to a decrease in the initial rate of oxygen uptake, which indicates the antioxidant effect of the studied triterpenoids. As an example, Fig. 2 shows the kinetic curves of oxygen uptake during the oxidation of ethylbenzene in the presence of different concentrations of the AO1 compound. The results on the effect of additives of AO2–AO4 compounds on the oxidation rate have a similar form. Typical kinetic curves of oxygen uptake in the presence of all the studied AO1–AO4 triterpenoids are shown in Fig. 3.
The results obtained provide a basis for assuming the mechanism of the reaction of radical chain oxidation of organic compounds in the presence of an inhibitor in accordance with the known literature data [15, 21, 22]:
Mechanism 1
Here I is an initiator, RH is a substrate, and InH is an inhibitor (antioxidant).
Note that the molecule of any antioxidant within the framework of the proposed mechanism has an active center that participates in the act of chain termination (V), which provides the effect of inhibition of the oxidative process. In this case, the peroxyl radical RO*2, active in the chain continuing reaction, is exchanged for the antioxidant radical In*, which is incapable of continuing the substrate oxidation chain. Therefore, reaction (V) is one of the key reactions, the value of the rate constant of which quantitatively determines the efficiency of the inhibitory action of the antioxidant. The most probable active center in the studied compounds is the N–H bond present in the structure of the studied triterpenoids AO1–AO4.
According to the kinetic curves of oxygen uptake, the values of the initial rates of oxidation of the model substrate at various concentrations of the studied substances were determined, the values of which are given in Table 1. At the same time, the effect of the concentration of added substances on the length of the reaction chain ν = V/Vi was established.
Note that a decrease in the oxygen uptake rate in the studied AO1–AO4 concentration range is accompanied by the preservation of the chain oxidation mode, for which Eq. 1 is valid:
Here F is the inhibition parameter; V0 and V are the rates of oxygen uptake in the absence and presence of an antioxidant, respectively; f is the stoichiometric coefficient of inhibition; kInH is the effective inhibition rate constants of the oxidation chain termination on the inhibitor; [InH] is the inhibitor concentration; 2kr is the rate constant of the quadratic chain termination on hydroperoxide radicals; and Vi is the initiation rate and the value of 2kr for ethylbenzene is 1.9 × 108 l/mol s [14].
Fig. 4 shows the dependence of the air oxygen uptake rate on the concentration of the studied substances.
These results are used to determine the value of the key rate constant kInH. Fig. 5 shows the straightening of these dependencies in the coordinates of Eq. 1, from which the values of the rate constant f·kInH are determined, the values of which are presented in Table 2.
The studied triterpanoids AO1–AO4 are biologically active substances, promising as biologically active compounds to food (BAC) and drugs [23, 24]. The effectiveness of these substances as antioxidants is determined by the ratio of the rate constants of the stages that make up the reaction mechanism, among which the reaction rate constant kInH plays the role. The value of this constant is determined by a number of reasons, the main of which is the strength of the bond in the active center of the molecule, which is responsible for the presence of the antioxidant effect. For the most common inhibitors, such bonds are N–H or O–H—a bond in aromatic amines or phenols and compounds close to them in structure. In each particular case, the bond strength is a property inherent in a given molecule, and the reaction rate constant kInH is closely related to this value. Nevertheless, there are two ways to influence the effectiveness of the antioxidant action of a particular antioxidant:
-
1.
Using the known effect of inhibitor regeneration from its radical, which leads to an increase in the inhibition efficiency factor f.
-
2.
Using the phenomenon of synergism in the reaction of inhibited oxidation, when two antioxidants of different nature are present in the reaction medium [25]. In the first case, the cause of the effect is the peculiarity of the structure of the oxidation substrate radical, which has both the properties of an oxidizing agent and a reducing agent. This, for example, is characteristic of hydroxyperoxyl radicals or, as we have previously shown, the one of triterpenoids of the lupane series containing an O–H bond in their structure [6].
In the second case, this is due to the ratio of the bond strengths of the active centers of the molecules—the components of the mixture. In this work, we have investigated the possibility of increasing the efficiency of inhibition due to a synergistic effect. For this purpose, a mixture of triterpenoid AO1 with 2-methyl-4,6-bis(octylsulfanylmethyl)phenol (AO5) was used as an antioxidant. This compound is well known as a polymer stabilizer under the commercial name Irganox 1520L [26]. Thus, there were two substances in the reaction medium—AO1, the active center of which is the N–H bond, and AO5, a representative of the class of sterically hindered phenols with an active center inhibiting the O–H bond.
Under the conditions of our experiments, AO5 demonstrated antioxidant properties, which is confirmed by the results presented in Supplementary information.
Fig. 6 shows the results of studying the dependence of the rate of ethylbenzene oxidation inhibited by the addition of a mixture of AO1 and AO5 compounds depending on the ratio of their concentrations in the mixture with the total amount of antioxidant.
The results obtained indicate the presence of a synergistic effect in the mixtures of two antioxidants AO1 + AO5, as a result of which, at a mole fraction of 50% AO1, it is 14 times greater than the effect of the total effect of individual components.
To explain the nature of this phenomenon in a mixture of AO1 and AO5 compounds, the reaction mechanism should be added with stages [25]:
Here In1H is the irganox molecule, In1O is its phenoxyl radical.
The proposed mechanism was analyzed by kinetic modeling with the help of a software package, which we successfully tested earlier [6, 27, 28]. The results of kinetic modeling confirming the validity of the reaction mechanism are presented in Supplementary information.
Thus, the presence of a mixture of the two antioxidants, among which AO5 acts as a more effective antioxidant (Supplementary information), leads not only to an additive increase in the efficiency of inhibition of the oxidative process, but also to a sharp increase in the inhibitory action as a result of a synergistic effect.
Previously, we studied triterpenoids that are similar in structure, but their antioxidant activity was viewed upon using the model reaction of 1,4-dioxane oxidation as an example [6]. The fkInH values obtained in oxidizable 1,4-dioxane are significantly higher than those found in ethylbenzene (Table 2).
The reason for this difference probably lies in the difference in the nature of the oxidized substrate. The radical-chain oxidation of ethylbenzene includes a key step of chain continuing (III), which is realized by the arylperoxyl radical of the substrate. According to the classical mechanism1 the value of the inhibitor efficiency factor f, which is proportional to the number of radicals killed on one antioxidant molecule, is equal to two. As we found earlier, 1,4-dioxane as a substrate forms an intermediate product during oxidation, the product is a source of oxyperoxyl radicals and ensures the regeneration of the inhibitor from its radical [6], which leads to the fact that f ≫ 2. The above considerations clearly show the difference between the two mechanisms for increasing the effectiveness of an antioxidant: the mechanism of a synergistic effect and the mechanism of inhibitor regeneration.
Conclusion
The effectiveness of a number of derivatives of betulinic acid as inhibitors of the reaction of radical-chain oxidation of ethylbenzene has been studied. The rate constants of the key chain termination reaction have been determined, which makes it possible to predict the ability to inhibit the rate of substrate oxidation depending on the nature of the antioxidant and its concentration in the solution. A synergistic effect has been found when using the mixture of [2, 3]b-Indolo-loop-20(29)-en-28-N-methylpiperazine amide and sterically hindered phenol as an antioxidant, which makes it possible to more than ten times increase the inhibitory effect and at the same time allows you to vary the concentration of triterpenoid when using them as biologically active substances or drugs. At the same time, the effect of biologically active substances can be preserved with an increase in the antioxidant effect of the composition.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Halliwell B, Gutterige JMC (1999) Free radical in biology and medicine. Oxford University Press, Oxford
Barclay LRC, Ingold KU (1981) Autoxidation of biological molecules. 2. The autoxidation of a model membrane. A comparison of the autoxidation of egg lecithin phosphatidylcholine in water and in chlorobenzene. J Am Chem Soc 103(21):6478–6486
Yeung AWK, Tzvetkov NT, El-Tawil OS, Bungǎu SG, Abdel-Daim MM, Atanasov AG (2019) Antioxidants: scientific literature landscape analysis. Oxid Med Cell. https://doi.org/10.1155/2019/8278454
Martynova YuZ, Khairullina VR, Garifullina GG, Nasretdinova RN, Mitsukova DS, Gerchikov AYa, Mustafin AG (2020) Determination of chain termination rate constants of radical-chain oxidation of organic compounds on antioxidant molecules by the QSPR method. Proc Acad Sci Chem Ser 9:1679–1691
Sidiropoulou GA, Metaxas A, Kourti M (2023) Natural antioxidants that act against Alzheimer’s disease through modulation of the NRF2 pathway: a focus on their molecular mechanisms of action. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2023.1217730
Gerchikov AY, Sharipova GM, Safarova IV, Safarov EF, Petrova AV (2021) Kinetics and mechanism of antioxidant action of triterpenoids in the liquid-phase oxidation reaction of 1,4-dioxane. Reac Kinet Mech Cat 134:629–640. https://doi.org/10.1007/s11144-021-02103-8
Periasamy G, Teketelew G, Gebrelibanos M, Sintayehu B, Gebrehiwot M, Karim A, Geremedhin G (2014) Betulinic acid and its derivatives as anti-cancer agent: a review. Arch Appl Sci Res 6(3):47–58
Król SK, Kiełbus M, Rivero-Müller A, Stepulak A (2015) Comprehensive review on betulin as a potent anticancer agent. Biomed Res Int. https://doi.org/10.1155/2015/584189
Bildziukevich U, Özdemir Z, Wimmer Z (2019) Recent achievements in medicinal and supramolecular chemistry of betulinic acid and its derivatives. Molecules 24(19):3546. https://doi.org/10.3390/molecules24193546
Medvedeva NI, Kazakova OB, Lopatina TV, Smirnova IE, Giniyatullina GV, Baikova IP, Kataev VE (2018) Synthesis and antimycobacterial activity of triterpenic A-ring azepanes. Eur J Med Chem 143:464–472. https://doi.org/10.1016/j.ejmech.2017.11.035
Kędzia B (1991) Antimicrobial activity of chamomille and its components. Herba Pol 37:29–38
Giniyatullina GV, Kazakova OB (2021) Synthesis and cytotoxicity of lupane mono- and bis-piperazinylamides. Chem Nat Compd 57:698–705. https://doi.org/10.1007/s10600-021-03453-4
Khusnutdinova EF, Smirnova IE, Kazakova OB, Petrova AV, Ha NTT, Viet DQ (2017) Synthesis and evaluation of 2,3-indolotriterpenoids as new α-glucosidase inhibitors. Med Chem Res 26:2737–2742. https://doi.org/10.1007/s00044-017-1972-0
Emanuel NM, Gal D (1984) Oxidation of ethylbenzene: (model reaction). USSR Academy of Sciences, Section of chemical, technological and biol sciences Hungerian Academy of Sciences Central Research Institute of Chemistry, Moscow
Amorati R, Baschieri A, Valgimigli L (2017) Measuring antioxidant activity in bioorganic samples by the differential oxygen uptake apparatus: recent advances. J Chem. https://doi.org/10.1155/2017/6369358
Sharipova GM, Safarova IV, Ya GA, Khairullina VR, Savchenko RG, Limantseva RM (2022) Kinetics and mechanism of antioxidant action of polysubstituted tetrahydroquinolines in liquid-phase oxidation reactions of organic compounds by oxygen. Int J Chem Kinet 54(7):435–443. https://doi.org/10.1002/kin.21572
Weisberg A, Proskauer E, Riddick J, Toups E (1958) Organic solvents. Physical properties and methods of purification. Publishers of Foreign Literature, Moscow
Lucarini M, Pedulli GF (2010) Free radical intermediates in the inhibition of the autoxidation reaction. Chem Soc Rev 39:2106–2119. https://doi.org/10.1039/B901838G
Denisov ET (1974) Liquid-phase reaction rate constants. Springer US, London
Moroni AF (1967) On the influence of the solvent in the thermal decomposition of azoisobutyric acid dinitrile. Makromol Chem 105(6):43–49
Denisov ET, Azatyan VV (2000) Inhibition of chain reactions. Gordon & Breach, London
Denisov ET, Afanas’ev IB (2005) Oxidation and antioxidants in chemistry and biology. CRC Press. Taylor and Francis, Boca Raton, London
Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K (2002) Methods for testing antioxidant activity. Analyst 127:183–198
Hossain MM, Shaha SK, Aziz F (2009) Antioxidant potential study of some synthesized N-heterocycles. Bangladesh Med Res Counc Bull 35:49–52. https://doi.org/10.3329/bmrcb.v35i2.2564
Emanuel NM, Denisov ET, Maizus ZK (1967) Liquid phase oxidation of hidrocarbons. Plenum Press, New York
Irganox 1520L. Guanan Industrial. BASF
Gerchikov AY, Sharipova GM, Safarova IV, Sakhibgareeva MV, Spivak SI (2017) Reactivity and mechanism of the action of C60 fullerene used as an inhibitor for the radical chain oxidation of 1,4-dioxane. J Phys Chem 91:1010–1014. https://doi.org/10.1134/s0036024417060103
Gerchikov AYa, Sharipova GM, Safarova IV, Kurmakaeva NV, Hairullina VR, Spivak SI (2020) Reactivity and mechanism of action of selenochromenes used as an inhibitor for the radical chain oxidation of 1,4-dioxane. Reac Kinet Mech Cat 131(1):1–12. https://doi.org/10.1007/s11144-020-01836-2
Acknowledgements
The research was supported by the Russian Science Foundation Grant No. 19-73-20073, https://rscf.ru/project/19-73-20073/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garifullina, G.G., Nasretdinova, R.N., Gerchikov, A.Y. et al. Antioxidant efficiency of triterpenoids in radical chain oxidation of organic compounds. Reac Kinet Mech Cat 137, 39–51 (2024). https://doi.org/10.1007/s11144-023-02516-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02516-7