Abstract
Kinetic and mass transfer aspects about the adsorption of tartrazine on a porous chitosan sponge (PCS) were elucidated. PCS was prepared and characterized. The kinetic curves were obtained in batch mode at different stirring rates and initial dye concentrations. The kinetic data were evaluated by the pseudo-first order, pseudo-second order (PSO), Elovich and Avrami models. The mass transfer aspects were evaluated by the external mass transfer coefficient (kf), intraparticle diffusion coefficient (Dint) and modified Biot number (Bi). The results revealed that PCS presented specific surface area around of 1150 m2 g−1 and porosity of 92 %. The adsorption kinetics was favored at higher values of stirring rate and initial dye concentration. PSO and Elovich models were suitable to represent the kinetic data. The kf values ranged from 7.38 × 10−3 to 5.25 × 10−1 m s−1 and the Dint values ranged from 8.93 × 10−10 to 4.72 × 10−9 m2 s−1. The Bi values at lowest stirring rates and initial dye concentrations showed that the external mass transfer should be considered. Otherwise, a complete dominance of intraparticle diffusion occurred at highest values of stirring rate and initial dye concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes are important additives in food processing. These compounds are widely used, and the consumers tend to expect and accept its presence. However, during food processing, a considerable amount dye containing effluents is generated [1]. Furthermore, several azo dyes, such as tartrazine, have been reported to cause allergic reactions [2]. Thus, colored effluents should be carefully treated. These effluents are difficult to treat since the dyes are stable and have a synthetic origin and also a complex molecular structure [3]. Among the existing technologies, adsorption is an attractive way to remove dyes from aqueous effluents, because it is effective, environmentally and economically viable [4–6].
Chitosan is a biodegradable, non-toxic and hydrophilic biopolymer, which is obtained from the chitin deacetylation. It is recognized in the literature that the adsorption onto chitosan is an emerging alternative technology to remove dyes from aqueous media [7, 8]. However, the majority of studies uses chitosan in powder form [5, 7, 9], but in this form, chitosan shows disadvantages, such as, low values of specific surface area and porosity [7]. Therefore, it is interesting to develop porous chitosan based materials for use as adsorbents. Recently, our research group developed a chitosan scaffold to remove five food dyes from aqueous media [10]. In this previous study, the equilibrium and thermodynamic data were evaluated. However, there is no available data in the literature regarding the kinetic and mass transfer aspects of tartrazine adsorption onto a porous chitosan sponge.
In solid/liquid homogeneous systems, the investigation of kinetic and mass transfer aspects is fundamental to determine the residence time, the factors affecting the operation and the rate controlling steps [5, 9, 11–14]. Generally, in the studies about dye removal on chitosan based materials, the kinetic investigation is based on the adsorption reaction models [5, 7, 9, 11]. These models provide a good representation of the experimental data and are simple from the mathematical viewpoint [11]. However, this work assumes that the adsorption kinetics can be represented in the same manner as the rate of a chemical reaction [11, 12]. Based on these models, it is not possible to obtain the mass transfer parameters and identify the rate controlling steps. On the other hand, the diffusional mass transfer models are constructed on the basis of three consecutive steps: external mass transfer, intraparticle diffusion and adsorption on active sites; and so represents more realistically the adsorption kinetics [15–17]. The use of both adsorption reaction models and diffusional models provides a good interpretation of the kinetic and mass transfer aspects in an adsorption process [5, 9, 11, 12, 16].
The objective of this study was to investigate the adsorption kinetics and mass transfer aspects of the system PCS-tartrazine. PCS was obtained and characterized by scanning electron microscopy (SEM), BET surface area and Fourier transform infrared spectroscopy (FT-IR). Adsorption kinetic curves were obtained at different stirring rates (50 and 150 rpm) and initial dye concentrations (50, 200 and 400 mg L−1). The models of pseudo-first order (PFO), pseudo-second order (PSO), Elovich and Avrami were fitted with the experimental data. An alternative procedure was used for the estimation of external mass transfer coefficient (kf), intraparticle diffusion coefficient (Dint) and modified Biot number (Bi).
Experimental
Adsorbate
The food azo dye tartrazine (FD&C Yellow 5, color index 19,140, molecular weight 534.4 g mol−1, molecular size 18 Å and wavelength 425 nm) was used as adsorbate. The dye was supplied by Duas Rodas Ind (Brazil) and had purity higher than 85 %.
Preparation and characterization of porous chitosan structure
Chitosan powder (deacetylation degree of 85 ± 1 % and molecular weight of 147 ± 5 kDa) was obtained from shrimp wastes (P. brasiliensis) [18]. PCS was prepared by dissolving 2 g of chitosan powder in 100 ml of 1 % (w/w) acid acetic solution. This solution was maintained under magnetic stirring for 24 h at room temperature, and then it was homogenized (Dremel, 1100–01, Brazil) for 5 min at 10,000 rpm. Subsequently, the solution was maintained at 193 K during 48 h in an ultrafreezer (Indrel, IULT 90–D, Brazil) and afterward, it was freeze dried (Liobras, l108, Brazil) at 219 K for 48 h under vacuum of 44 mm Hg (Liobras, l108, Brazil) [10].
The textural characteristics of PCS were observed by scanning electron microscopy (SEM) (Jeol, JSM–6060, Japan) [19]. The specific surface area and porosity were determined by a volumetric adsorption analyzer (Quantachrome instruments, New Win 2, USA) using the BET method [12]. The functional groups were identified by Fourier transform infrared spectroscopy (FT-IR) (Prestige, 21210045, Japan) [20].
Adsorption experiments
The adsorption experiments were carried out using solutions prepared from stock solutions of the dye tartrazine. The pH was adjusted to 6.0 by buffer disodium phosphate/citric acid solution (0.1 mol L−1), which did not present interaction with the dye. The reagents used in the experimental assays were of analytical grade. Distilled water was used to prepare all solutions. The kinetic assays were carried out in a thermostated shaker (Innova 44, New Brunswick Scientific, USA) at different stirring rates (50 and 150 rpm) and different initial dye concentration (50, 200 and 400 mg L−1) under the following fixed conditions: temperature of 298 ± 1 K and PCS concentration of 250 mg L−1. Aliquots were taken at predetermined time intervals (from 0 to 630 min) and analyzed. The dye concentration was determined by spectrophotometry (Quimis, Q108 DRM, Brazil), and blanks were performed. All experiments were carried out in triplicate (n = 3). The amount of dye adsorbed at time t (qt) was determined as [7]:
Here, Co, and Ct (mg L−1) are the dye concentrations at t = 0 and at any time, respectively, W (g) is the adsorbent amount and V (L) is the volume of the solution.
Kinetic models
In order to verify the adsorption kinetic behavior, the pseudo-first order (PFO), pseudo-second order (PSO), Elovich and Avrami models were fitted with the experimental data.
The kinetic models of pseudo-first order (PFO) [21] and pseudo-second order (PSO) [22] are based in the adsorption capacity. The PFO model (Eq. 2) is generally applicable over the initial 20–30 min of the sorption process, while the PSO model (Eq. 3) is suitable for the whole range of contact time.
Here, k1 and k2 are the rate constants of pseudo-first order and pseudo-second order models, respectively in (min−1) and (g mg−1 min−1), q1 and q2 are the theoretical values for the adsorption capacity (mg g−1).
The characteristic curve based on Elovich model (Eq. 4) is between those PFO model and intraparticle diffusion model [23].
being, ‘a’ is the initial velocity due to dq/dt with qt = 0 (mg g−1 min−1), ‘b’ is the desorption constant of the Elovich model (g mg−1).
Avrami [24], proposed an alternative kinetic equation, based on the thermal decomposition, which has been used also for adsorption systems [9], as shown in Eq. 5:
Here, kAV is the Avrami kinetic constant (min−1), qAV is the theoretical Avrami adsorption capacity (mg g−1), ‘n’ is a fractional exponent.
Mass transfer aspects
The mass transfer aspects of tartrazine adsorption on PCS were elucidated by the estimation of external mass transfer coefficient (kf), intraparticle diffusion coefficient (Dint) and modified Biot number (Bi). These mass transfer parameters were estimated from the kinetic curves, using a new simplified method, which was proposed by Yao and Chen [25]. Based on Eq. 1 and the film surface diffusion model [12, 15], the early-stage kinetic equation was proposed as [25]:
Here, α is a fitting parameter (min−1) and r0 is the initial adsorption rate (mg g−1 min−1), given by Eq. 7:
Here, R is the adsorbent radius (m) and ρp is the adsorbent density (kg m−3). According to Yao and Chen [25], the early-stage kinetic data, which corresponds to the range of the adsorbed amount (qt) from zero to about half of the equilibrium value (qe), can be fitted with Eq. 6, and then, the kf values can be found by Eq. 7.
In the same way, the Dint values (m2 s−1) were estimated by fitting the late-stage kinetic data [which corresponds to the range of the adsorbed amount (qt) from 70 to 100 % of the equilibrium value (qe)] with the late-stage kinetic equation (Eq. 8):
The late-stage kinetic equation was based on an approximation of Reichenberg equation used for Boyd plot at long adsorption times. In Eq. 8, ‘f’ is a fitting parameter and ke (min−1) is defined as the late-stage adsorption rate constant, given by Eq. 9:
To verify the predominance of intraparticle diffusion in relation to the external mass transfer, the modified Biot number (Bi) was calculated by Eq. 10 [15, 16]:
Here, q0 (mg g−1) is the solid phase concentration in the adsorbent in equilibrium with a residual hypothetical liquid concentration.
Statistical evaluation of the fitted models
The kinetic and mass transfer parameters were determined by the fit of the models with the experimental data through nonlinear regression. The Simplex/Quasi–Newton estimation method was used and the calculations were performed through the Statistic software (Statistic 9.1, Statsoft, USA). The fit quality was measured by the coefficient of determination (R2), adjusted determination coefficient (\({\text{R}}_{\text{adj}}^{2}\)) and average relative error (ARE) [26].
Results and discussion
Main characteristics of PCS
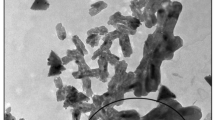
Fig. 1 shows the SEM image of PCS. In this figure it can be observed that PCS presented a porous structure. This porous structure is important for adsorption purposes once it allows the access of the dye molecules into the internal sites [7, 10]. In addition, the specific surface area was around of 1150 m2 g−1 and the porosity was 92 %. The literature presents values of specific surface area of 4.2 m2 g−1 and porosity lower than 1 % for chitosan powder [27]. This shows that the preparation of PCS is efficient to improve the chitosan characteristics for adsorption purposes. The PCS radius (R) was 0.00125 m and ρp was 1550 kg m−3. The large increases in specific surface area and porosity can be attributed to the preparation method as follows: under acid conditions the chitosan NH3 groups are protonated, leading to the expansion of polymeric chains. Coupled to this, the intense agitation can provided more effective shocks between the polymeric chains, forming new links. These new links formed a porous structure. The solvent was removed by freeze dried, maintaining the structure.
The main functional groups of PCS were identified by FT-IR (Fig. 2). At 3200 cm−1 the broad band is relative to the O–H and N–H stretchings. At 1700 cm−1 can be observed the vibration of amide band I. The bands at 1550 and 1400 cm−1 can be assigned the C–N stretchings of amide and the angular deformation of C–O–H and H–C–H links, respectively. The band at 1000 cm−1 represents the C–O links, and the N–H links are shown at 680 cm−1 band. From the bands obtained on FT-IR, it can be affirmed that PCS presents the typical groups of chitosan (NH2 and OH), which are potential sites for dyes adsorption [5, 7, 8, 10].
Kinetic results
Fig. 3 shows the adsorption kinetic curves of tartrazine onto PCS at different initial dye concentrations (50, 200 and 400 mg L−1) and stirring rates [(a) 50 and (b) 150 rpm]. The kinetic curves show that the adsorption capacity was enhanced when the stirring rate and initial dye concentration were increased. The adsorption equilibrium was faster attained at highest initial dye concentrations and stirring rates. The equilibrium adsorption capacities were 80.0, 202.0 and 373.7 mg g−1 for the initial dye concentrations of 50, 200 and 400 mg L−1, respectively. The stirring rate effect can be explained by the highest power dissipation in the system at 150 rpm. This stirring rate increase also lead to a decrease in the external mass transfer resistance, facilitating the migration of the dye molecules to the PCS surface and, increasing the adsorption capacity in the early steps [5]. Dotto and Pinto [16] obtained similar results in the biosorption of synthetic dyes by Spirulina platensis. Regarding to the initial dye concentration, its increase provides the necessary driving force to overcome the resistance to the mass transfer of dye between the aqueous and the solid phases. Therefore, an increase in the initial concentration of dye enhances the adsorption capacity [14]. Chiou and Li [28], observed the effect of dye concentration on the adsorption kinetics of reactive red 189 by cross-linked chitosan, and found similar trend.
To investigate the tartrazine adsorption onto PCS, the experimental data were fitted with different kinetic models, like pseudo-first order (PFO), pseudo-second order (PSO), Elovich and Avrami. The kinetic parameters are shown in Table 1. Based on the high values of coefficient of determination (R2 > 0.92), adjusted determination coefficient (\({\text{R}}_{\text{adj}}^{2} > \, 0. 9 1\)) and in the low values of the average relative error (ARE < 14.0 %) presented in Table 1, it was found that the PSO and Elovich models were the most appropriate to represent the kinetic data of tartrazine adsorption by PCS. Table 1 shows that the parameters q2, k2, ‘b’, and ‘ab’ increased with initial dye concentration and stirring rate, confirming the experimental observations. The h0 (h0 = k2(q2)2) parameter increased with initial dye concentration and stirring rate, indicating that, at the initial stages, the adsorption was faster at 400 mg L−1 and 150 rpm.
Mass transfer results
The mass transfer aspects of tartrazine adsorption on PCS were elucidated by the estimation of external mass transfer coefficient (kf), intraparticle diffusion coefficient (Dint) and modified Biot number (Bi). It was found that the early-stage kinetic equation (Eq. 6) fitted very well with the experimental data (R2 > 0.96 and ARE < 10.0 %). In the same way, the late-stage kinetic equation (Eq. 8) fitted very well with the experimental data (R2 > 0.97 and ARE < 7.0 %). This confirms the mass transfer steps (external mass transfer and intraparticle diffusion) and allows the accurate estimation of kf, Dint and Bi. The results are shown in Table 2.
It was found in Table 2 that the external mass transfer coefficient (kf) increased with the stirring rate and initial dye concentration. This shows that the transference of the tartrazine molecules from the bulk solution to the external surface of PCS was facilitated at highest values of stirring rate and initial dye concentration. The explanation for this behavior was presented in the above section (section Kinetic results). Similar behavior was found by Dotto et al. [15] in the adsorption of food dyes onto chitosan films. The Dint values ranged from 8.93 × 10−10 to 4.72 × 10−9 m2 s−1 and increased with the initial dye concentration. These values are high in relation to the following solid/liquid adsorption systems: chitin–tartrazine, chitosan–tartrazine [9], activated carbon-organic compounds [12], chitosan films–food dyes [15], Spirulina–synthetic dyes [16], Chitosan–FD&C red 40 [27] and others [25]. This probably is a result of the PCS characteristics, which is composed for larger porous, facilitating the mass transfer inside the particles. From the Bi values (Table 2), it was found that at lowest stirring rates and initial dye concentrations, the external mass transfer should be considered (Bi < 10). On the other hand, a complete dominance of intraparticle diffusion occurred at highest values of stirring rate and initial dye concentration.
SEM images
The SEM images of PCS before and after the adsorption of tartrazine are presented in Fig. 4. PCS presented porous network before the adsorption process Fig. 4a. However, these textural characteristics were modified after adsorption (Fig. 4b). The pores were filled and the surface was smoothed. This occurred due to the filling pores by water and dye molecules. These results confirm that the tartrazine were densely and homogeneously adhered onto the adsorbent.
Comparison with other adsorbents
In order to verify the efficiency of PCS, the adsorption capacity of PCS was compared with other adsorbents used for tartrazine removal. These results are shown in Table 3. It can be observed in Table 3 that PCS presented higher adsorption capacities than all adsorbents, including activated carbon. Another important fact is that the high adsorption capacity was attained at pH of 6.0. According to Hessel et al. [34] the pH of colored effluents is in the range from 5.5 to 8.5. This high adsorption capacity probably occurred due the high porosity and surface area of PCS.
Conclusion
In this work, the kinetic and mass transfer aspects about the tartrazine adsorption on a porous chitosan sponge (PCS) were studied. The results revealed that the adsorption was favored at highest stirring rates and initial dye concentrations. The PSO and Elovich models were the most suitable to represent the experimental kinetic curves. The kf values ranged from 7.38 × 10−3 to 5.25 × 10−1 m s−1 and the Dint values ranged from 8.93 × 10−10 to 4.72 × 10−9 m2 s−1.
The external resistance should be considered at lowest values of stirring rate and initial dye concentration. However, at the highest values of stirring rate and initial dye concentration, a complete dominance of intraparticle diffusion occurred. The SEM images showed that tartrazine was densely and homogeneously adhered to the PCS adsorbent. The adsorption capacity was 373.7 mg g−1 at pH of 6.0, 298 K, 150 rpm and 400 mg L−1 of dye. PCS presented higher adsorption capacity than other adsorbents shown in literature for the tartrazine removal.
Abbreviations
- a:
-
Initial velocity due to dq/dt with qt = 0 (mg g−1 min−1)
- ARE:
-
Average relative error (%)
- b:
-
Desorption constant of the Elovich model (g mg−1)
- Bi :
-
Biot number (dimensionless)
- C0 :
-
Initial dye concentration (mg L−1)
- Ct :
-
Dye concentration at time t (mg L−1)
- Dint :
-
Intraparticle diffusion coefficient (m2 s−1)
- f:
-
Fitting parameter of Eq. (8) (dimensionless)
- k1 :
-
Rate constant of pseudo-first order model (min−1)
- k2 :
-
Rate constant of pseudo-second order model (g mg−1 min−1)
- kAV :
-
Avrami kinetic constant (min−1)
- ke :
-
Late-stage adsorption rate constant (min−1)
- kf :
-
External mass transfer coefficient (m s−1)
- n:
-
Fractional exponent of the Avrami model (dimensionless)
- q0 :
-
Concentration in the adsorbent in equilibrium with a liquid concentration (mg g−1)
- q1 :
-
Theoretical value of the adsorption capacity for the PFO model (mg g−1)
- q2 :
-
Theoretical value of the adsorption capacity for the PSO model (mg g−1)
- qAV :
-
Theoretical value of the adsorption capacity for the Avrami model (mg g−1)
- qe :
-
Experimental equilibrium adsorption capacity (mg g−1)
- qt :
-
Amount of dye adsorbed at time t (mg g−1)
- r0 :
-
Initial adsorption rate (mg g−1 min−1)
- R:
-
Adsorbent radius (m)
- R2 :
-
Coefficient of determination (dimensionless)
- \({\text{R}}_{\text{adj}}^{2}\) :
-
Adjusted determination coefficient (dimensionless)
- t:
-
Time (min)
- V:
-
Volume of solution (L)
- W:
-
Adsorbent amount (g)
- α:
-
Fitting parameter of Eq. (6) (min−1)
- ρp :
-
Adsorbent density (kg m−3)
References
Yagu MT, Sen TK, Afroze S, Ang HM (2014) Adv Colloid Interface Sci 209:172–184
Kobylewski S, Jacobson MF (2010) Food dyes a rainbow of risks. Center for Science in the Public Interest, Washington
Dotto GL, Pinto LAA, Hachicha MA, Knani S (2015) Food Chem 171:1–7
Gupta VK, Suhas (2009) J Environ Manag 90:2313–2342
Dotto GL, Pinto LAA (2011) J Hazard Mater 187:164–170
Ali I, Asim M, Khan TA (2012) J Environ Manag 113:170–183
Crini G, Badot PM (2008) Prog Polym Sci 33:399–447
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB, Gholami Z, Amouzgar P (2014) Carbohydr Polym 113:115–130
Dotto GL, Vieira MLG, Pinto LAA (2012) Ind Eng Chem Res 51:6862–6868
Esquerdo VM, Cadaval TR, Dotto GL, Pinto LAA (2014) J Colloid Interface Sci 424:7–11
Qiu H, Pan LL, Zhang QJ, Zhang W, Zhang Q (2009) J Zhejiang Univ Sci A10:716–724
Leyva-Ramos R, Ocampo-Perez R, Mendoza-Barron J (2012) Chem Eng J 183:141–151
Wu JT, Kuo CY, Wu CH (2015) Reac Kinet Mech Cat 114:341–355
Haji S, Khalaf M, Shukrallah M, Abdullah J, Ahmed S (2015) Reac Kinet Mech Cat 114:795–815
Dotto GL, Buriol C, Pinto LAA (2014) Chem Eng Res Des 92:2324–2332
Dotto GL, Pinto LAA (2012) Biochem Eng J 68:85–90
Ruthven DM (1984) Principles of Adsorption and Adsorption Processes. Wiley, New York
Dotto GL, Souza VC, Pinto LAA (2011) LWT Food Sci Technol 44:1786–1792
Goldstein JI, Newbury DE, Echil P, Joy DC, Romig AD, Lyman CE (1992) Scanning electron microscopy and X-Ray microanalysis. Plenum Press, New York
Silverstein RM, Webster FX, Kiemle DJ (2007) Spectrometric identification of organic compounds. Wiley, New York
Lagergren S (1898) Kung Sven Vetensk Hand 24:1–39
Ho YS, McKay G (1998) Process Saf Environ Prot 76B:332–340
Wu FC, Tseng RL, Juang RS (2009) Chem Eng J 150:366–373
Avrami M (1939) J Chem Phys 7:1103–1112
Yao C, Chen T (2015) Chem Eng J 265:93–99
El–Khaiary MI, Malash GF (2011) Hydrometallurgy 105:314–320
Piccin JS, Dotto GL, Vieira MLG, Pinto LAA (2011) J Chem Eng Data 56:3759–3765
Chiou MS, Li HY (2002) J Hazard Mater B93:233–248
Weber CT, Collazzo GC, Mazutti MA, Foletto EL, Dotto GL (2014) Water Sci Technol 70:102–107
Zhou Z, Lin S, Yue T, Lee TC (2014) J Food Eng 126:133–141
Gautam RK, Gautam PK, Banerjee S, Rawat V, Soni S, Sharma SK, Chattopadhyaya MC (2015) J Environ Chem Eng 3:79–88
Mittal A, Kurup L, Mittal J (2007) J Hazard Mater 146:243–248
Ngah WSW, Ariff NFM, Hanafiah MAKM (2010) Water Air Soil Pollut 206:225–236
Hessel C, Allegre C, Maisseu M, Charbit F, Moulin P (2007) J Environ Manag 83:171–180
Acknowledgments
The authors would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel) and CNPq (National Council for Scientific and Technological Development) for the financial support. Furthermore, the authors would like to thank CEME–SUL/FURG (Electron Microscopy Center of South/Federal University of Rio Grande/RS/Brazil) due to the scanning electron microscopy images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esquerdo, V.M., Quintana, T.M., Dotto, G.L. et al. Kinetics and mass transfer aspects about the adsorption of tartrazine by a porous chitosan sponge. Reac Kinet Mech Cat 116, 105–117 (2015). https://doi.org/10.1007/s11144-015-0893-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0893-5