Abstract
Dwarf elder (Sambucus ebulus L.) berries are rich in health-promoting phytochemicals such as polyphenols and anthocyanins, and display a significant antioxidant activity. They are also rich in two lectins (ebulin f and SELfd) that share amino acid sequence homology with the elderberry allergen Sam n1 present in Sambucus nigra pollen and fruits. Ebulin f displays toxicity by oral ingestion. This study was aimed at eliminating the toxicity of these lectins whilst having little or no effect on the antioxidant properties of dwarf elder berries. We thus investigated the potential effects of incubation in a boiling water bath of extracts from several parts of the plant on total polyphenol content, antioxidant activity, total anthocyanins, cyanidin-3-glycoside content, and the sensitivity of purified dwarf elder fruit lectins to a simulated gastric fluid. The study shows that five minutes of said heat treatment fully sensitized both lectins to pepsin digestion, whilst minimally reducing phenol and antioxidant as well as free radical scavenging activities to below 13 %. It proved possible to eliminate the potential risks derived from the presence of lectins in dwarf elder juices without any significant reduction in the content of the antioxidant compounds. Dwarf elder berries may thus be a valuable nutritional source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consumption of berries in the diet has been linked to strong human health due to the presence of bioactive constituents thought to be especially relevant in the Mediterranean diet. Berries are rich in antioxidant compounds such as phenols and vitamins [1]. Antioxidant rich intake is thought to have a beneficial effect on human health, particularly vis-à-vis the risk of cancer and other metabolic diseases such as obesity [2]. Anthocyanins promote positive effects on cardiovascular diseases [3, 4]. Coloured berries contain high amounts of anthocyanins such as cyanidin-3-glycoside that exhibits anticancer properties [5].
Consuming elderberry and dwarf elderberry as food and medicine has been reported since ancient times [6], and these are currently consumed as food complements [7, 8]. The berries of Sambucus spp. contain a variety of bioactive-like flavonoids, vitamins and lectins [8, 10]. Lectins have sugar-binding proteins usually with sugar specificity and cell-agglutinating activity and have been found in a variety of foods, especially in vegetables, and may prove to be anti-nutrients [10] and toxins [11]. Members of the elderberry family (Sambucaceae) contain a number of lectins, such as ebulins [12] and nigrins [13], which exhibit ribosome-inactivating activity.
Unripe elder fruits, and particularly dwarf elder fruits, have been traditionally been considered toxic. However, no scientifically sound data are available on elder toxicity. Elderberries contain sambunigrin, a cyanide producing glycoside, purported to be the toxic agent, since it can be hydrolysed leading to the release of cyanide [14]. A study carried out with species of Sambucus canadiensis indicated that most individuals from nine populations of east-central Illinois did not produce cyanogenic glycosides [15]. The only toxicity studies carried out so far with specific compounds of S. ebulus are those conducted with purified lectins, especially ebulin f, which exhibits anti-ribosomal activity. Ebulin f administration of 5 mg/kg body weight by gavage to Swiss mice displays acute toxicity after 3–4 days and leads to a 50 % death rate after 10 days [16]. In addition, ebulin f resists digestion by a simulated gastric fluid for at least 30 min [12]. We therefore hypothesise that dwarf elder fruit toxicity might be due to ebulin f.
Despite the toxicity of S. ebulus, raw fruits have been widely used in traditional medicine in Europe [17]. It has been used as an expectorant, antiseptic, diuretic, anti-inflammatory in joint diseases and rheumatic pain [17], as well as for its wound-healing potential [18]. In a study on various Bulgarian medicinal plants it was found to be the second richest plant in polyphenols and antioxidant activity [19]. On the other hand, Sam n1-dependent type I allergy to pollen elderberry has also been described [20]. The allergen Sam n1 is a protein that shares amino acid sequence homology with ebulin. On the basis of these findings, it may be conjectured that dwarf elder fruit lectins might be considered part of a wide family of structurally related proteins (Sambucus lectins) carrying that allergen. Since the toxicity and potential allergenicity of dwarf elder fruits may reduce their use as a source of antioxidants including polyphenols, we investigated the possibility of inactivating the ebulin f and SELfd lectins present in fruits [12] without significantly inactivating total phenolic compounds and with no reduction in antioxidant activity. Inactivation of lectins was explored as sensitisation to a simulated gastric fluid upon short time incubation.
Materials and Methods
All reagents were of the highest available purity. Chromatography supports were purchased from Pharmacia Ibérica (Madrid, Spain). Dwarf elder species were harvested from Mansilla de las Mulas (León) and Barruelo del Valle (Valladolid, Spain) in mid-August and early July (2012), respectively, and stored frozen at −20 °C. Dwarf elder lectins ebulin f and SELfd were isolated as previously described, and judged homogeneous by SDS-PAGE [12].
Extract Preparation and Heat Treatment
25 g of the corresponding dwarf elder aerial parts of the plant were minced, ground in a mortar with pestle, and extracted with 100 mL of buffer solution (0.28 M NaCl and 5 mM sodium phosphate pH 7.4). The resulting material was strained through cheesecloth and the supernatant was centrifuged at 7,500 g for 30 min at 4 °C. The supernatant was centrifuged again in the same conditions and used immediately for the subsequent experiments or stored at −20 °C and thawed once. Heat treatment was performed by incubating the extracts in a boiling water bath for 0, 30, 60 and 120 s in the case of bovine serum albumin (BSA), and for 0, 5, 15, 45 and 60 min in the case of purified lectins, and analyses were then conducted immediately. For SDS-PAGE analysis, extracts were concentrated five-fold by ultracentrifugation with Millipore Amicon ultra 0.5 centrifugal filter units (Ultracel® 10 K) and stored at −20 °C until use.
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
Analysis of proteins by SDS-PAGE was performed with the miniVE Amersham Bioscience system (Pharmacia Biotec), using 4 % staking gel and 15 % polyacrylamide separation slab gels. Samples were incubated for 5 min at 100 °C in the loading buffer (62.5 mM Tris–HCl (pH 6.8), 2 % (w/v) SDS, 10 % (v/v) glycerol and 0.025 % (w/v) bromophenol blue). 18 μL of sample buffer containing the protein sample was loaded into each well and electrophoresis was performed for 150 min at 20 °C and 25 mA per gel, using a buffer containing 25 mM Tris–HCl (pH 8.3), 192 mM glycine, and 0.1 % (w/v) SDS. Gels were then stained overnight with a solution containing 1 % (w/v) Coomassie Brilliant Blue R-250, 50 % (v/v) methanol, and 10 % (v/v) acetic acid, and then further destained with a solution containing 5 % (v/v) methanol and 7 % (v/v) acetic acid.
Total Phenolic Content
Total phenolic compounds present in elderberry extracts were determined with Folin-Ciocalteau’s reagent for phenolics [21]. Reaction mixtures of 1.5 mL contained 0.6 mL of sodium carbonate saturated solution, 0.2 mL of Folin-Ciocalteau’s reagent and 0.1 mL of sample. The resulting tubes were incubated at 50 °C for 10 min and cooled prior the measurement of the absorbance at a wavelength of 760 nm. Gallic acid was used as standard for calibration, and sample reaction with Folin-Ciocalteau’s phenol reagent and values are expressed as mg of gallic acid equivalents (GAE) per gram (wet weight) of plant material.
CUPRAC Assay for Antioxidant Activity
Antioxidant activity was determined by the CUPRAC assay [22]. Reaction mixtures of 4 mL containing 0.02 mL of sample, 1 mL of 10 mM CuCl2, 1 mL of 7.5 mM neocuproin in ethanol, and 1 mL of 1 M ammonium acetate (pH 7.0) were prepared and kept for 60 min to allow colour development. The resulting colour was measured spectrophotometrically at a wavelength of 450 nm. Gallic acid was used as standard for calibration. The results were expressed as gallic acid equivalents (GAE) per gram (wet weight) of plant material.
DPPH Radical-Scavenging Activity
The capacity to scavenge free radicals was assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) colour decay after 10-min incubation [23]. Reaction mixtures containing 0.03 mL of sample and 2.9 mL of DPPH dissolved in methanol were kept in the dark for colour development, and colour reduction was determined by measuring the absorbance signal at a wavelength of 515 nm. Trolox® was used as standard and results were expressed as Trolox® equivalents per gram (wet weight) of plant material.
Total Anthocyanin Content
Total anthocyanins were determined using the differential pH procedure [24]. Two solutions of 1.2 mL each of different pH containing 0.05 mL of sample were prepared. One solution containing 0.2 M KCl was adjusted to pH 1.0 with HCl. The other, containing 0.2 M sodium acetate, was adjusted to pH 4.5 with acetic acid. Absorbance at 510 and 700 nm was recorded at pH 1.0 and 4.5. A* value was calculated using the following equation: A* = (A510 - A700) pH 1.0 - (A510 - A700) pH 4.5. Finally, monomeric anthocyanidin (AM) was calculated using the following expression: AM (mg/L) = A* · Mr · dilution factor · 1,000)/26,900 (molar extinction coefficient in L · mol · cm−1), where Mr denotes the relative molecular mass. Cyanidine-3-glycoside (C3G) was used as standard, and results were expressed as C3G equivalents (C3GE) per gram (wet weight) of plant material.
Determining Cyanidin-3-Glycoside by HPLC Analysis
Cyanidin-3-glycoside assay was carried out adapting a previously described HPLC method [25]. Briefly, a Jasco modular HPLC system (Jasco International Co. Ltd., Japan) was used. A C18 column Spherisorb ODS-1 (5 μm, 250.0 × 4.6 mm) (Waters Cromatografía, S.A., Spain) with a 4.0 × 3.0 mm C18 guard column (Phenomenex, USA) was selected as stationary phase. The mobile phase consisted of acetonitrile (Scharlab SL, Spain) (A) and 1 % (v/v) phosphoric acid (Panreac Química SA, Spain) and 10 % (v/v) acetic acid (Scharlab SL, Spain) in milli-Q water (B). The program followed a linear gradient from 2 to 20 % of A in 10 min and then from 20 to 2 % of A in 15 min. Flow rate was set at 1.0 mL min−1 and injection volume was 20 μL. Absorbance of the eluent was monitored at 520 nm with a detection sensitivity of 0.250 aufs.
Pepsin Digestion and SDS-PAGE Analysis
Digestion by simulated gastric fluid was performed as reported elsewhere [12]. Reaction mixtures of 90 μL contained 0.3 mg of pepsin, 0.066 N HCl, 30 mM NaCl, herein called simulated gastric fluid (SGF), and the test protein (either purified lectin or BSA). Incubations were carried out at 37 °C for the times indicated in the figure legends [Electronic Supplementary Material 1]. Digestion was stopped at appropriate times by mixing with 30 μL of 200 mM NaHCO3 (pH 11). From these mixtures, 18 μL was taken and mixed with 6 μL of four-times concentrated electrophoresis buffer containing 40 % glycerol, 20 % SDS, 0.33 M Tris (pH 6.8), and 0.05 % bromophenol blue, and processed for SDS-PAGE analysis as described elsewhere [12].
Results
One goal of preparing and processing juices from berries is to affect, as little as possible, antioxidant content and other medicinal compounds that might prove sensitive to lengthy heat treatments [26, 27]. In an initial step, the content in the different aerial parts of the plant was studied. Ripe fruits are richest in phenolic compounds measured by reaction with Folin-Ciocalteau’s phenolic reagent and also displayed the highest antioxidant and free-radical scavenging activities measured using CUPRAC and DPPH assays, respectively [Electronic Supplementary Material 2].
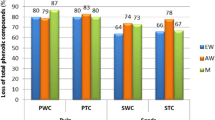
The effect of boiling water bath heat pre-treatment of the unripe (green) and ripe (cyan) fruit extracts on total phenolic degradation and reduction in antioxidant and free scavenging activities was also elucidated. As shown in Table 1, 10-min heat pre-treatment on green fruit extracts led to a 7.6 % reduction in phenolic content, an 8.8 % reduction in antioxidant activity by CUPRAC assay, and a 9.5 % reduction in free-radical scavenging activity by DPPH assay.
For ripe fruits after the same pre-treatment, results showed reductions of 4.0 % for total phenolic compounds and 5.0 % for total anthocyanins (Table 1). Reductions in antioxidant by CUPRAC assay and free-radical scavenging activities at the same time were 5.4 and 13.0 %, respectively (Table 1). Anthocyanins were present only in the stalks of ripe fruits and showed extremely low concentrations compared to ripe fruits, although these were high enough to give some cyan colour. Perhaps the most significant polyphenol from a health point of view is cyanidin-3-glycoside, which has been reported to display anticancer properties [5]. Extracts were analysed by HPLC to assess the presence of said anthocyanin and to determine its stability upon heating [25]. As shown in Fig. 1, a symmetrical peak moved at 16.8 min which concurred with standard cyanidin-3-glycoside. In elderberry (S. nigra), cyanidin-3-glycoside has been reported to move in exactly the same position as cyanidin-3-sambubioside [26]. The possibility that our peak might consist of a mixture of these two glycosides cannot therefore be ruled out. Heat treatment for 5-min reduced its concentration by nearly 40 %, by 50 % after 15 min, and completely after 80 min. The degradation time-course suggests a second order rate. Our data agree with previous reports indicating that these polyphenols are sensitive to heat [27, 28].
Ebulin f and SELfd, the lectins present in dwarf elder, displayed differential sensitivity to pepsin in a simulated gastric fluid, the main feature being that SELfd proved resistant to pepsin digestion up to 60 min, whereas ebulin f was digested partially after 40 min incubation [12]. Ebulin f has been reported to be toxic orally and intraperitoneally [12, 16]. Since gastric resistance of lectins might determine their fate and effects on the intestinal epithelium, we sought to explore the effects of moist heat on the stability of S. ebulus fruit lectins in simulated gastric fluid to best define their potential as toxin and food allergens.
As shown in Fig. 2, SDS-PAGE analysis of extracts from several parts of dwarf elder revealed that ripe fruits, late, and early green fruits, blossoms and late fruit stalks contain a large proportion of these lectins. They are comparable since all the extracts were prepared in the same conditions and the amount of protein in each well was nearly the same. SELfd lectin is the major protein present in fruits and blossoms; meanwhile, ebulin f is the major protein in late stalk fruits. A small amount of ebulin f is also present in ripe fruits.
SDS-PAGE analysis of extracts of several aerial parts of dwarf elder (S. ebulus). (Left panel) (a), tips; (b), shoots; (c), leaf; (d), early green fruits; (e), late green fruits; (f), ripe fruits; (g), blossoms; (h), early fruit stalks; (i), late fruit stalks. (Right panel) SDS-PAGE of extracts obtained from the corresponding aerial parts; each lane contained 18 μL of crude extract in sample buffer. The amounts of protein markers were SELfd (Mr 68,000), 9.8 μg; nigrin b (Mr 58,000), 8.2 μg and SNAIV (Mr 30,000), 11.1 μg
It is well known that certain plants display toxicity which disappears after cooking. Examples of these are those that contain protein lectins and enzyme inhibitors [29]. We therefore investigated whether short-time heat treatment in a boiling water bath might change the sensitivity of the two lectins to pepsin. As shown in the Supplementary Figure, without heat treatment, ebulin f was poorly hydrolysed at least after 45 min, in agreement with previous report [12]. Under these conditions, BSA was already completely degraded at 30 s and, based on a previous study SELfd, was shown to be stable for up to 60 min (data not shown; [12]). It was found that heat treatment in a boiling water bath made ebulin f and SELfd fully sensitive to pepsin after just 5 min incubation.
Discussion
Dwarf elder is extremely rich in phenolic compounds, antioxidants and other beneficial components and has therefore been proposed as a rich source of these substances [19]. However, dwarf elder fruits have traditionally been considered as risky due to the presence of heat sensitive toxics, particularly in unripe fruits, which might lead to gastrointestinal disturbance. Fruits contain ebulin whose oral intake by mice shows important toxicity on the small intestine which is able to kill the animals [16]. Ebulin shares amino acid sequence homology with the elderberry allergen Sam n1 that triggers type I allergy [20]. Benefitting from them whilst avoiding lectin-related risks thus involves finding a procedure able to trigger lectin inactivation without affecting antioxidant properties. In our hands, dwarf elder ripe fruits have by far the highest concentration of phenolics and display antioxidant and free scavenger activity in parts of the plant. Mature leaves and late fruit stalks are also rich parts to be considered as sources of phenols and antioxidants. The advantage of the short-time incubation assayed here is that this treatment promotes no significant reduction in either total phenols or antioxidant capacity. Overall, short-time heat treatment may be assumed as being not too destructive, at least as regards phenols and antioxidant capacity. In contrast to phenols, the effects of heat treatment on lectins seem to determine their fate since they become fully sensitized to the hydrolytic action of pepsin and will therefore not activate the gastrointestinal immune system or display toxicity. Thermal processing has also been investigated as a way to reduce immunogenicity of cow and buffalo milk caseins in mice [30], and phytohemagglutinins from beans (Phaseolus vulgaris) are totally inactivated [29].
When selecting individual ripe fruits in the laboratory, care is taken to obtain homogeneous populations and avoid green fruits and fruit stalks. However, this proves quite difficult at field harvesting level. In such cases, most probably ripe fruits are mixed to a variable degree with unripe fruits and stalks of ripe fruits, both of which contain far more ebulin f than ripe fruits (Fig. 2). A further difficulty is that both unripe green and ripe fruits coexist in the same umbel. The resulting preparations would contain significant amounts of ebulin f which would increase the risks of toxicity in addition to the allergenicity risks dependent on both SELfd and ebulin f lectins. That is why tagging for elimination by heat treatment prior to digestion proves so important. The present experiments are thus of major importance from the plant food safety and industrial standpoint as regards of berry juice processing since they eliminate: a) potential food allergy risks caused by the potential allergenic activity of Sambucus lectins; b) potential toxicity or abnormal growth effects due to ingestion of raw elderberry juices containing lectins. In summary, ripe dwarf elder fruits are a rich source of phenols and antioxidant compounds. However, they coexist with two-chain lectins called SELfd and ebulin f that are resistant to pepsin digestion. Furthermore, ebulin f displays a significant oral toxicity to mice. The present study shows that it is possible to sensitise both lectins to pepsin by short-time heating of ripe fruit extracts which only reduces total phenol content and antioxidant and free-radical scavenging activities up to 13.0 %. This finding is very important when large-scale juice preparation is considered since sensitisation to pepsin will abolish potential risks of allergenicity due to both lectins and toxicity thanks to the low amounts of ebulin f present in the juices. Taking the present results into account, dwarf elder fruits may be useful as a source of nutraceuticals. Work is ongoing to study in vivo digestibility in mice and to ascertain meal factors which might change the pattern found in vitro. In addition, the allergenicity of these lectins and the effects of heat treatment on the binding of IgE will be studied further.
References
Szajdek A, Borowska EJ (2008) Bioactive compounds and health-promoting properties of berry fruits: a review. Plant Foods Hum Nutr 63(4):147–156. doi:10.1007/s11130–008–0097–5
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306. doi:10.1080/1040869059096
Tsuda T (2012) Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res 56(1):159–170. doi:10.1002/mnfr.201100526
Wallace TC (2011) Anthocyanins in cardiovascular disease. Adv Nutr 2(1):1–7. doi:10.3945/an.110.000042
Ding M, Feng R, Wang SY et al (2006) Cyanidin-3-Glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem 281(25):17359–17368. doi:10.1074/jbc.M600861200
Mariotti Lippi L, Bellini C, Mori Secci M (2010) Palaeovegetational reconstruction based on pollen and seeds/fruits from a bronze age archaeological site in Tuscany (Italy). Plant Biosyst 144(4):902–908. doi:10.1080/11263504.2010.491978
Kaack K, Austed T (1998) Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum Nutr 52:187–198. doi:10.1023/A:1008069422202
Veberic R, Jakopic J, Stampar F, Schmitzer V (2009) European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem 114:511–515. doi:10.1016/j.foodchem.2008.09.080
Girbés T, Ferreras JM, Arias FJ, Stirpe F (2004) Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev Med Chem 4:461–476. doi:10.2174/1389557043403891
Liener IE (1994) Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr 34(1):31–67. doi:10.1080/10408399409527649
Stirpe F, Battelli MG (2006) Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci 63(16):1850–1866
Jimenez P, Tejero J, Cabrero P, Cordoba-Diaz D, Girbes T (2013) Differential sensitivity of D-galactose-binding lectins from fruits of dwarf elder (Sambucus ebulus L.) to a simulated gastric fluid. Food Chem 136:794–802. doi:10.1016/j.foodchem.2012.09.011
Ferreras JM, Citores L, Iglesias R, Jimenez P, Souza AM, Gayoso MJ, Girbes T (2011) Occurrence of the type two ribosome-inactivating protein nigrin b in elderberry (Sambucus nigra L.) bark. Food Res Int 44:2798–2805. doi:10.1016/j.foodres.2011.06.004
Bromley J, Hughes BGM, Leong DCS, Buckley NA (2005) Life-threatening interaction between complementary medicines: cyanide toxicity following ingestion of amygdalin and vitamin C. Ann Pharmacother 39:1566–1569. doi:10.1345/aph.1E634
Buhrmester RA, Ebingerla JE, Seigler DS (2000) Sambunigrin and cyanogenic variability in populations of Sambucus canadensis L. (Caprifoliaceae). Biochem Syst Ecol 28(7):689–695. doi:10.1016/S0305–1978(99)00105–2
Jiménez P, Gayoso MJ, Tejero J et al (2013) Toxicity in mice of lectin ebulin f present in dwarf elderberry (Sambucus ebulus L.). Toxicon 61:26–29. doi:10.1016/j.toxicon.2012.10.009
Schwaiger S, Zeller I, Pölzelbauer P et al (2011) Identification and pharmacological characterization of the anti-inflammatory principal of the leaves of dwarf elder (Sambucus ebulus L.). J Ethnopharmacol 133(2):704–709. doi:10.1016/j.jep.2010.10.049
Süntar IP, Akkol EK, Yalçin FN, Koca U, Keleş H, Yesilada E (2010) Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-o-Glucoside. J Ethnopharmacol 129(1):106–114. doi:10.1016/j.jep.2010.01.051
Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T (2006) Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from bulgarian herbs. Phytother Res 20:961–965. doi:10.1002/ptr.1985
Förster-Waldl E, Marchetti M, Schöll I et al (2003) Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clin Exp Allergy 33(12):1703–1710. doi:10.1111/j.1365–2222.2003.01811.x
Prior RL, Wu XL, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302. doi:10.1021/jf0502698
Apak R, Guclu K, Ozyurek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52:7970–7981. doi:10.1021/jf048741x
Reis FS, Barros L, Martins A, Ferreira ICFR (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 50:191–197. doi:10.1016/j.fct.2011.10.056
Giusti MM, Wrolstad RE (1996) Characterization of red radish anthocyanins. J Food Sci 61:322–326. doi:10.1111/j.1365–2621.1996.tb14186.x
Terry LA, Chope GA, Bordonaba JG (2007) Effect of water deficit irrigation and inoculation with Botrytis cinerea on strawberry (Fragaria x ananassa) fruit quality. J Agric Food Chem 55(26):10812–10819. doi:10.1021/jf072101n
Bermúdez-Soto MJ, Tomás-Barberán FA (2004) Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur Food Res Technol 219(2):133–141. doi:10.1007/s00217–004–0940–3
Oliveira C, Amaro LF, Pinho O, Ferreira IM (2010) Cooked blueberries: anthocyanin and anthocyanidin degradation and their radical-scavenging activity. J Agric Food Chem 58(16):9006–9012. doi:10.1021/jf101923w
Yue X, Xu Z (2008) Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. J Food Sci 73(6):494–499. doi:10.1111/j.1750–3841.2008.00845.x
Carvalho MRB, Sgarbieri VC (1997) Heat treatment and inactivation of trypsin-chymotrypsin inhibitors and lectins from beans (Phaseolus vulgaris L.). J Food Biochem 21(4):219–233. doi:10.1111/j.1745–4514.1997.tb00216.x
Shandilya UK, Kapila R, Haq RM, Kapila S, Kansal V (2013) Effect of thermal processing of cow and buffalo milk on the allergenic response to caseins and whey proteins in mice. J Sci Food Agric 93(9):2287–2292. doi:10.1002/jsfa.6041
Acknowledgments
This work has been supported by grants from the Regional Government of Castilla y León (GR106 and the Regional Health Ministry) and UVa-GIR.
Conflict of Interest
The authors declare no conflict of interest.
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jimenez, P., Cabrero, P., Basterrechea, J.E. et al. Effects of Short-term Heating on Total Polyphenols, Anthocyanins, Antioxidant Activity and Lectins of Different Parts of Dwarf Elder (Sambucus ebulus L.). Plant Foods Hum Nutr 69, 168–174 (2014). https://doi.org/10.1007/s11130-014-0417-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-014-0417-x