Abstract
In this study, the sol–gel method is utilized to produce TiO2–In2O3 composite (Ti–In). The generated Ti–In was separately co-doped with C–N and S–N to form novel TiO2–In2O3–C–N (Ti–In–C–N) and TiO2–In2O3–S–N (Ti–In–S–N). The sources of N, C and S dopants were urea, powder activated carbon and Na2S2O3. The dye C.I. reactive red 2 (RR2) was used as a model compound that was exposed to various composites and its removal by photocatalytic degradation and adsorption was measured. The percentage of anatase phase in Ti–In–C–N and Ti–In–S–N exceeded that in Ti–In; conversely, the particle diameter and band gap energy of Ti–In exceeded those of Ti–In–C–N and Ti–In–S–N. Bonds of Ti–O–N, Ti–N–O and Ti–O–C were detected in Ti–In–C–N and bonds of Ti–O–N, Ti–N–O and Ti–O–S were found in Ti–In–S–N. The pseudo-first order RR2 removal rate constants in UV/Ti–In, UV/Ti–In–C–N and UV/Ti–In–S–N were 0.43, 1.68 and 1.70 h−1, in order. The specific oxygen uptake rates of the RR2 effluent in UV/Ti–In, UV/Ti–In–C–N and UV/Ti–In–S–N were determined to be 4.4, 7.3 and 5.3 mg O2/g-MLVSS h. Ti–In–C–N and Ti–In–S–N were more effective in decolorizing and detoxifying RR2 than was their parent Ti–In.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced oxidation refers to a set of chemical treatment procedures for removing organic and inorganic materials from wastewater by oxidation. Photocatalysis using a nanosized metal oxide semiconductor is regarded as one of the most effective and simplest ways to mitigate environmental pollution that is caused by humans. Titanium dioxide (TiO2) is currently considered to be the most promising photocatalyst because of its chemical and biological stability, high abundance and non-toxicity. The photocatalytic degradation of pollutants in water has been successfully performed using a suspension of TiO2. The generally accepted mechanisms of the photodegradation of organics by UV/TiO2 are as follows [1]. Photogenerated holes are formed when TiO2 particles are irradiated by UV light. Hydroxyl radicals are produced by the oxidation of OH− or H2O by these photogenerated holes, and are primarily responsible for the destruction of the organic species. Oxygen is used as an efficient electron trap to prevent the recombination of photogenerated electrons and holes. If the amount of available oxygen is limited, then the rapid recombination of photogenerated electrons and holes in TiO2 reduces the efficiency of the photocatalytic reactions.
A different method for preparing photocatalytic TiO2 efficiently involves coupling it with other semiconductor oxides, such as CdSe [2], SnO2 [3, 4], WO3 [4], and In2O3 [5–7]. These composite systems demonstrate considerably enhanced photocatalytic performance in the degradation of pollutants, as the composite facilitates charge transfer and suppresses the recombination of electron–hole pairs in the photocatalysts systems. Coupling In2O3 with TiO2 enables the transfer of the photogenerated electrons from the surface of In2O3 to the conduction band of TiO2 because the band gap of In2O3 (2.64 eV) is lower than that of TiO2 (2.95 eV) and the conduction band of In2O3 (−0.63 eV vs. NHE) is higher than that of TiO2 (−0.4 eV vs. NHE). This process results in the effective capture of photogenerated electrons and retardation of the recombination of photogenerated electron–hole pairs [7, 8]. Various studies have demonstrated that the photocatalytic activity of TiO2–In2O3 composite (Ti–In) exceeds that of In2O3 and TiO2 [5, 6].
Various metal and nonmetal ions have been doped into photocatalysts to reduce the recombination of photogenerated electrons and holes in them and to increase their absorption of light. However, metal ion-doped photocatalysts have some serious drawbacks, such as thermal instability and the metal centers acting as electron traps, reducing photocatalytic efficiency [9]. Sojic et al. [10] showed that nonmetals are more efficient dopants than metals. Accordingly, doping nonmetal ions into photocatalysts has recently attracted considerable attention. Nonmetal-doped forms of TiO2, including C-doped [11–13], N-doped [10–12] and S-doped TiO2 [12, 14–16], has been found to exhibit superior photocatalytic activity because C, N and S atoms can effectively narrow the energy band gap of TiO2. Moreover, C–N [11] and S–N co-doped TiO2 [17, 18] have been investigated and co-doped TiO2 has been found to have a higher photocatalytic activity than single-doped TiO2 [11, 18].
Ma et al. [8] demonstrated that the photocatalytic activity of Pt/Ti–In exceeded that of Ti–In because the separation of the photogenerated electron–hole pairs in the former is more efficient, owing to the incorporation of In2O3 and Pt into the TiO2 framework. Ag/Ti–In has been found to exhibit a similar enhancement [19]. However, to the best of our knowledge, the photocatalytic activities of Ti–In that is co-doped with nonmetal dopants have not been examined. In this study, the Ti–In photocatalyst was synthesized via the sol–gel method, doped separately with C and S, and finally the C-doped and S-doped Ti–In were calcined with urea to form C–N co-doped and S–N co-doped Ti–In. To determine the photocatalytic activities of C–N-co-doped and S–N-co-doped Ti–In, their removal of a common azo dye, C.I. reactive red 2 (RR2), was studied. The objectives of this study are (i) to prepare novel C–N-co-doped Ti–In (abbreviated as Ti–In–C–N) and S–N-co-doped Ti–In (Ti–In–S–N), (ii) to characterize the surface properties of Ti–In–C–N and Ti–In–S–N, (iii) to determine changes associated with the adsorption of RR2 onto Ti–In–C–N and Ti–In–S–N, and (iv) to evaluate the photocatalytic activities of the doped Ti–In–C–N and Ti–In–S–N in decolorizing RR2, relative to that of their parent Ti–In.
Materials and methods
Materials
In this investigation, the sources of Ti and In are TiCl4 (Acros) and InCl3 (Alfa Aesar). The sources of N, C and S dopants were urea (Katayama), powder activated carbon (PAC) (Katayama) and Na2S2O3 (Merck. The parent compound, RR2, was purchased from Sigma Aldrich. Its formula, molecular weight, and maximum light absorption wavelength were C19H10Cl2N6Na2O7S2, 615 g/mol, and 538 nm. The pH of the solution was adjusted by adding 0.1 M HNO3 (Merck) or NaOH (Merck). All compounds were used as received (without further purification), and all solutions were prepared using deionized water (Milli-Q) and reagent-grade chemicals.
Preparation of Ti–In, Ti–In–C–N and Ti–In–S–N
Ti–In was prepared with a Ti/In mole ratio of 54 using the sol–gel method, as previously described [7]. The molar ratios of Ti/C and Ti/S in Ti–In–C–N and Ti–In–S–N were both 7.5; the molar ratios of Ti/N in both Ti–In–C–N and Ti–In–S–N were both 0.07.
To prepare Ti–In–C–N and Ti–In–S–N, C-doped Ti–In (Ti–In–C) and S-doped Ti–In (Ti–In–S) were first produced. To prepare the Ti–In–C, PAC (0.184 g) was first mixed with HCl (20 mL; 0.65 M) and the mixture was placed in an ultrasonic bath for 10 min; then this solution was added InCl3 (0.4805 g; 99.9 %) and TiCl4 (13 mL; 98 %). The pH of the solution was adjusted to pH 8 by adding NH4OH solution (28 %), forming a solid precipitate. This precipitate was collected by filtration and washed repeatedly using distilled water; it was dried at 110 °C for 24 h and then calcined at 450 °C for 2 h to yield the Ti–In–C. To prepare Ti–In–S, PAC was replaced with Na2S2O3 (1.212 g) and the other steps were the same as those used to prepare Ti–In–C. To prepare Ti–In–C–N (or Ti–In–S–N), 2.5 g Ti–In–C (or Ti–In–S) and urea (12.5 g) were thoroughly mixed and the mixture was calcined at 450 °C for 2 h.
Characterization of Ti–In–C–N and Ti–In–S–N
The crystalline structures of various photocatalysts were analyzed by X-ray diffraction (XRD) (Bruker D8 SSS, Germany). The XRD patterns were recorded over 2θ values of 10–90°, with an accelerating voltage and applied current of 40 kV and 30 mA, respectively. Diffuse reflectance UV–Vis spectra of the photocatalysts were obtained using a UV–Vis spectrophotometer (JAS.CO-V670, Japan) and these were used to calculate the band gap energies. Specific surface areas of the samples were determined by the BET method from the nitrogen adsorption isotherms that were obtained using a Micrometrics ASAP 2020 apparatus. X-ray photoelectron spectroscopy (XPS) measurements were made using a PHI Quantum 5000 XPS system (USA) with a monochromatic Al Kα source and a charge neutralizer.
Decolorization of RR2
Experiments were performed using 0.2 g/L of each photocatalyst, with [RR2] = 20 mg/L, at pH 3 and 25 °C. Decolorization experiments were conducted in a 3 L hollow cylindrical glass reactor, illuminated by an 8 W UV lamp (254 nm, Philips) with a light intensity of 1.12 W/m2 that was placed inside a quartz tube. Adsorption experiments were performed in the dark. The reaction medium was continuously stirred at 300 rpm to maintain the suspension. Aliquots each with a volume of 10 mL were withdrawn from the reactor at intervals to monitor reaction progress. Following sampling, solids were separated by filtration using a 0.22 µm filter (Millipore), and the RR2 that remained in the filtrate was analyzed by measuring absorbance at 538 nm using a spectrophotometer (Hitachi U-5100, Japan).
Tests of specific oxygen uptake rate (SOUR)
The biodegradability of RR2 effluent was evaluated by performing a SOUR test. Activated sludge was collected from a sewage treatment plant in Kaohsiung. Before testing, the activated sludge was aerated for 24 h. The concentration of mixed liquor volatile suspended solids (MLVSS) in activated sludge was measured to be 3.5 g/L. The SOUR (mg O2/g-MLVSS h) of aerobic microorganisms was measured for the RR2 effluent (after 180 min reaction and separated the suspended photocatalyst) to be the biodegradability index in the UV/Ti–In, UV/Ti–In–C–N and UV/Ti–In–S–N systems. SOURs were determined by dividing abatement rates in dissolved oxygen (DO) to MLVSS values.
Results and discussion
Characteristics of the surface of prepared photocatalysts
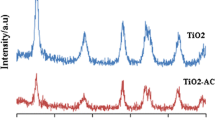
Fig. 1 displays the XRD patterns of Ti–In, Ti–In–C–N and Ti–In–S–N. The anatase peaks were at 25.4° and 48.2°. The peaks at 27.5°, 36.2°, 41.3°, 54.4°, and 69.2° were attributable to rutile crystal phases. The peaks at 21.5°, 30.6°, 35.5°, 51.0°, and 60.7° were attributable to In2O3. No pattern included any In2O3-derived, N-derived, S-derived, or C-derived peaks.
The anatase content was determined from the integrated intensity of the anatase diffraction peak at 2θ = 25.4°, IA, and the rutile content was determine from the rutile diffraction peak at 2θ = 27.5°, IR, using Eq. 1 below [20]. From the XRD patterns, the crystalline size was calculated using the Scherrer equation, Eq. 2 [21]:
Here, D represents the crystalline size (nm); λ is the wavelength of the X-rays (0.15418 nm); β is the line-width at the half maximum of the anatase peak at 2θ = 25.4° or of the rutile peak at 2θ = 27.5°, and θ is the diffraction angle (°). Table 1 lists the surface properties of various photocatalysts. The surface areas followed the order Ti–In–S–N > Ti–In ≒ Ti–In–C–N. The percentage of anatase phase in Ti–In–C–N and Ti–In–S–N exceeded that in Ti–In. Clearly, C–N and S–N co-doping in Ti–In inhibited the conversion of TiO2 from anatase to rutile. This retardation of phase transformation may be due to stabilization of the anatase phase by surrounding N ions via formation of Ti–O–N bonds. Anatase is regarded as the photoactive form of TiO2, whereas rutile exhibits a low photocatalytic activity. Hurum et al. [22] proposed that electron-trapping sites are more stable in anatase than in rutile, so anatase exhibits greater photocatalytic activity. Accordingly, C–N and S–N co-doping in Ti–In may improve the photocatalytic activity of Ti–In. Additionally, C–N and S–N co-doping in Ti–In reduced the particle diameter, suggesting that the C–N and S–N co-doping retard the agglomeration of Ti–In crystals. Liu et al. [16] indicated that the doping with S effectively inhibited the growth of TiO2 crystalls and Chen et al. [11] that doping with N had a similar effect. Li et al. [23] also demonstrated that carbon acts as a barrier that controls the growth of TiO2 powder and prevents its agglomeration.
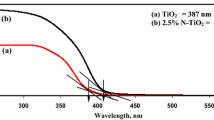
The band gap energy of Ti–In exceeded those of Ti–In–C–N and Ti–In–S–N (Table 1). N-doping was effective in reducing the band gap of TiO2 by creating an isolated N2p narrow band above the O2p valence, by the incorporation of N atoms into the TiO2 lattice [24]. The reduction of the band gap of C-doped TiO2 was attributed to the formation of Ti–O–C bonds [25]. C–N co-doping generated the intragap localized state of C2p and N2p above the valence band gap of TiO2. Therefore, electrons were promoted from the valence band to the conduction band, leaving holes behind [26]. The role of S in Ti–In–S–N was same as that of C in Ti–In–C–N; therefore, co-doping of C–N and S–N in Ti–In reduced the band gap energy.
XPS was utilized to determine the chemical compositions of the photocatalysts. Fig. 2a–e display the XPS spectra of Ti2p, In3d, O1s, C1s, and N1s for Ti–In–C–N. The Ti2p3/2 and Ti2p1/2 spin-orbital-splitting photoelectrons were observed at binding energies of 455.7–458.8 and 462.1–464.5 eV [26–28]. The binding energy of the Ti2p3/2 and Ti2p1/2 spin-orbital-splitting photoelectrons in Ti–In–C–N was 458.2 and 463.9 eV (Fig. 2). These binding energies are close to those reported for Ti4+ in TiO2 [29]. The In3d5/2 and In3d3/2 spin-orbital splitting photoelectrons were observed at binding energies of 443.9–445 [19, 28, 30, 31] and 451.5–452.0 eV [19, 28, 30]. The binding energy of the In3d5/2 and In3d3/2 spin-orbital-splitting photoelectrons of Ti–In–C–N was 444.3 and 451.9 eV (Fig. 2b). Peaks at 528.5–529.5 [26, 28, 32] and 529.6–531.0 eV [27, 30, 31] were assigned to O1s; the former was associated with Ti–O and the latter was attributable to In–O. Peaks at 531.4–531.9 eV indicated other oxygen species, possibly belonging to OH− on the surface of the photocatalyst [32, 33]. The binding energy of Ti–O, In–O, and OH− in Ti–In–C–N was 529.4, 529.7, and 531.6 eV (Fig. 2c). The binding energies of C1s, which were 284.8, 285.4 and 288 eV, were attributed to C–C, C–O [34], and Ti–O–C [27] bonds (Fig. 2d). The 284.8 eV peak is due to the carbon-containing species adsorbed on the surface, which is usually ascribed to the adventitious carbon or carbon residues from the organic precursor [12]. Kang et al. [35] assigned the binding energy at 282 eV to the Ti–C bond that was formed by the substitution of carbon at an oxygen site in TiO2; they assigned the binding energy at 288 eV to the presence of carbonate species that were formed by the substitution of carbon at the Ti site in TiO2. The prepared Ti–In–C–N formed C–C and C–O bonds, but no Ti–C bonds. Chen et al. [11] reported that carbonate species that formed on the TiO2 surface served as a photosensitizer, like an organic dye, promoting the absorption of TiO2.
Fig. 3a–e present the XPS spectra of Ti2p, In3d, O1s, N1s, and S2p for Ti–In–S–N. The XPS spectra of Ti2p, In3d and O1s in Ti–In–S–N (Fig. 3a–c all revealed similar binding energies to those in Ti–In–C–N (Fig. 2a–c). Binding energies of 397.0–397.4 [36–38] and 398.6 eV [36, 37] in the N1s region were assigned to Ti–N–O; the binding energy at 399–400.7 eV was attributed to the Ti–O–N bond [28, 31]. For Ti–In–C–N, Ti–N–O and Ti–O–N were obtained at the binding energy of 397.3 and 399.6 eV (Fig. 2e). Moreover, for Ti–In–S–N, Ti–N–O and Ti–O–N were obtained at the binding energy of 398.7 and 400 eV (Fig. 3d). The XPS spectra of Ti2p and In3d for Ti–In–C–N and Ti–In–S–N were similar to those for Ti–In [7] and Ti–In–N [39] ; moreover, the XPS spectra of O1s and N1s for Ti–In–C–N and Ti–In–S–N were similar to those obtained for Ti–In–N by Wu et al. [39] (data not shown). Zhang et al. [40] indicated the post-nitridation of TiO2 by NH3 takes only place on the surface layer and sublayer of TiO2 and maintains the original crystal phase of TiO2 below 500 °C; accordingly, no crystalline phase of Ti–N was obtained. The present study identified two crystalline phases, Ti–O–N and Ti–N–O, which may be attributable to the use of urea rather than NH3 as the precursor of N. Asahi et al. [24] utilized urea as a dopant and concluded that nitrogen atoms substituted at the lattice oxygen sites, forming an isolated narrow band above the valence band and narrowing the band gap. Notably, the preparation procedure varied among the studies cited above, and this variation may explain the various XPS observations.
The S atoms herein are in the S6+ state, yielding a peak at 168.2–169 eV [14, 16, 41]. The binding energy of S2p in Ti–In–S–N is 168.7 eV (Fig. 3e). When S atoms replace O atoms on the TiO2 surfaces, no peak in the range 160–163 eV, which corresponds to the Ti–S bond, is obtained [14]. Umebayashi et al. [42] suggested that sulfur was doped as an anion and replaced the lattice oxygen in TiO2 to form Ti–S bond, conversely, Ohno et al. [41] reported that S atoms were incorporated as cations and replaced Ti ions in the S-doped TiO2. Ho et al. [15] suggested that the oxidation state of the S-dopant depends on the preparation route. Anionic S doping may be difficult to perform because S2− (0.17 nm) has a significantly larger ionic radius than does O2− (0.122 nm). Hence, the substitution of Ti4+ by S6+ is chemically more favorable than replacing O2− with S2− [14], so Ti–In–S–N becomes positively charged. Therefore, the efficiency of adsorption of RR2 by Ti–In–S–N may exceed those of Ti–In–C–N and Ti–In.
Li et al. [18] found that the absorption spectra of S–N co-doped TiO2 exhibited a red shift in the adsorption edge. This shift was attributed to the fact that calcination induces the doping of S and N atoms into the lattice of TiO2, narrowing the band gap. Wang and Lim [26] observed a red shift of the absorption edge of C–N co-doped TiO2 owing to the coexistence of Ti–C bonds, carbonate and oxynitride species in the TiO2 lattice. The experimental results for Ti–In–C–N and Ti–In–S–N herein were similar to those for C–N co-doped TiO2 and S–N co-doped TiO2.
Photocatalytic properties of Ti–In, Ti–In–C–N and Ti–In–S–N
At pH 3, no significant decolorization occurred during direct photolysis (<5 %) (data not shown herein). Hence, the disappearance of RR2 was attributed to adsorption and/or photodegradation reactions. Fig. 4 presents the decolorization of RR2 by adsorption and photodegradation in different systems. After 180 min, the adsorptions of RR2 (in the dark) by Ti–In, Ti–In–C–N and Ti–In–S–N were 34, 20 and 43 %, while the total removals of RR2 (adsorption and photodegradation under illumination) were 78, 97 and 99 %. Therefore, the net removal of RR2 that was attributable to photodegradation by various photocatalysts followed the order Ti–In–C–N > Ti–In–S–N > Ti–In. The order of RR2 adsorption by these photocatalysts was Ti–In–S–N > Ti–In > Ti–In–C–N, which agrees with the order of the surface areas of these photocatalysts. The removal of RR2 in all experiments followed pseudo-first order kinetics, as has been reported in various studies of RR2 decolorization [7, 13]. Table 1 lists the pseudo-first order RR2 removal rate constants (k), which follow the order Ti–In–S–N > Ti–In–C–N > Ti–In. The k values in UV/Ti–In, UV/Ti–In–N [39], UV/Ti–In–C–N, and UV/Ti–In–S–N were 0.43, 1.60, 1.68 and 1.70 h−1. Clearly, the promotion of photocatalytic activity of Ti–In by co-doping with C–N or S–N was greater than that by N doping.
This study suggested that N atoms could form Ti–N–O and Ti–O–N, while C atoms could form a mixed layer deposited PAC and Ti–O–C bond at the surface of Ti–In–C–N particles. Furthermore, S atoms were incorporated as cations and replaced Ti ions in Ti–In–S–N. Based on the experimental analysis, C, S and N were successfully doped into Ti–In. The improved photocatalytic activity can be evidenced by an increase in either the number of Ti4+ defects or the number oxygen vacancies upon doping, the form of the impurity states, which inhibit the recombination of photogenerated hole and electron pairs, reduced band gap energy and increased lifetime of holes and electrons [43]. Yu et al. [17] suggested that the high activity of N–S co-doped TiO2 can be attributed to the red shift of the adsorption edge, a large surface area and the two phase structures of N–S co-doped TiO2. Large surface areas facilitate contact between holes (or electrons) and the pollutant molecules, improving photocatalytic activity.
Fig. 5 plots the DO profiles that were obtained by respirometric measurements of the RR2 effluent in different systems. The SOURs for the RR2 effluent (after 180 min reaction) in UV/Ti–In, UV/Ti–In–C–N and UV/Ti–In–S–N were determined to be 4.4, 7.3 and 5.3 mg O2/g-MLVSS h. The C–N and S–N co-doped Ti–In improved the biodegradation of RR2 effluent. The enhancement of biodegradation by Ti–In–C–N exceeded that by Ti–In–S–N. The experimental results revealed that Ti–In–C–N effectively decolorized and detoxified RR2.
Conclusions
Novel Ti–In–C–N and Ti–In–S–N photocatalysts were fabricated in this study. C–N and S–N-co-doping in Ti–In inhibited the transformation of TiO2 from anatase to rutile and particle agglomeration. The bang gap energy and particle diameter of Ti–In–C–N and Ti–In–S–N were smaller than those of Ti–In. The RR2 removal rates followed the order Ti–In–S–N > Ti–In–C–N > Ti–In. In this investigation, C, S and N were successfully doped into Ti–In, and the resulting improved photocatalytic activity was attributed to an increase in either the number of Ti4+ defects or the number of oxygen vacancies, which inhibited the recombination of photogenerated hole and electron pairs. Ti–In–C–N and Ti–In–S–N effectively decolorized and detoxified RR2.
References
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations—a review. Appl Catal B 49:1–14
Lo SC, Lin CF, Wu CH, Hsieh PH (2004) Capability of coupled CdSe/TiO2 for photocatalytic degradation of 4-chlorophenol. J Hazard Mater 114:183–190
Ou XH, Wu CH, Lo SL (2006) Photodegradation of 4-chlorophenol by UV/photocatalysts: the effect of the interparticle electron transfer process. React Kinet Catal Lett 88:89–95
Lin CF, Wu CH, Onn SN (2008) Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J Hazard Mater 154:1033–1039
Chakraborty AK, Rhaman MM, Hossain ME, Sobahan KMA (2014) Preparation of WO3/TiO2/In2O3 composite structures and their enhanced photocatalytic activity under visible light irradiation. Reac Kinet Mech Cat 111:371–382
Li C, Ming T, Wang J, Wang J, Yu JC, Yu SH (2014) Ultrasonic aerosol spray-assisted preparation of TiO2/In2O3 composite for visible-light-driven photocatalysis. J Catal 310:84–90
Wu CH, Kuo CY, Lai CH, Chung WY (2014) Surface characteristics and photocatalytic properties of TiO2-In2O3 composite powders by using the sol-gel method: effects of preparation conditions. Reac Kinet Mech Cat 112:543–557
Ma F, Zhang S, Yang X, Guo W, Guo Y, Huo M (2012) Fabrication of metallic platinum and indium oxide codoped titania nanotubes for the simulated sunlight photocatalytic degradation of diethyl phthalate. Catal Commun 24:75–79
Choi W, Termin A, Hoffmann MR (1994) The role of metal-ion dopants in quantum-sized TiO2 - Correlation between photoreactivity and charge-carrier recombination dynamics. J Phys Chem B 98:13669–13679
Sojic DV, Despotovic VN, Abazovic ND, Comor MI, Abramovic BF (2010) Photocatalytic degradation of selected herbicides in aqueous suspensions of doped titania under visible light irradiation. J Hazard Mater 179:49–56
Chen D, Jiang Z, Geng J, Wang Q, Yang D (2007) Carbon and nitrogen co-doped TiO2 with enhanced visible-light photocatalytic activity. Ind Eng Chem Res 46:2741–2746
Wang P, Yap PS, Lim TT (2011) C-N-S tridoped TiO2 for photocatalytic degradation of tetracycline under visible-light irradiation. Appl Catal A 399:252–261
Kuo CY, Wu CH, Chen ST (2014) Decolorization of C.I. reactive red 2 by UV/TiO2/PAC and visible-light/TiO2/PAC systems. Desalin Water Treat 52:834–843
Yu JC, Ho W, Yu J, Yip H, Wong PK, Zhao J (2005) Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ Sci Technol 39:1175–1179
Ho W, Yu JC, Lee S (2006) Low-temperature hydrothermal synthesis of S-doped TiO2 with visible light photocatalytic activity. J Solid State Chem 179:1171–1176
Liu SY, Tang QL, Feng QG (2011) Synthesis of S/Cr doped mesoporous TiO2 with high active visible light degradation property via solid state reaction route. Appl Surf Sci 257:5544–5551
Yu J, Zhou M, Cheng B, Zhao X (2006) Preparation, characterization and photocatalytic activity of in situ N, S-codoped TiO2 powders. J Mol Catal A 246:176–184
Li X, Xiong R, Wei G (2008) S-N co-doped TiO2 photocatalysts with visible-light activity prepared by sol-gel method. Catal Lett 125:104–109
Yang X, Wang Y, Xu L, Yu X, Guo Y (2008) Silver and indium oxide codoped TiO2 nanocomposites with enhanced photocatalytic activity. J Phys Chem C 112:11481–11489
Spurr RA, Myers H (1957) Quantitative analysis of anatase-rutile mixtures with an Xray diffractometer. Anal Chem 29:760–762
Klug P, Alexander LE (1974) X-ray diffraction procedures. Wiley, New York
Hurum DC, Agrios AG, Gray KA, Rajh T, Thurnauer MC (2003) Explaining the enhanced photocatalytic activity of degussa P25 mixed-phase TiO2 using EPR. J Phys Chem B 107:4545–4549
Li Y, Zhang S, Yu Q, Yin W (2007) The effects of activated carbon supports on the structure and properties of TiO2 nanoparticles prepared by a sol–gel method. Appl Surf Sci 253:9254–9258
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Min Y, Zhang K, Zhao W, Zheng F, Chen Y, Zhang Y (2012) Enhanced chemical interaction between TiO2 and grapheme oxide for photocatalytic decolorization of methylene bule. Chem Eng J 193–194:203–210
Wang X, Lim TT (2011) Effect of hexamethylenetetramine on the visible-light photocatalytic activity of C-N codoped TiO2 for bisphenol A degradation: evaluation of photocatalytic mechanism and solution toxicity. Appl Catal A 399:233–241
Xiao Q, Ouyang L (2009) Photocatalytic activity and hydroxyl radical formation of carbon-doped TiO2 nanocrystalline: Effect of calcination temperature. Chem Eng J 148:248–253
Sasikala R, Shirole AR, Sudarsan V, Jagannath Sudakar C, Naik R, Rao R, Bharadwaj SR (2010) Enhanced photocatalytic activity of indium and nitrogen co-doped TiO2-Pd nanocomposites for hydrogen generation. Appl Catal A 377:47–54
Wang X, Hu Z, Chen Y, Zhao G, Liu Y, Wen Z (2009) A novel approach towards high performance composite photocatalyst of TiO2 deposited on activated carbon. Appl Surf Sci 255:3953–3958
Tominaga M, Hirata N, Taniguchi I (2005) UV-ozone dry-cleaning process for indium oxide electrodes for protein electrochemistry. Electrochem Commun 7:1423–1428
Reyes-Gil KR, Reyes-Garcia EA, Raftery D (2007) Nitrogen-doped In2O3 thin film electrodes for photocatalytic water splitting. J Phys Chem C 111:14579–14588
Eshaghi A, Pakshir M, Mozaffarinia (2010) Preparation and photo-induced superhydrophilicity of composite TiO2–SiO2–In2O3 thin film. Appl Surf Sci 256:7062–7066
Zheng J, Liu Z, Liu X, Yan X, Li D, Chua W (2011) Facile hydrothermal synthesis and characteristics of B-doped TiO2 hybrid hollow microspheres with higher photo-catalytic activity. J Alloy Compd 509:3771–3776
Le HA, Linh LT, Chin S, Jurng J (2012) Photocatalytic degradation of methylene blue by a combination of TiO2-anatase and coconut shell activated carbon. Powder Technol 225:167–175
Kang IC, Zhang Q, Yin S, Sato T, Saito F (2008) Preparation of a visible sensitive carbon doped TiO2 photo-catalyst by grinding TiO2 with ethanol and heating treatment. Appl Catal B 80:81–87
Saha NC, Tompkins HG (1992) Titanium nitride oxidation chemistry: An x-ray photoelectron spectroscopy study. J Appl Phys 72:3072–3079
Gyorgy E, Pino AP, Serra P, Morenza JL (2003) Depth profiling characterisation of the surface layer obtained by pulsed Nd:YAG laser irradiation of titanium in nitrogen. Surf Coat Technol 173:265–270
Ismail IM, Abdallah B, Abou-Kharroub M, Mrad O (2012) XPS and RBS investigation of TiNxOy films prepared by vacuum arc discharge. Nucl Instrum Method Phys Res B 271:102–106
Wu CH, Kuo CY, Wu JT, Hong PKA, Lai CH, Chung WY (2014) Effects of nitrogen and carbon dopings on properties and photocatalytic activity of TiO2–In2O3 composite. Korean J Chem Eng (revised)
Zhang Z, Wang X, Long J, Gu Q, Ding Z, Fu X (2010) Nitrogen-doped titanium dioxide visible light photocatalyst: Spectroscopic identification of photoactive centers. J Catal 276:201–214
Ohno T, Akiyoshi M, Umebayashi T, Asai K, Mitsui T, Matsumura M (2004) Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl Catal A 265:115–121
Umebayashi T, Yamaki T, Yamamoto S, Miyashita A, Tanaka S, Sumita T, Asai K (2003) Sulfur-doping of rutile-titanium dioxide by ion implantation: photocurrent spectroscopy and first-principles band calculation studies. J Appl Phys 93:5156–5160
Lu X, Tian B, Chen F, Zhang J (2010) Preparation of boron-doped TiO2 films by autoclaved-sol method at low temperature and study on their photocatalytic activity. Thin Solid Films 519:111–116
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 101–2221–E–151–038–MY3. Additionally, Mr. Lai and Chung of National Kaohsiung University of Applied Sciences are appreciated for assistance in conducting some of experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, JT., Kuo, CY. & Wu, CH. Photodegradation of C.I. reactive red 2 in UV/TiO2–In2O3–C–N and UV/TiO2–In2O3–S–N systems. Reac Kinet Mech Cat 114, 341–355 (2015). https://doi.org/10.1007/s11144-014-0774-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0774-3