Abstract
Insomnia, as a difficulty in initiating and maintaining sleep, coupled with cardiovascular diseases (CVDs) increase the risk of aggravate daytime symptoms, mortality, and morbidity. Cognitive behavioral therapy (CBT) is thought to have a significant impact on insomnia treatment, but in patients with CVDs, there is a paucity of data. To provide a comprehensive appraisal on the impact of CBT on the treatment of insomnia in patients with CVDs. We searched Ovid, Scopus, Web of science, and Cochrane central, to randomized controlled trials (RCTs) from inception till November 2022. Outcomes of interest were insomnia severity index (ISI), Pittsburgh Sleep Quality Index (PSQI), sleep efficiency (SE), Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS), and sleep disorders questionnaire (SDQ). Pooled data were analyzed using mean difference (MD) with its 95% confidence interval (CI) in a random effect model using STATA 17 for Mac. Nine RCTs comprising 365 patients were included in the analysis. CBT significantly reduced scores of ISI (MD = − 3.22, 95% CI − 4.46 to − 1.98, p < 0.001), PSQI (MD = − 2.33, 95% CI − 3.23 to − 1.44, p < 0.001), DBAS (MD = − 0.94, 95% CI − 1.3 to − 0.58, p < 0.001), SDQ (MD = − 0.38, 95% CI − 0.56 to − 0.2, p < 0.001). Also, it increased the score of SE (MD = 6.65, 95% CI 2.54 to 10.77, p < 0.001). However, there was no difference in terms of ESS. CBT is an easy and feasible intervention with clinically significant improvement in insomnia symptoms. Further large-volume studies are needed to assess sustained efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia, defined as difficulty initiating, maintaining sleep, and waking up prematurely associated with daytime cognitive impairment, is common in the general population with a prevalence of approximately 10–30% (Morin & Benca, 2012). Moreover, insomnia in cardiovascular diseases (CVD) is a frequent comorbidity, with an estimated prevalence of 37% in patients with acute coronary artery disease, and about 50% in heart failure patients (Redeker et al., 2010).
Cardiac symptoms coupled with insomnia can aggravate disabling symptoms such as fatigue, depression, anxiety, and pain (Costa et al., 2017), increase mortality rates and other cardiovascular events; and further poor quality of life (Javaheri & Redline, 2017). Although there is an increased risk of mortality and morbidity in insomnia patients with CVD, only few studies with limited data available addressed the need for treatment of these patients (Conley & Redeker, 2015; Javaheri & Redline, 2017).
Cognitive Behavioral Therapy for Insomnia (CBT-I) has proven effective in various populations, from general cases to those with comorbidities like psychiatric disorders, chronic pain, cancer, and depression (Javaheri & Redline, 2017). Furthermore, studies exploring CBT interventions for different conditions, including CVD, have demonstrated positive outcomes, suggesting a viable approach for patients with both CVD and insomnia. European guidelines advocate training healthcare professionals to widen access to insomnia treatment. Also, interventions targeting depression in patients with heart failure and CVD have shown positive effects, particularly when deliverers underwent brief training and had access to guideline scripts (Conley & Redeker, 2015). Tailoring CBTI interventions for CVD patients, incorporating cardiac-relevant components, presents a potential avenue to address both insomnia and cardiac health.
However, there is a paucity of data concerning the effect of CBT-I on patients with insomnia and cardiac diseases on sleep-related outcomes, including cognitive function, quality of life, or functional status. Moreover, no prior meta-analysis reported the effect of this intervention in such population.
So, in this systematic review and meta-analysis, we aimed to address the impact of CBT-I on sleep-related outcomes in patients diagnosed with insomnia and cardiovascular diseases.
Patients and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines during this systematic review and meta-analysis (Liberati, et al., 2009). The methods were done in strict accordance with the Cochrane Handbook of Systematic Reviews and Meta-analysis of Interventions (version 5.1.0).
Eligibility criteria
We included all randomized controlled trials (RCT) that assessed CBT in the treatment of insomnia in patients with CVD compared to control patients who were allocated to self-management sleep monitoring and reported outcomes of interest in an intention-to-treat analysis. Our outcomes of interest were Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), Sleep Efficiency (SE), Epworth Sleepiness Scale (ESS), Dysfunctional Beliefs and Attitudes about Sleep (DBAS), and Sleep Disorders Questionnaire (SDQ).
Studies that were not in English, observational studies, protocols, and abstracts were excluded.
Literature search
The following databases were comprehensively searched from inception until November 2022 for relevant studies: Web of Science, Scopus, Cochrane, and Ovid.
Search was done using the following strategy: (“Cognitive behavioral therapy” OR “CBT” OR “CBT-I”) AND (“insomnia” OR “disturbed sleep”) AND (“cardiovascular disease” OR “heart disease”). All Duplicates were removed using Endnote. Manual backward citation analysis was done for all the references of the included studies.
Screening and selection process
Screening of literature for eligibility was performed using Rayyan by two independent reviewers. First by title and abstract and then full text screening. Any conflicts were resolved by discussion with a third reviewer if necessary.
Data extraction
Data were extracted in a uniform sheet including characteristics of the included studies, characteristics of the population of included studies, risk of bias domains and outcomes measures including efficacy endpoints such as: ISI, PSQI, SE, ESS, DBAS, and SDQ.
Risk of bias assessment
We used the Cochrane Risk of Bias 2 tool for RCTs to check for randomization process (selection bias), deviation from intended interventions (performance bias), outcome measurement (detection bias), missing outcome data (attrition bias), selection of reported results (reporting bias) and other potential biases (‘Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training’, 2022; Sterne, 2019). Two independent reviewers decided either low, some concerns or high risk of bias and provided a quote from the study report together with a justification for each judgement. Any conflicts were resolved by discussion with a third reviewer if necessary.
To explore the publication bias across studies, we constructed funnel plots to present the relationship between effect size and standard error. Two methods assessed evidence of publication bias: (1) Egger’s regression test and (2) the Begg and Mazumdar rank correlation test (Kendall’s tau).
Synthesis of results
Dichotomous data were analyzed as odds ratios and continuous data as mean differences or standardized mean differences. The following outcome measures were analyzed: ISI, PSQI, SE, ESS, DBAS, and SDQ. We performed meta‐analyses using STATA 17 only if the interventions, participants, and the underlying clinical question were similar enough for pooling. Where multiple trial arms were reported in a single trial, we included only the relevant arms. We used a regression model as a quantitative research method to deal with several variables including dependent and independent variables. A random‐effects model was used when there is heterogeneity due to differences in study populations and methods.
Heterogeneity assessment
Chi-square test was used to evaluate the statistical heterogeneity among studies. A Chi-square P value less than 0.1 was considered as significant heterogeneity. I-square values ≥ 50% were considered as high heterogeneity.
Certainty assessment
We conducted a certainty assessment through sensitivity analysis (also called leave-one-out meta-analysis) to test the evidence’s robustness. For every outcome in the meta-analysis, we ran sensitivity analysis in multiple scenarios, excluding one study in each scenario to make sure the overall effect size was not dependent on any single study.
Given the limited amount of data reported, and due to the cumulative pooling of trials; there is an increased risk of type 1 and type 2 errors. Trial Sequential Analysis (TSA) was used to assess whether the evidence of the pooled trials is conclusive and reliable. The level of confidence in the intervention is conclusive and sufficient when the z-line on the curve crosses the conventional boundary and boundary of sequence monitoring; indicating that no further studies are needed. Moreover, if the z-line on the curve does not cross any boundary, the evidence is insufficient and more studies are still required. In this meta-analysis, we used an alpha error of 0.05, and a beta error of 80% power. We calculated the mean difference in the current meta-analysis to obtain the sample size needed for TSA.
To evaluate the strength and level of evidence, he Grades of Recommendations Assessment Development and Evaluation (GRADE) scale was used, and was stratified as follow: high quality which indicates no further trials are needed, as the confidence level is unlikely to be changed; moderate quality which indicates that more trials may needed to affect the overall estimates; low quality which indicates more trials are needed to have a crucial impact on the overall confidence of the overall estimate; and very low quality which indicates that we are not certain about this estimate.
Results
Literature search
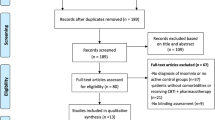
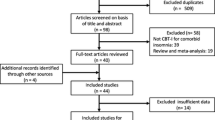
A total of 242 records were retrieved from databases systemic search and a total of 9 studies were included in the final analysis after title, abstract, full text screening, and citation analysis (Redeker et al., 2015)–(Redeker, et al., 2022). The PRISMA flow diagram for this systematic review and meta-analysis is illustrated in Fig. 1.
Characteristics of included studies
This systematic review and meta-analysis included 9 RCTs comprising 192 patients in the CBT group and 173 participants in the control group. Characteristics and summary of the included articles are summarized in Table 1.
Risk of bias assessment
The risk of bias assessment and the graph are illustrated in Fig. 2. The Cochrane risk of bias assessment tool-2 (ROB-2) was used in this meta-analysis.
Outcomes
The ISI total score was assessed in five studies with a total of 314 participants; CBT significantly reduced the ISI score compared to the control group (MD = − 3.22 [95% CI: − 4.46 to − 1.98]; p < 0.001). The pooled studies were homogenous (I2 = 0.00%, p = 0.95) as shown in Fig. 3.
We tested the heterogeneity using Galbraith plot, and all five studies were visualized inside the 95% CI of the regression, indicating there was no heterogeneity between studies as shown in Figure S1.
We used the DOI plot to detect a possible bias, and by inspection, we found minor asymmetry indicating a possible publication bias (LFK index = − 2.51), as shown in Figure S2. Our findings may be explained by insufficient literature search or clinical heterogeneity.
We performed a trial sequential analysis (TSA) on five studies that assessed ISI of which the cumulative Z-line on the curve crossed both the conventional boundaries of benefit and the trial sequential monitoring boundaries, suggesting that CBT-I is effective and reliable intervention; however, further large volume RCTs should be carried out to validate our results, as shown in Fig. 4.
The PSQI total score was pooled based on three studies, with a total of 237 participants, in which CBT significantly reduced the overall score of PSQI compared to the control (MD = − 2.33 [95% CI: − 3.23 to − 1.44]; p < 0.001); the pooled studies were homogenous (I2 = 0.00%, p = 0.45), as shown in Fig. 5.
Regarding SE, three studies with a total of 237 participants showed that CBT significantly increased the overall score compared to the control (MD = 6.65 [95% CI: 2.54 to 10.77]; p < 0.001); the pooled studies were homogenous (I2 = 0.00%, p = 0.55), as shown in Fig. 6.
On the other hand, there was no significant difference between CBT intervention and the control regarding ESS (MD = − 0.91 [95% CI: − 1.85 to 0.03]; p = 0.06); the pooled studies were homogenous (I2 = 0.00%, p = 0.74) as shown in Figure S3.
Of note, only two studies assessed SDQ and DBAS in which CBT reduced total scores of SDQ and DBAS compared to the control (MD = − 0.38 [95% CI: − 0.56 to − 0.2]; p < 0.001) for SDQ and (MD = − 0.94 [95% CI: − 1.3 to − 0.58]; p < 0.001) for DBAS. The pooled studies were homogenous (I2 = 0.00%, p = 0.71; I2 = 0.00%, p = 0.68) for SDQ and DBAS respectively as shown in Figure S4 and S5.
GRADE assessment
The GRADE rating results are shown in Table 2.
Discussion
Our study comprised nine RCTs whose analyses revealed a significant impact favoring CBT over control for insomnia in patients with cardiovascular diseases. The CBT group showed significantly better results regarding ISI, PSQL, SE, SDQ, and DBAS, while there was no significant difference between CBT and the control group regarding ESS outcome.
Regarding insomnia relation with cardiovascular disease and how CBT could be beneficial, previous literature showed that sleeping only 6 h per night consistently has been associated with a higher risk for hypertension (Gottlieb et al., 2006). While some studies suggest that insomnia does not increase mortality risk, other research has found that frequent insomnia symptoms can increase the risk of death and cardiovascular events (Kripke et al., 2002; Schwartz et al., 1999). People who struggle to fall or stay asleep may have a higher risk of acute myocardial infarction and coronary cardiovascular disease-related death (Chien et al., 2010; Laugsand et al., 2011; Schwartz et al., 1999). The biological mechanisms underlying this link are not fully understood. Still, they may be related to increased activity in the sympathetic nervous system or the hypothalamic–pituitary–adrenal axis, leading to increased inflammation (Spiegel et al., 1999; Vgontzas et al., 2001). Patients with implantable cardioverter-defibrillator devices also experience significant sleep disruption, which can increase their risk for anxiety and insomnia due to fears of device malfunction or other issues (Camm et al., 1999; Cross et al., 2010; Pedersen et al., 2010).
Regarding ISI, Redeker et al., in their most recent study (2022), were in line with our pooled results favoring CBT over the control group for insomnia, while their previous RCT and other literature did not detect any significant difference between the two groups (Harris et al., 2019; Javaheri et al., 2020; Redeker et al., 2015, 2022; Siebmanns et al., 2021). However, this discrepancy could be justified by the small sample size of previous studies compared to the most recent study of Redeker et al. and our pooled sample; this makes our analysis more powerful and less vulnerable to type two error (Harris et al., 2019; Javaheri et al., 2020; Redeker et al., 2015, 2022; Siebmanns et al., 2021). Also, sleep effectiveness outcomes showed similar results to the previous outcome; Redeker et al. (2022) agreed with our results, but Harrison et al. and Redeker et al. (2015) were not (Harris et al., 2019; Redeker et al., 2015, 2022).
As to PSQL, our pooled analysis, Redeker et al. (2022) and Harris et al. favored CBT, while Redeker et al. (2015) showed no difference (Harris et al., 2019; Redeker et al., Oct. 2015; Redeker, et al., 2022). Different patients’ characteristics could explain these different findings among studies as patients in Harris et al. and Redeker et al. (2022) there were only 20% of patients with class III NYHA class, while in Redeker et al. there were 37% of patients with class III NYHA (Harris et al., 2019; Redeker et al., 2015, 2022).
To the best of our knowledge, this is the first meta-analysis to be conducted regarding the role of CBT in treating insomnia in cardiovascular patients. Furthermore, all of our studied outcomes were homogenous. Additionally, we represented the publication bias by Doi and Galbraith plots though, making the detection of bias visually easier, enhancing the transparency and reproducibility. However, our study is not free of limitations such as: (1) The way of endpoint detections was subjective and mostly questionnaire-based; further studies may try more objective ways, such as measuring melatonin or orexin levels (Levenson et al., 2015). (2) we need more subgrouping to the patients to avoid confounding biases as there are various categories under cardiovascular diseases and even more categories inside the same disease as dyspnea degrees in heart failure patients that may affect the validation of the results. (3) We have a relatively small sample size that could handle the generalizability of our results. (4) Finally, the variations in CBT methods between studies need to be stratified in order to know exactly the efficacy of each method in improving insomnia.
Conclusion
Our research analyzed nine RCTs and found that CBT was more effective than the control group in improving insomnia symptoms in patients with cardiovascular diseases. Specifically, the CBT group demonstrated better outcomes in ISI, PSQL, SE, SDQ, and DBAS measures, but there was no significant difference between the CBT and control groups regarding ESS. We recommended further studies to be conducted on that topic to pool larger samples and enhance our evidence. Also, the coming RCTs should stratify their patients according to their disease category and severity.
References
‘Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training’. https://training.cochrane.org/handbook (accessed Jun. 21, 2022).
Camm, A. J., Sears, S. F., Todaro, J. F., Lewis, T. S., Sotile, W., & Conti, J. B. (1999). Examining the psychosocial impact of implantable cardioverter defibrillators: A literature review. Clinical Cardiology, 22, 481–489. https://doi.org/10.1002/clc.4960220709
Chien, K.-L., et al. (2010). Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: Report from a community-based cohort. Sleep, 33, 177–184. https://doi.org/10.1093/sleep/33.2.177
Conley, S., & Redeker, N. S. (2015). Cognitive behavioral therapy for insomnia in the context of cardiovascular conditions. Curr Sleep Med Rep, 1, 157–165. https://doi.org/10.1007/S40675-015-0019-7/TABLES/2
Cross, N. J., McCrae, C. S., Smith, K. M., Conti, J. B., & Sears, S. F. (2010). Comparison of actigraphic and subjective measures of sleep in implantable cardioverter defibrillator and coronary artery disease patients. Clinical Cardiology, 33, 753–759. https://doi.org/10.1002/clc.20827
Da Costa, D., Allman, A. A., Libman, E., Desormeau, P., Lowensteyn, I., & Grover, S. (2017). Prevalence and determinants of insomnia after a myocardial infarction. Psychosomatics, 58, 132–140. https://doi.org/10.1016/J.PSYM.2016.11.002
Gottlieb, D. J., et al. (2006). Association of usual sleep duration with hypertension: The sleep heart health study. Sleep, 29, 1009–1014. https://doi.org/10.1093/sleep/29.8.1009
Harris, K. M., Schiele, S. E., & Emery, C. F. (2019). Pilot randomized trial of brief behavioral treatment for insomnia in patients with heart failure. Heart and Lung, 48, 373–380. https://doi.org/10.1016/j.hrtlng.2019.06.003
Javaheri, S., & Redline, S. (2017). Insomnia and risk of cardiovascular disease. Chest, 152, 435–444. https://doi.org/10.1016/j.chest.2017.01.026
Javaheri, S., Reid, M., Drerup, M., Mehra, R., & Redline, S. (2020). Reducing coronary heart disease risk through treatment of insomnia using web-based cognitive behavioral therapy for insomnia: a methodological approach. Behavioral Sleep Medicine, 18, 334–344. https://doi.org/10.1080/15402002.2019.1584896
Kripke, D. F., Garfinkel, L., Wingard, D. L., Klauber, M. R., & Marler, M. R. (2002). Mortality associated with sleep duration and insomnia. Archives of General Psychiatry, 59, 131. https://doi.org/10.1001/archpsyc.59.2.131
Laugsand, L. E., Vatten, L. J., Platou, C., & Janszky, I. (2011). Insomnia and the risk of acute myocardial infarction. Circulation, 124, 2073–2081. https://doi.org/10.1161/CIRCULATIONAHA.111.025858
Levenson, J. C., Kay, D. B., & Buysse, D. J. (2015). The pathophysiology of insomnia. Chest, 147, 1179–1192. https://doi.org/10.1378/chest.14-1617
Liberati, A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine, 151, 65. https://doi.org/10.1136/bmj.b2700
Moon, J. R., et al. (2021). The effects of rational emotive behavior therapy for depressive symptoms in adults with congenital heart disease. Heart and Lung, 50, 906–913. https://doi.org/10.1016/j.hrtlng.2021.07.011
Morin, C. M., & Benca, R. (2012). Chronic insomnia. The Lancet, 379, 1129–1141. https://doi.org/10.1016/S0140-6736(11)60750-2
Pedersen, S. S., Theuns, D. A. M. J., Jordaens, L., & Kupper, N. (2010). Course of anxiety and device-related concerns in implantable cardioverter defibrillator patients the first year post implantation. Europace, 12, 1119–1126. https://doi.org/10.1093/europace/euq154
Redeker, N. S., Jeon, S., Andrews, L., Cline, J., Mohsenin, V., & Jacoby, D. (2019). Effects of cognitive behavioral therapy for insomnia on sleep-related cognitions among patients with stable heart failure. Behavioral Sleep Medicine, 17, 342–354. https://doi.org/10.1080/15402002.2017.1357120
Redeker, N. S., et al. (2022). Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: the HeartSleep Study. Sleep, 45, 1. https://doi.org/10.1093/sleep/zsab252
Redeker, N. S., et al. (2020). Effects of cognitive behavioral therapy for insomnia on sleep, symptoms, stress, and autonomic function among patients with heart failure. Behavioral Sleep Medicine, 18, 190–202. https://doi.org/10.1080/15402002.2018.1546709
Redeker, N. S., Jeon, S., Andrews, L., Cline, J., Jacoby, D., & Mohsenin, V. (2015). Feasibility and efficacy of a self-management intervention for insomnia in stable heart failure. Journal of Clinical Sleep Medicine, 11, 1109–1119. https://doi.org/10.5664/jcsm.5082
Redeker, N. S., Jeon, S., Muench, U., Campbell, D., Walsleben, J., & Rapoport, D. M. (2010). Insomnia symptoms and daytime function in stable heart failure. Sleep, 33, 1210. https://doi.org/10.1093/SLEEP/33.9.1210
Rossman, J. (2019). Cognitive-behavioral therapy for insomnia: An effective and underutilized treatment for insomnia. American Journal of Lifestyle Medicine, 13, 544. https://doi.org/10.1177/1559827619867677
Sato, D., Yoshinaga, N., Nagai, E., Hanaoka, H., Sato, Y., & Shimizu, E. (2018). Randomised controlled trial on the effect of internet-delivered computerised cognitive–behavioural therapy on patients with insomnia who remain symptomatic following hypnotics: A study protocol. British Medical Journal Open, 8, 18220. https://doi.org/10.1136/BMJOPEN-2017-018220
Schwartz, S., Anderson, W. M., Cole, S. R., Cornoni-Huntley, J., Hays, J. C., & Blazer, D. (1999). Insomnia and heart disease. Journal of Psychosomatic Research, 47, 313–333. https://doi.org/10.1016/S0022-3999(99)00029-X
Siebmanns, S., Johansson, P., Ulander, M., Johansson, L., Andersson, G., & Broström, A. (2021). The effect of nurse-led Internet-based cognitive behavioural therapy for insomnia on patients with cardiovascular disease: A randomized controlled trial with 6-month follow-up. Nursing Open, 8, 1755–1768. https://doi.org/10.1002/nop2.817
Spiegel, K., Leproult, R., & Van Cauter, E. (1999). Impact of sleep debt on metabolic and endocrine function. The Lancet, 354, 1435–1439. https://doi.org/10.1016/S0140-6736(99)01376-8
Sterne, J. A. C., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/BMJ.L4898
Vgontzas, A. N., et al. (2001). Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. Journal of Clinical Endocrinology and Metabolism, 86, 3787–3794. https://doi.org/10.1210/jcem.86.8.7778
Funding
There is no fund.
Author information
Authors and Affiliations
Contributions
AA: Conceptualization, Supervision, data collection revision, statistical analysis, writing—original draft and editing. AH: screening, writing—original draft. MRR: screening, data collection, revision of data collection, quality assessment. MA: quality assessment, full-text screening, data collection, writing—revision. MAE: summary and characteristics, quality assessment, writing—revision. HE: Conceptualization, screening, writing—original draft, GRADE assessment. YES,: screening, data collection, data revision for analysis, GRADE assessment. MA quality assessment, GRADE assessment, summary and characteristics Abdullah AH writing—original draft, data revision, GRADE assessment. HA: quality assessment, GRADE assessment, screening. RJEH: draft finalizing, data revision, analysis revision. NIH: screening, data collection, data revision, analysis revision. HSG: data revision, data analysis, finalizing the draft.
Corresponding author
Ethics declarations
Competing interests
Ahmed Abdelaziz, Abdelrahman H. Hafez, Merna Raafat Roshdy, Mohamed Abdelaziz, Moemen A. Eltobgy, Hanaa Elsayed, Youssef El-Sherif, Mohamed Atef, Abdullah Ashraf Hamad, Hany Atwan, Rama jamal eddin haboush, Nada Ibrahim Hendi, Alyaa Hefny, Hazem S. Ghaith declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelaziz, A., Hafez, A.H., Roshdy, M.R. et al. Cognitive behavioral therapy for the treatment of insomnia in patients with cardiovascular diseases: a meta-analysis with GRADE analysis. J Behav Med 47, 819–827 (2024). https://doi.org/10.1007/s10865-024-00490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-024-00490-6