Abstract
This is a meta-analysis of randomized controlled trials (RCTs) comparing cognitive behaviour therapy for insomnia (CBT-I) monotherapy with active control treatment for insomnia in patients with medical or psychiatric comorbidities. Both international (PubMed, EMBASE, PsycINFO, Cochrane Library) and Chinese (WanFang, and CNKI) databases were systematically searched. The random effects model was used. Thirteen RCTs comparing CBT-I (n = 441) and active controls (n = 412) groups were included. CBT-I group showed significant advantage over active controls at post-treatment assessment in terms of Insomnia Severity Index (ISI; SMD = -0.74), sleep onset latency (SMD = -0.36), wake after sleep onset (SMD = -0.21), sleep quality (SMD = 0.56), Pittsburgh sleep quality index total scores (PSQI; SMD = -0.76) and the total score of dysfunctional beliefs and attitudes about sleep scale (DBAS; SMD = -1.09). Subgroup analyses revealed significant improvement in sleep onset latency in patients with psychiatric disorders (SMD = -0.45), while significant reduction of number of wakeup after sleep onset was found in patients with medical conditions (SMD = -0.31). This meta-analysis found that CBT-I monotherapy had greater efficacy than other active control treatment for insomnia in patients with medical or psychiatric comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia is a common sleep disorder [1] that can lead to negative health outcomes, such as fatigue, increased daytime irritability, cognitive deficits and poor health status [2, 3]. Insomnia often co-exists with major medical conditions, such as diabetes [4], hypertension [5] and cancer [6], which increase personal suffering and even the risk of all-cause mortality [7]. In addition, insomnia also occurs in up to 40%–50% of patients with psychiatric disorders [8], such as depression [9], bipolar and anxiety disorder [10], and increases the risk of suicide [11, 12].
There is a complex association between insomnia and major medical/ psychiatric disorders. Medical and psychiatric disorders could precipitate the development of insomnia, while insomnia could increase the risk of medical/ psychiatric conditions. Therefore both insomnia and major medical/ psychiatric disorders need to be treated concurrently [1, 13]. Psychotropic medications, such as benzodiazepines and non-benzodiazepine hypnotics (e.g. Zolpidem), are commonly prescribed, although the long-term use is not encouraged due to the risk of dependency [14, 15].
Psychosocial interventions, such as cognitive behavioural therapy for insomnia (CBT-I), have been increasingly used in treating patients with insomnia disorder [16,17,18,19]. The efficacy of CBT-I in primary insomnia has been shown in several meta-analyses [20,21,22,23,24,25,26] and CBT-I is recommended for insomnia by the American Academy of Sleep Medicine [17, 27].
Research findings on the efficacy of CBT-I in patients with major medical conditions (e.g. cancer [28]) or psychiatric disorders (e.g. depression (Manber et al., 2008) and posttraumatic stress disorder (Ger-main, Shear, Hall, & Buysse, 2007; Talbot et al., 2014)), have been mixed. One recent meta-analysis [29] examined the efficacy of CBT-I for patients with medical or psychiatric comorbidities. However, the diagnosis of insomnia was based on either patients’ complaints or standardized diagnostic scales, such as the insomnia severity index (ISI), and only a proportion of participants in the included studies had comorbid medical and/or psychiatric disorders. Due to the heterogeneity in diagnostic criteria and study samples across studies, the findings could be biased to an uncertain extent.
Thus we conducted this meta-analysis of randomized controlled trials (RCTs) comparing CBT-I monotherapy with active control treatment for insomnia in patients with major medical or psychiatric disorders using stringent diagnostic criteria.
Methods
Selection Criteria
Following the PICOS acronym [30], the inclusion criteria were as follows: participants (P): patients with a diagnosis of insomnia and major medical or psychiatric comorbidities; insomnia was diagnosed using standardized diagnostic criteria, such as the Diagnostic Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [31], the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) [32], or the International Classification of Sleep Disorders (ICSD) [33, 34]. Intervention (I): CBT-I monotherapy. Comparison (C): active control group, such as behavioural desensitization and sleep hygiene education. Outcomes (O): the primary outcome measure was the improvement of insomnia symptoms as assessed with standardized scales, such as the Insomnia Severity Index (ISI). Secondary outcome measures included the Pittsburgh Sleep Quality Index (PSQI), dysfunctional attitudes and beliefs about sleep scale (DBAS) and sleep parameters based on polysomnography, actigraphy or sleep diaries, such as sleep efficiency, total sleep time, sleep latency, wake after sleep onset, time in bed, sleep quality and number of awakenings. Study design (S): Randomized controlled trials (RCTs). Studies that did not specifically mention CBT-I were excluded, although certain components of CBT-I were used.
Search Methods
Literature search was independently conducted by three authors (FCZ and YY) in PubMed, EMBASE, PsycINFO, Cochrane Library, WanFang, and CNKI databases from the inception date until November 12, 2017, using the following terms: CBTI, CBT-I, cognitive behavioural therapy for insomnia, cognitive behavioural for insomnia, cognitive behavioural treatment for insomnia, cognitive behavioural treatment for insomnia, cognitive behavioural therapy of insomnia, cognitive behavioural therapy of insomnia, insomnia, sleep, maintenance disorder, dyssomnia, sleepless, early morning awakening, Randomized controlled trials, Randomized controlled trial, RCT, and RCTs. In addition, we manually reviewed the reference list of relevant reviews for additional studies.
Data Extraction
Relevant data were independently extracted by two authors (FCZ and YY). Any discrepancies were resolved by consensus or a discussion with the third author (YTX). If both polysomnography or actigraphy and rating scales were used, polysomnography and actigraphy were preferred as they are more objective than scales. Several studies had several follow-up assessments. In order to reduce heterogeneity caused by study periods, only the data at 3 months follow-up were extracted and analyzed.
Quality Assessment
The study quality was assessed using the Cochrane risk of bias [35] and the Jadad scale [36, 37]. The total score of the Jadad scale ranges from 1 to 5, with a higher score indicating higher quality. Studies with a Jadad total score of <3 was considered as low-quality; otherwise they were considered as high-quality [36]. The system grading of recommendations assessment, development, and evaluation (GRADE) was used in evaluating the evidence level of outcomes [38, 39].
Data Synthesis and Statistical Analyses
Due to the discrepancy in sampling methods, measurements and demographic and clinical characteristics between studies, the random effects model was used for meta-analytic outcomes [40]. Compared to fixed-effects model, the random-effects model is more conservative [40]. Intention-to-treat (ITT) analyses were preferred if available in included studies [41,42,43]. The heterogeneity across included studies was assessed using I2 index [44], with I2 of 25%, 50% and 75% indicating mild, moderate, and high heterogeneity between studies, respectively. The standardized mean difference (SMD) with 95% confidence intervals (CIs) was used for continuous outcome variables. Funnel plots and Egger’s test were performed [45] for publication bias of primary outcome. A significance level of 0.05 was set for all meta-analytic outcomes (two-sided). Review Manager Version 5.3 (http://www.cochrane.org) and Comprehensive Meta-Analysis V2.0 (www.meta-analysis.com) were used for all analyses.
Results
Literature Search and Study Characteristics
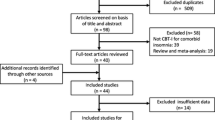
A total of 250 relevant articles were initially identified in the literature search. Finally, 13 RCTs with 27 treatment arms were included in the analyses (Fig. 1). One study [46] included three arms (e.g., an internet CBT-I, an in-person CBT-I and a control group), and so data from both CBT-I groups were combined for analyses. Eight hundred and fifty-three participants were included in this meta-analysis, with 441 patients in the CBT-I group and 412 in the active control group. The included studies were carried out in the United States (7 RCTs, n = 481), Sweden (1 RCT, n = 64), Canada (2 RCTs, n = 183), and Spain (3 RCTs, n = 125). The diagnosis of insomnia was established using DSM-IV, DSM-IV-TR, DSM-III-R, International Classification of Sleep Disorders (ICSD), ICSD-2, or American Academy of Sleep Medicine criteria. Treatment frequency varied from weekly to biweekly, and the number of sessions of CBT-I treatment ranged from 3 to 12 (Table 1).
Assessment Quality and Quality of Evidence
The risk of bias in the 13 RCTs is shown in Table S1. Five studies were double blinded, 8 studies were single blinded or partial blinded, and the rest did not provide any information of blinding. All the included RCTs described the random sequence generation, and 5 mentioned allocation concealment. All studies were rated as “low risk” in terms of attrition and reporting bias. Jadad total score ranged from 3 to 4 (Table 1). All included RCTs were rated as “high quality”. The quality of evidence of outcome measures were evaluated as “very low” (5.9%, 1/17), “low” (29.4%, 5/17), and “moderate” (64.7%, 11/17) according to the GRADE approach (Table 3).
Publication Bias
As the number of studies was less than 10 for analyses on efficacy measures, publication bias could not be evaluated [47].
Primary Outcome
Insomnia Severity Index (ISI)
Seven studies reported insomnia data assessed by ISI at post-treatment assessment. Compared with the control group, the CBT-I group showed more significant improvement at post-treatment (n = 527; SMD: -0.74, 95% CI: −0.92 to −0.56, I2 = 39%, p < 0.0001, Table 2, Fig. S1.1). Subgroup analyses found superiority of CBT-I over active control group in patients with major medical conditions at post-treatment (N = 3, n = 275, SMD: -0.58, 95% CI: −0.82 to −0.34, I2 = 33%, p < 0.001), as well as in those with psychiatric disorders at post-treatment (N = 4, n = 252, SMD: -0.93, 95% CI: −1.20 to −0.66, I2 = 7%, p < 0.001) (Fig. S1.2). Additional follow-up data were only available in 2 studies, which also found superiority of CBT-I group over active control group at 3-month follow-up (n = 168, SMD: -0.33, 95% CI: −0.64 to −0.01, I2 = 0%, p = 0.04, Table 2, Fig. S1.3).
Secondary Outcomes
Sleep Efficiency
Data on sleep efficiency were available in 7 RCTs (n = 498). Compared with control group, CBT-I was associated with small but significant improvement at post-treatment assessment (n = 498, SMD: 0.18, 95% CI: 0.00 to 0.36, I2 = 36%, p = 0.05, Table 2, Fig. S2.1). Compared with the control group, CBT-I did not show significant benefits in patients with psychiatric disorders at post-treatment assessment (2 RCTs, n = 158, SMD: 0.13, 95% CI: −0.19 to 0.46, I2 = 2%, p = 0.42, Table 2). There were also non-significant findings in patients with medical conditions at post-treatment assessment (5 RCTs, n = 340, SMD: 0.14, 95% CI: −0.19 to 0.47, I2 = 52%, p = 0.40)(Fig. S2.2). Three studies reported additional follow-up assessments and found a significant improvement of sleep efficiency in CBT-I group that persisted at 3 months (n = 234, SMD: 0.31, 95% CI: 0.04 to 0.58, I2 = 0%, p = 0.03, Table 2, Fig. S2.3).
Total Sleep Time
Eight RCTS reported data on total sleep time. There was no significant group difference at post-treatment assessment (n = 531, SMD: -0.13, 95% CI: −0.30 to 0.04, I2 = 45%, p = 0.14, Table 2, Fig. S3.1). In the subgroup analyses, neither patients with major medical conditions nor those with psychiatric disorders showed any significant improvement in CBT-I group at post-treatment assessment (major medical conditions: 5 RCTs, n = 309, SMD: -0.15, 95% CI: −0.49 to 0.18, I2 = 51%, p = 0.37; psychiatric disorders: 3 RCTs, n = 222, SMD: -0.04, 95% CI: −0.44 to 0.35, I2 = 51%, p = 0.83, Fig. S3.2). Three studies reported additional follow-up data at 3 months, but there was no significant group difference (n = 235, SMD: -0.20, 95% CI: −0.47 to 0.07, I2 = 15%, p = 0.14, Table 2, Fig. S3.3).
Sleep Onset Latency
Data were available from 6 RCTs on sleep latency. Compared with control group, the CBT-I group showed significant improvement at post-treatment assessment (n = 505, SMD: -0.36, 95% CI: −0.62 to −0.10, I2 = 51%, p = 0.007, Table 2, Fig. S4.1). No group difference was found in patients with major medical conditions at post-treatment assessment (3 RCTs, n = 283, SMD: -0.27, 95% CI: −0.78 to 0.24, I2 = 78%, p = 0.30). Nevertheless, subgroup analyses revealed a significant group difference in patients with psychiatric disorders at post-treatment assessment (3 RCTs, n = 222, SMD: -0.45, 95% CI: −0.73 to 0.18, I2 = 0%, p = 0.001, Fig. S4.2). Three studies reported data in additional follow-up assessment, but no group difference was found (n = 234, SMD: -0.13, 95% CI: −0.40 to 0.14, I2 = 32%, p = 0.33, Table 2, Fig. S4.3).
Wake after Sleep Onset
Data were available from 7 RCTS on wake after sleep onset at the post-treatment assessment. The CBT-I group showed significant improvement compared with active control group (n = 531, SMD: -0.21, 95% CI: −0.38 to −0.04, I2 = 34%, p = 0.02, Table 2, Fig. S5.1). Significant group difference was found in patients with major medical conditions (4 RCTs, n = 309, SMD: -0.31, 95% CI: −0.54 to −0.09, I2 = 0%, p = 0.006). However, in patients with psychiatric disorders, no significant group difference was found (3 RCTs, n = 222, SMD: -0.08, 95% CI: −0.51 to 0.35, I2 = 59%, p = 0.72, Fig. S5.2). In the three studies which reported additional 3 months follow-up, and the superiority of CBT-I group was found (n = 250, SMD: -0.30, 95% CI: −0.56 to 0.05, I2 = 47%, p = 0.02, Table 2, Fig. S5.3).
Time in Bed
Two RCTs reported data on time in bed between CBT-I group and active control group at post-treatment assessment, but no significant group difference was found (n = 84, SMD: -0.09, 95% CI: −0.72 to 0.90, I2 = 67%, p = 0.83, Table 2, Fig. S6).
Sleep Quality
Data were available in 2 RCTS on sleep quality at the post-treatment assessment, and the CBT-I group showed significant improvement (n = 164, SMD: 0.56, 95% CI: 0.23 to 0.88, I2 = 0%, p < 0.001, Table 2, Fig. S7).
Number of Awakenings
Data were available from 2 RCTs on number of awakenings at the post-treatment assessment, but no significant group difference was found (n = 126, SMD: 0.20, 95% CI: −0.16 to 0.57, I2 = 0%, p = 0.28, Table 2, Fig. S8).
Pittsburgh Sleep Quality Index (PSQI)
Seven RCTs reported the changes of PSQI total score at post-treatment assessments. CBT-I group showed significant improvement (n = 417; SMD: -0.76, 95% CI: −1.09 to −0.42, I2 = 61%, p < 0.001, Table 2, Fig. S9.1). Subgroup analyses revealed a significant advantage of CBT-I for patients with major medical conditions (5 RCTs, n = 329, SMD: -0.76, 95% CI: −1.22 to −0.30, I2 = 74%, p = 0.001) and for those with psychiatric disorders (2 RCTs, n = 88, SMD: -0.76, 95% CI: −1.19 to −0.32, I2 = 0%, p = 0.006, Fig. S9.2). Follow-up assessments were reported in 3 RCTs, and the superiority of CBT-I persisted for three months (n = 215, SMD: -0.56, 95% CI: −1.01 to −0.12, I2 = 55%, p = 0.01, Table 2, Fig. S9.3).
Dysfunctional Attitudes and Beliefs about Sleep Scale (DBAS)
The DBAS total scores were available in 3 RCTs at post-treatment assessment. CBT-I group had significant improvement at post-treatment assessment (n = 283; SMD: -1.09, 95% CI: −1.48 to −0.71, I2 = 60%, p < 0.001, Table 2, Fig. S10.1). Three studies reported data at additional follow-up, but there were no significant group difference (n = 251; SMD: -0.8, 95% CI: −1.35 to −0.25, I2 = 76%, p = 0.004, Table 2, Fig. S10.2).
All Cause Discontinuation
Eleven studies reported discontinuation rates at post-treatment assessments, but no significant group difference was found (n = 773, RR = 0.81, 95% CI: 0.44 to 1.47, I2 = 60%, p = 0.49, Fig. S11).
Discussion
This was the first meta-analysis of RCTs specifically comparing CBT-I monotherapy with active control treatment for insomnia in patients with medical or psychiatric comorbidities.
CBT-I usually contains five core components [27]: stimulus control, sleep restriction, sleep hygiene, relaxation training and cognitive restructuring. Of the included studies, 4 studies used all the five components [46, 48,49,50], 8 studies used four components [51,52,53,54,55,56,57,58] and 1 study used two components [59]. Of the five core components, stimulus control was used in all studies. Four studies also used an additional component (relapse prevention for insomnia) [50, 52, 56, 57], and psycho-education about the association between sleep and fibromyalgia was used in 3 studies [50, 56, 57]. This meta-analysis consistently found superiority of CBT-I over active control group in treating insomnia in patients with major medical or psychiatric comorbidities. The effect size was medium as measured by the primary outcome measure (SMD = −0.74). In addition, the advantage of the CBT-I group remained in most secondary outcome measures, such as sleep onset latency (SMD = -0.36), wake after sleep onset (SMD = -0.21), sleep quality (SMD = 0.56), PSQI total scores (SMD = -0.76) and DBAS total scores (SMD = -1.09). However, no group difference was found in total sleep time, time in bed, number of awakening and sleep quality. This meta-analysis also found that the superiority of CBT-I persisted at 3 months in the following measures: the wake after sleep onset (SMD = -0.30), PSQI (SMD = -0.56) and ISI (SMD = −0.33). The effect sizes in these measures were generally larger than those reported in the Geiger-Brown et al.’s study [29], in which the benefits in the CBT-I group were less pronounced (effect size: −0.17 to 0.10) at 3 months follow-up.
As for the primary outcome measure, the effect size between CBT-I and active control treatment in this meta-analysis (SMD = -0.74) was smaller compared to the Geiger-Brown et al. study (2015) (effect size = 1.22), which could be due to different proportion of comorbidities between study samples. In the 2015 study [29] the majority of the participants suffered from chronic pain syndrome and mixed medical and/or psychiatric conditions. CBT-I was associated with an effect size of 1.00 in patients with medical conditions and with an effect size of 1.51 in those with psychiatric disorders [29]. Subgroup analyses of this meta-analyses revealed similar findings, which suggests that CBT-I appears to be more effective in treating insomnia patients with psychiatric disorders. The present study revealed that CBT-I was associated with a greater improvement in patients with psychiatric comorbidities (SMD: −0.93) than those with medical comorbidities (SMD: −0.58) as measured by the ISI total score.
In this meta-analysis CBT-I showed superiority over control group in several secondary outcome measures, such as sleep onset latency, wake after sleep onset, sleep quality, PSQI and DBAS total scores. The effect sizes were generally smaller than most findings published in previous meta-analyses regarding CBT-I [20, 22, 25, 26]. Moreover, this meta-analysis did not find any advantage of CBT-I in the following domains: total sleep time, time in bed, and number of awakening. These inconsistent findings may be due to different proportion of comorbidities between study samples.
Subgroup analyses revealed that CBT-I had an advantage on sleep onset latency only in patients with psychiatric disorders, while CBT-I had an advantage regarding number of wakeup after sleep onset only in those with medical conditions. This finding was not reported previously. This meta-analysis also found that the advantage of CBT-I persisted at 3-month follow-up in the following measures: wake after sleep onset, PSQI, and ISI. However, the effect sizes appeared to decrease over time.
The strengths of this meta-analysis include the use of stringent diagnostic criteria for insomnia, inclusion of insomnia patients with major medical conditions or psychiatric disorders, and administration of a variety of secondary outcome measures. However, several limitations need to be addressed. First, there was a discrepancy in study designs, participant characteristics, insomnia definition, and outcome measures between studies, although the random effects model and subgroup analyses have been performed. Second, some relevant factors, such as prescriptions of medications and severity of major medical conditions and psychiatric disorders, were not analysed due to insufficient data. Third, the long-term effect of CBT-I could not be examined due to lack of data. Finally, only English and Chinese databases were searched.
Conclusion
This meta-analysis found that CBT-I monotherapy generally had greater efficacy than other active control treatment for insomnia in patients with medical or psychiatric comorbidities. CBT-I was more efficacious in improving sleep onset latency in patients with mental disorders, while it also has an advantage in reducing number of wakeup after sleep onset in patients with medical conditions. However, the advantage of CBT-I was only maintained in some measurements at 3-month assessment, with decreased effect sizes over time.
Data Availability
Data will be provided by the corresponding author upon reasonable request.
References
American Psychiatric Association, Diagnostic and statistical manual of mental disorders (DSM-5®). 2013: American Psychiatric Pub.
Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51(3):229–34.
Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69–82.
Vgontzas AN, et al. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes care, 2009.
Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–7.
Anderson KO, Getto CJ, Mendoza TR, Palmer SN, Wang XS, Reyes-Gibby CC, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manag. 2003;25(4):307–18.
Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–64.
Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–9.
Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation sleep in America survey. J Psychosom Res. 2004;56(5):497–502.
Sylvia LG, Dupuy JM, Ostacher MJ, Cowperthwait CM, Hay AC, Sachs GS, et al. Sleep disturbance in euthymic bipolar patients. J Psychopharmacol. 2012;26(8):1108–12.
Ivbijaro G, et al. Preventing suicide, promoting resilience: Is this achievable from a global perspective? Asia Pac Psychiatry, 2019: p. e12371.
Fuller-Thomson E, West KJ, Baiden P. The tide does turn: predictors of remission from suicidal ideation and attempt among Canadians who previously attempted suicide. Psychiatry Res. 2019;274:313–21.
Zorick F, Walsh J. Evaluation and management of insomnia: an overview. Principles and Practice of Sleep Medicine (3rd ed). Philadelphia: Saunders, 2000: p. 615–623.
Kripke DF. Chronic hypnotic use: deadly risks, doubtful benefit. Sleep Med Rev. 2000;4(1):5–20.
Bajaj V et al. A case of zolpidem dependence with extremely high daily doses. Asia Pac Psychiatry., 2019: p. e12356.
Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577–601.
Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004). Sleep. 2006;29(11):1398–414.
Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13(3):205–14.
Espie CA, Barrie LM, Forgan GS. Comparative investigation of the psychophysiologic and idiopathic insomnia disorder phenotypes: psychologic characteristics, patients' perspectives, and implications for clinical management. Sleep. 2012;35(3):385–93.
Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–80.
Montgomery P, Dennis JA. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2003;1:CD003161.
Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9(1):24–34.
Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63(1):79–89.
van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3–16.
Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14.
Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16.
American Academy of Sleep Medicine, Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of sleep medicine report. Sleep, 2006. 29(11): p. 1415–1419.
O'Donnell JF. Insomnia in cancer patients. Clin Cornerstone. 2004;6(1):S6–S14.
Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54–67.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edition). Washington, DC: American Psychiatric Association Press; 1994.
World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992.
American Academy of Sleep Medicine. International classification of sleep disorders. Westchester, IL: American Academy of Sleep Medicine; 2005.
American Academy of Sleep Medicine. International classification of sleep disorders–third edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2014.
Higgins, J. and S. Green, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2014.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
White A, Ernst E. A systematic review of randomized controlled trials of acupuncture for neck pain. Rheumatology (Oxford, England). 1999;38(2):143–7.
Atkins D, et al. Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed.). 2004;328(7454):1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Dossing A, Tarp S, Furst DE, Gluud C, Beyene J, Hansen BB, et al. Interpreting trial results following use of different intention-to-treat approaches for preventing attrition bias: a meta-epidemiological study protocol. BMJ Open. 2014;4(9):e005297.
Higuchi T, Ishigooka J, Iyo M, Yeh CB, Ebenezer EG, Liang KY, et al. Lurasidone in the treatment of schizophrenia: results of a double-blind, placebo-controlled trial in Asian patients. Asia Pac Psychiatry. 2019;11(2):e12352.
Wang X, Jiang H, Zhao M, Li J, Gray F, Sheng L, et al. Treatment of opioid dependence with buprenorphine/naloxone sublingual tablets: a phase 3 randomized, double-blind, placebo-controlled trial. Asia Pac Psychiatry. 2019;11(1):e12344.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557–60.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Taylor DJ, et al. Internet and in-person cognitive behavioral therapy for insomnia in military personnel: a randomized clinical trial. Sleep. 2017:40(6).
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ-British Medical Journal. 2011;343:d4002.
Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Nicassio P, Ganz PA, et al. Tai chi Chih compared with cognitive behavioral therapy for the treatment of insomnia in survivors of breast Cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2017;35(23):2656–65.
Garland SN, Rouleau CR, Campbell T, Samuels C, Carlson LE. The comparative impact of mindfulness-based Cancer recovery (MBCR) and cognitive behavior therapy for insomnia (CBT-I) on sleep and mindfulness in cancer patients. Explore (NY). 2015;11(6):445–54.
Martinez MP, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2014;37(4):683–97.
Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015;67(5):1221–33.
Norell-Clarke A, Jansson-Fröjmark M, Tillfors M, Holländare F, Engström I. Group cognitive behavioural therapy for insomnia: effects on sleep and depressive symptomatology in a sample with comorbidity. Behav Res Ther. 2015;74:80–93.
Lee JY, Harvey AG. Memory for therapy in bipolar disorder and comorbid insomnia. J Consult Clin Psychol. 2015;83:92–102. https://doi.org/10.1037/a0037911.
Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee J, Kanady J, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: a pilot randomized controlled trial. J Consult Clin Psychol. 2015;83(3):564–77.
Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–57. https://doi.org/10.1200/JCO.2012.47.7265.
Sánchez AI, et al. Effects of cognitive-behavioral therapy for insomnia on polisomnographic parameters in fibromyalgia patients. Int J Clin Health Psychol. 2012;12(1):39–53.
Miro E, et al. Cognitive-behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: a pilot, randomized controlled trial. J Health Psychol. 2011;16(5):770–82.
Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61:664–75. https://doi.org/10.1111/j.1365-2648.2007.04560.x.
Smitherman TA, Walters AB, Davis RE, Ambrose CE, Roland M, Houle TT, et al. Randomized controlled pilot trial of behavioral insomnia treatment for chronic migraine with comorbid insomnia. Headache. 2016;56:276–91. https://doi.org/10.1111/head.12760.
Contributors
Fu-Chun Zhou: Conceptualization, Data curation, Formal analysis, Writing - original draft, Validation. Yuan Yang: Conceptualization, Data curation, Formal analysis, Writing - original draft, Validation. Yuan-Yuan Wang, Wen-Wang Rao, and Shu-Fang Zhang: Data curation, Formal analysis. Liang-Nan Zeng and Wei Zheng: Data curation. Chee H. Ng: Writing - original draft, Validation. Gabor S. Ungvari: Writing - original draft, Validation. Ling Zhang: Writing - review and editing. Yu-Tao Xiang: Conceptualization, Writing - original draft, Validation.
Funding Source
The study was supported by the University of Macau (MYRG2015-00230-FHS; MYRG2016-00005-FHS), the National Key Research & Development Program of China (No. 2016YFC1307200), the Beijing Municipal Administration of Hospitals Incubating Programme (No. PX2016028), Beijing Municipal Administration of Hospitals' Youth Programme (QML20161902), and the Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20151801).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
N/A.
Consent for Publication
All co-authors approve the final version for publication.
Code Availability
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PROSPERO Registration Number: CRD42020171039
Rights and permissions
About this article
Cite this article
Zhou, FC., Yang, Y., Wang, YY. et al. Cognitive Behavioural Therapy for Insomnia Monotherapy in Patients with Medical or Psychiatric Comorbidities: a Meta-Analysis of Randomized Controlled Trials. Psychiatr Q 91, 1209–1224 (2020). https://doi.org/10.1007/s11126-020-09820-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11126-020-09820-8