Abstract
The freshwater macrophyte, Ottelia alismoides, is a bicarbonate user performing C4 photosynthesis in the light, and crassulacean acid metabolism (CAM) when acclimated to low CO2. The regulation of the three mechanisms by CO2 concentration was studied in juvenile and mature leaves. For mature leaves, the ratios of phosphoenolpyruvate carboxylase (PEPC) to ribulose-bisphosphate carboxylase/oxygenase (Rubisco) are in the range of that of C4 plants regardless of CO2 concentration (1.5–2.5 at low CO2, 1.8–3.4 at high CO2). In contrast, results for juvenile leaves suggest that C4 is facultative and only present under low CO2. pH-drift experiments showed that both juvenile and mature leaves can use bicarbonate irrespective of CO2 concentration, but mature leaves have a significantly greater carbon-extracting ability than juvenile leaves at low CO2. At high CO2, neither juvenile nor mature leaves perform CAM as indicated by lack of diurnal acid fluctuation. However, CAM was present at low CO2, though the fluctuation of titratable acidity in juvenile leaves (15–17 µequiv g−1 FW) was slightly but significantly lower than in mature leaves (19–25 µequiv g−1 FW), implying that the capacity to perform CAM increases as leaves mature. The increased CAM activity is associated with elevated PEPC activity and large diel changes in starch content. These results show that in O. alismoides, carbon-dioxide concentrating mechanisms are more effective in mature compared to juvenile leaves, and C4 is facultative in juvenile leaves but constitutive in mature leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The low rate of diffusion and frequently depleted concentration of CO2 in water coupled with the kinetic inefficiency and poor specificity for CO2 of the primary carboxylating enzyme ribulose 1,5-bisphosphate carboxylase-oxygenase (Rubisco) can restrict the productivity of submerged macrophytes (Vadstrup and Madsen 1995; Maberly and Gontero 2017). However, the presence of carbon dioxide-concentrating mechanisms (CCMs) in some freshwater plants can reduce or overcome the problem of limited inorganic carbon supply (Maberly and Madsen 2002). The most frequent CCM in freshwater plants is based on the biophysical active uptake of bicarbonate, which is found in ~ 45% of tested species (Maberly and Gontero 2017). In addition, crassulacean acid metabolism (CAM) and C4 metabolism are also present in some freshwater plants (Keeley 1981; Bowes and Salvucci 1989; Bowes et al. 2002; Keeley and Rundel 2003). In terrestrial plants adapted to xeric conditions, CAM can be widespread and plays a major role in the water-use efficiency of the plant (Silvera et al. 2010). In aquatic plants, CAM occurs in about 8% of tested species (Maberly and Gontero 2017) and acts as a carbon-conserving and -concentrating mechanism that reduces the loss of respiratory carbon at night and exploits nocturnal CO2 concentration that is often higher than during the day (Klavsen et al. 2011). Terrestrial C4 carbon fixation is known in about 3% of species and involves spatial separation of carboxylation and decarboxylation between different cells, mesophyll and bundle-sheath, or occasionally within one type of cell (Sage 2016). In aquatic plants, C4 photosynthesis is found in about 4% of tested species, occurs within a single cell, and reduces the effects of photorespiration especially when water column concentrations of oxygen are high and CO2 are low (Bowes et al. 2002).
In CAM and C4 photosynthesis, the first carboxylating enzyme is usually phosphoenolpyruvate carboxylase (PEPC) (Osmond 1978). In C4 photosynthesis, PEPC is active during the day but in CAM it is only active during the night (Hatch 1987). PEPC uses phosphoenolpyruvate (PEP) and external or internal respiratory CO2 to produce the C4 compound oxaloacetate (OAA) that in turn is converted into malate. In C4 photosynthesis, the malate is decarboxylated close to Rubisco while in CAM, it is transported and stored in the vacuole resulting in the well-known acidification observed during the night. In CAM, during the day, the malic acid is decarboxylated into pyruvate, a C3 compound and CO2, that is then fixed by Rubisco within the Calvin–Benson–Bassham cycle. Nocturnal CO2 fixation via PEPC requires an adequate pool of carbohydrates to generate PEP, in addition to that needed for other metabolic processes including dark respiration, organic carbon export and growth (Nobel and Hartsok 1983). In several CAM species, PEPC-mediated carboxylation is indeed closely linked to the diel turnover of starch, since the amount of CO2 taken up during the night depends on the availability of C3-carbon substrate (PEP) produced from the nocturnal degradation of starch accumulated during the day (Ceusters et al. 2014). Thus, in these plants, there is a large diel change in transitory starch content as well as acidity (Neuhaus and Schulte 1996).
CAM metabolism is a plastic process in freshwater and terrestrial plants. In freshwater plants, its expression can be regulated by a range of environmental parameters, e.g. irradiance, CO2 availability, temperature and nutrient availability (Keeley et al. 1983; Aulio 1985; Madsen 1987; Bowes and Salvucci 1989; Robe and Griffiths 1990; Hostrup and Wiegleb 1991; Klavsen and Maberly 2010; Zhang et al. 2014; Shao et al. 2017). The activity of CAM may also be dependent on leaf maturity and in many terrestrial species CAM is only fully expressed in mature leaves (Jones 1975; Ting et al. 1996; Wen et al. 1997; Taybi et al. 2002). Whether comparable ontogenetic change occurs in aquatic CAM plants has not been widely studied. There is very little information on the influence of leaf maturity on aquatic CAM plants (Klavsen and Madsen 2008) or on the interplay between developmental and environmental factors. In the terrestrial plant, Mesembryanthemum crystallinum, salinity was originally reported to induce CAM (Winter and von Willert 1972), but it was later shown that CAM was a genetically controlled developmental programme that was just accelerated by the salinity stress (Adams et al. 1998). C4 photosynthesis is usually constitutive in terrestrial plants but is facultative in the freshwater macrophytes Hydrilla verticillata (Bowes et al. 2002) and Egeria densa (Casati et al. 2000). Use of bicarbonate can also be plastic and in Elodea canadensis and Ranunculus peltatus is down-regulated at high CO2 concentration (Sand-Jensen and Gordon 1986; Madsen et al. 1996).
It has been shown that in Ottelia alismoides from the Hydrocharitaceae, C4 metabolism and bicarbonate use are constitutive, while CAM metabolism is facultative and only induced at low CO2 (Zhang et al. 2014). O. alismoides operates CAM at night and C4 metabolism during the day. It is the only known aquatic species to operate CAM and C4 in the same tissue, and one of the only two known aquatic species to operate CAM and bicarbonate use (Shao et al. 2017) although six species of Portulaca are also known to have constitutive C4 metabolism and facultative CAM induced by water stress (Koch and Kennedy 1980; Guralnick et al. 2002; Holtum et al. 2017).
Ottelia alismoides leaves differ in size and shape and develop from a basal rosette (Cook and Urmi-König 1984; Yu and Yu 2009). The first juvenile leaves are only 2–4 cm wide and 18–20 cm long, and are linear or lanceolate and either sessile or attenuate (Fig. 1). Under summer conditions, juvenile leaves can develop into mature leaves that have a petiole that can be up to 50 cm long and are ovate to cordate, 18–20 cm long and up to 20 cm wide. In addition to these morphological characteristics, the fresh weight and leaf area of mature leaves is 4–5-times greater than juvenile leaves but the specific leaf area is 1.15-times greater in juvenile than in mature leaves, while the content of chlorophyll a and b on a fresh weight basis is similar (Table S1). Previously, we studied fully expanded mature leaves of O. alismoides, but we observed that there were significant differences in the diurnal change of acidity between mature and juvenile leaves (unpublished observations). Little is known about differential expression of CCMs in different types of leaf in freshwater plants although Maberly and Spence (1983) reported that linear leaves of Potamogeton x zizii were restricted to CO2 while broad leaves could also use bicarbonate.
In the present study, we tested the hypothesis that CAM activity, C4 metabolism and bicarbonate use, will differ between mature and juvenile leaves. We further hypothesised that there will be an interplay between leaf development and acclimation to CO2 concentration.
Materials and methods
Plant material and leaf sampling
Seeds of O. alismoides were germinated on 20 March 2017 in a 1-L glass beaker filled with sterile tap water with an alkalinity of about 1.9 mequiv L−1 and a low nutrient concentration (TP = 0.05 mg L−1; TN = 1.35 mg L−1). The beaker was placed in a growth chamber at 25 °C and the water was replaced daily. Six weeks after germination, two or three seedlings (about 8 cm tall) comprising two to four juvenile leaves were transplanted into a plant pot (15 cm diameter, 12 cm high) containing sterile soil from nearby Donghu Lake. The pots were placed in a tank (64 cm deep) that was located in a glasshouse on the flat roof of the laboratory, containing about 400 L of tap water. The water level was increased gradually as the plants grew in order to ensure that the whole plant was fully submerged in the water. Snails and moribund leaves were removed daily and the tap water in the tank was replaced every 2 days.
Acclimation to CO2
After 7–8 weeks, in mid-July 2017, the plants were 25–30 cm tall with mature and newly produced juvenile leaves. Four pots containing O. alismoides plants of similar height from the glasshouse were placed into each of the two white plastic tanks (65 × 45 × 35 cm) containing tap water that was renewed twice a week. The tanks were placed in a constant temperature room at 25 ± 2 °C and were illuminated with FSL T5/865 28 W fluorescence tubes, producing 140–150 µmol photon m−2 s−1 (photosynthetically available radiation; Li-Cor underwater sensor, UWQ, connected to a Li-Cor LI-1400 data logger) at the water surface with a 14-h light (08:00–22:00) and 10-h dark photoperiod. In each tank, pH was measured with a combination pH electrode (model IP-600-9 Jenco Instruments, USA) centrally located 15 cm below the water surface, connected to a microcomputer pH controller (model 6311, Jenco Instruments, USA). The plants were grown at two CO2 concentrations. In the high CO2 treatment (HC), the target pH of 7.0 was maintained between 6.90 and 7.18 by bubbling the growth medium with pure CO2 from four tubes, one in each corner of the tank, under the control of the computer. On approximately eight occasions during the daytime, the water was thoroughly mixed and the pH was recorded. In the low CO2 treatment (LC), the natural photosynthetic activity of the plants depleted the inorganic carbon concentration of the water. The water was thoroughly mixed and the pH measured as described above. The pH ranged from 8.65 to over 9.64. Alkalinity was measured every 2 days (see “Materials and methods” below). Over the whole period of acclimation, the CO2 concentration calculated from pH, alkalinity, and temperature using the equations in Maberly (1996) was between 302 and 604 µmol L−1 for the HC treatment, and 0.1 and 5 µmol L−1, for the LC treatment. These are equivalent to between 22 and 44 times and between 1 and 36% times the concentration at equilibrium with 400 ppm for the HC treatment and the LC treatment, respectively. After 14 days, juvenile leaves (newly produced, long, and narrow) and mature leaves (oval) from the plants treated with HC and LC were sampled at 07:30 (towards the end of the dark period, hereafter referred to as ‘dawn’) and 21:30 (towards the end of the photoperiod, hereafter referred to as ‘dusk’), respectively. Leaves grown at LC tended to have a layer of marl (calcite) on the upper surface which was gently removed by rubbing. After cleaning, the leaves were immediately placed on aluminium foil on top of ice and kept in the dark to reduce metabolic changes. Measurements were made either on one large mature leaf or on two to three juvenile leaves. Each leaf was photographed, blotted dry and weighed. Half of the material was used to determine enzyme activity and pigment content, the other half to determine acidity and starch content. Samples were taken in triplicate from different plants and stored in liquid nitrogen before measurement. Chlorophyll fluorescence and rates of oxygen exchange were measured on fresh leaves in the morning.
Overnight changes in gas exchange, enzyme activity and chemical composition in detached leaves
In order to analyse the capture of respired CO2 by different types of leaf, one mature leaf or three juvenile leaves (about 1.0 g fresh weight in both cases), detached from the plants grown under HC and LC at dusk, were placed in 580-mL gas-tight glass bottles, containing the tap water enriched with CO2 to a final concentration of 560 µmol L−1, and a dissolved oxygen concentration of 7.7 mg L−1. All the bottles were incubated in the dark, unstirred, at 25 °C for 11 h. In addition, at the start of the overnight incubation experiment, one mature or three juvenile leaves were collected from the plants grown either at high or low CO2, wrapped in foil envelopes and frozen in liquid nitrogen for later determination of acidity, starch and enzyme activity. After overnight incubation, the alkalinity, pH, and O2 concentration of the incubation medium were measured as described below. Meanwhile, the detached leaves in the bottles were removed and acidity, starch, and enzyme activity were measured. Measurements were made in triplicate.
pH-drift
About 0.2–0.3 g fresh weight of juvenile and mature leaves grown at HC and LC were washed in tap water and then incubated in 65-mL glass tubes with ground glass stoppers. The glass tubes contained 60 mL of test solution (equimolar concentrations of NaHCO3 and KHCO3 at an overall concentration of 1 mM) and about 5-mL air. The glass tubes were incubated, unstirred, in a growth room at 25 °C and 120–135 µmol photon m−2 s−1 PAR. After 24-h continuous irradiance, the final pH was measured with the pH electrode (model IP-600-9 Jenco Instruments, USA) and the final alkalinity in the solution was measured by Gran titration (see below).
Measurement of CAM activity
CAM activity was measured as a change in titratable acidity between minimal acidity levels at dusk and maximal levels at dawn. To measure acidity, 10 mL CO2-free milliQ water was added to the leaf samples (0.2–0.5 g fresh weight) that had been stored in liquid nitrogen and then boiled for 30 min. After cooling to room temperature, the samples were titrated to an end-point of pH 8.3 with 0.01 N NaOH (Madsen 1987; Zhang et al. 2014). Other methods, e.g. those used by Keeley and co-workers and others titrate to pH 6.4 and Keeley et al. (1983), report that this produced 8% lower estimates of acidity compared to titrating to pH 8.3.
Measurement of enzyme activity
The extraction and assay of PEPC, Rubisco, PPDK, NAD-ME and NADP-ME were based on the methods described by Zhang et al. (2014) and Shao et al. (2017). Enzyme activity was assessed from the rate of appearance or disappearance of NADPH or NADH at 340 nm at 25 °C, using a microplate reader (Tecan M200 PRO, Austria). A calibration curve for both cofactors was produced to convert absorbance to concentration.
Measurement of starch, alkalinity and pH
Starch content was determined by the amyloglucosidase assay according to Smith and Zeeman (2006) and Shao et al. (2017). Alkalinity was measured by Gran titration with a standardised solution of HCl (Shao et al. 2017). pH was measured with the pH electrode (model IP-600-9 Jenco Instruments, USA) connected to the microcomputer pH controller.
Measurement of leaf area, chlorophyll content and chlorophyll fluorescence
Projected (1-sided) leaf area was estimated from photographs analysed using AreaAna software (Huazhong University of Sciences and Technology, China). Chlorophyll a and b were extracted overnight in 90% ethanol from samples collected in the morning, and concentrations calculated according to the method of Brain and Solomon (2007). Parameters of chlorophyll variable fluorescence were determined with a pulse-modulated fluorometer PAM 2100 (Walz, Germany). Prior to measurements the leaves were kept in the dark for 15 min to minimise fluorescence quenching. The parameter Fv/Fm was used as an indicator of the maximal quantum yield of photosystem II (PSII) (Kitajima and Butler 1975). The effective quantum yield of PSII was also obtained using the Win Control software (Walz, Germany).
Oxygen exchange
Oxygen exchange was measured with an optical oxygen electrode system (Unisense OX-13298 and a Unisense microsensor multimeter Version 2.01) in a glass and Perspex chamber (62 mL) according to the method of Shao et al. (2017). The chamber was placed in a constant temperature water bath at 25 °C and illuminated from the side by fluorescent tubes (36 W, 6500 K colour temperature) producing a photon irradiance of 115 µmol m−2 s−1. The oxygen electrode was calibrated in the chamber with tap water (alkalinity ~ 1.9 mequiv L−1) that had been vigorously bubbled either with air from outside the laboratory (100% saturation) or with nitrogen (0% saturation). About 0.4 g fresh weight of leaf was placed in the chamber filled with water from the tank in which the plants were growing and hence measurements were made at the CO2 concentration at which they were grown. Changes of oxygen concentration were recorded for 5–10 min on a computer connected to the Unisense meter. After measuring photosynthesis, the chamber was placed in the dark and the decline in oxygen concentration was recorded for 15 min.
Statistical analysis
The data were analysed using SPSS 16.0 (SPSS Inc., Chicago, USA). The significance of CO2 treatment and leaf maturity were determined with one- and two-way ANOVA (with Duncan’s and Tukey’s post hoc tests).Comparisons of physiological parameters between attached and detached leaves were determined with t-tests. Pearson correlation was used to test the correlation between acidity and other parameters (e.g. PEPC activity, starch) and the significance level was set at 5%.
Results
Comparison of mature and juvenile leaves at different CO2 concentrations
At high CO2 at dawn, the activities of PEPC and PPDK were significantly lower in juvenile leaves than in mature leaves on a fresh weight basis. However, compared to mature leaves, the activity of Rubisco was markedly higher in juvenile leaves. NAD-ME and NADP-ME were not significantly different between the two types of leaf. The only significant difference in the PEPC to Rubisco ratio was at dusk when mature leaves had a significantly higher ratio than juvenile leaves (Fig. 2; Table 1).
Influence of CO2 concentration on activities of enzymes from O. alismoides collected at dawn and dusk for juvenile and mature leaves. a Rubisco, b PEPC, c PEPC:Rubisco ratio, d PPDK, e NAD-ME and f NADP-ME. There are four treatments: mature leaves grown at high CO2 (HC, 302–604 µmol L−1); juvenile leaves grown at high CO2 (HC, 302–604 µmol L−1); mature leaves grown at low CO2 (LC, 0.1–5 µmol L−1); juvenile leaves grown at low CO2 (LC, 0.1–5 µmol L−1). Mean values with their SD (n = 3) are presented and differences among means were tested using one-way ANOVA with Duncan’s and Tukey’s post hoc tests. Data with different letters are significantly different within the four treatments (P < 0.05)
At low CO2, the juvenile leaves had significantly lower PEPC (1.64-fold, SD = 1.72) and Rubisco (1.42-fold, SD = 0.64) activity than mature leaves on a fresh weight basis (Fig. 2; Table 1) and also on an area leaf basis (data not shown). In contrast, the activities of PPDK, NAD-ME and NADP-ME were not significantly affected by leaf maturity (Fig. 2; Table 1). The PEPC-to-Rubisco ratios in juvenile and mature leaves at dawn and dusk were not significantly different (Fig. 2). At low and high CO2, the content of chlorophyll a, chlorophyll b and total chlorophyll per unit fresh weight was not significantly different in juvenile and mature leaves (Fig. 3a) according to the one-way ANOVA analysis. However, a two-way ANOVA showed that leaf maturity has a significant effect on the content of chlorophyll a and total chlorophyll because of an interaction between leaf maturity and CO2 (Table 2).
Influence of CO2 concentration on pigment content for juvenile and mature leaves (a), chlorophyll fluorescence (b) and rate of O2 exchange (c) of O. alismoides. There are four treatments: mature leaves grown at high CO2 (HC, 302–604 µmol L−1); juvenile leaves grown at high CO2 (HC, 302–604 µmol L−1); mature leaves grown at low CO2 (LC, 0.1–5 µmol L−1); juvenile leaves grown at low CO2 (LC, 0.1–5 µmol L−1). Mean values with their SD (n = 3) are presented and differences among means were tested using one-way ANOVA with Duncan’s and Tukey’s post hoc tests. Data with different letters and symbols are significantly different within the four treatments (P < 0.05)
At low CO2, the maximal photochemical efficiency of PSII (Fv/Fm) and the actual photochemical efficiency of PSII, or yield, were slightly but significantly higher in the mature, compared to the juvenile leaves (ratio of 1.1, SD = 0.03 for Fv/Fm and 1.1, SD = 0.01 for yield). In contrast, at high CO2, the maximal photochemical efficiency of PSII was slightly but significantly higher in the juvenile leaves (ratio of 1.05, SD = 0.03), while the yield was similar in both types of leaf (Fig. 3b; Table 2). At both low and high CO2, the rate of net photosynthesis and the rate of respiration were not significantly different for juvenile and mature leaves (Fig. 3c; Table 2).
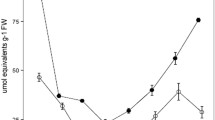
At low CO2, for both types of leaf, there were marked diel changes in acidity. The change was significantly lower in juvenile leaves (15 µequiv g−1 FW) than in mature leaves (19 µequiv g−1 FW) (Fig. 4a). In contrast, at high CO2, there was no significant difference in the diel change in acidity between juvenile and mature leaves and the average diel acidity change was only 8.2 µequiv g−1 FW (Fig. 4a). At dusk and dawn, at low CO2, starch content was similar in juvenile and mature leaves while at high CO2, it was significantly lower in juvenile leaves compared to mature leaves (Fig. 4b).
Influence of CO2 concentration on acidity (a) and starch content (b) of O. alismoides from dawn and dusk for juvenile and mature leaves. There are four treatments: mature leaves grown at high CO2 (HC, 302–604 µmol L−1); juvenile leaves grown at high CO2 (HC, 302–604 µmol L−1); mature leaves grown at low CO2 (LC, 0.1–5 µmol L−1); juvenile leaves grown at low CO2 (LC, 0.1–5 µmol L−1). Mean values with their SD (n = 3) are presented and differences among means were tested using one-way ANOVA with Duncan’s and Tukey’s post hoc tests. Data with different letters are significantly different within the four treatments (P < 0.05); NS not significant
Responses of juvenile and mature leaves to variable CO2
In mature leaves, the Rubisco activity was significantly higher at low, compared to high CO2 in both dusk (2.45-fold, SD = 0.64) and dawn (1.74-fold, SD = 0.89). However, in juvenile leaves, the Rubisco activity was significantly lower at low, compared to high CO2 (1.41-fold, SD = 0.62) at dusk (Fig. 2a). The PEPC activity at low CO2 was significantly higher than at high CO2 in mature leaves both at dawn and dusk (1.83-fold, SD = 1.72; 1.43-fold, SD = 1.30). The same tendency was found in juvenile leaves (1.76-fold, SD = 1.16), but only at dawn (Fig. 2b). The PPDK activity at low CO2 was significantly higher than at high CO2 in mature leaves at dusk (1.35-fold, SD = 1.87), the same tendency was found in juvenile leaves at dawn (1.59-fold, SD = 1.35) (Fig. 2d). There was no significant effect of CO2 concentration on NAD-ME and NADP-ME activities from mature and juvenile leaves at either dusk or dawn (Fig. 2e, f).
In mature leaves, Fv/Fm was slightly but significantly higher (1.1-fold, SD = 0.03) at low, compared to high CO2 but in juvenile leaves, the opposite, significant, response (0.93-fold, SD = 0.02) was found. The yield was not significantly affected by CO2 concentration in the mature leaves; however, it was slightly but significantly lower (1.2-fold, SD = 0.01) in the juvenile leaves at low compared to high CO2 (Fig. 3b; Table 2). The rate of net photosynthesis at high CO2 was significantly higher than at low CO2 in mature (1.4-fold, SD = 0.7) and juvenile leaves (1.3-fold, SD = 0.6). The respiration rate was significantly lower in juvenile leaves (1.5-fold, SD = 0.4) at low CO2 but in mature leaves, this rate was not affected by CO2 concentration (Fig. 3c; Table 2). There was no significant effect of CO2 concentration on chlorophyll content from mature and juvenile leaves at dusk or dawn (Fig. 3a; Table 2).
The diel change in acidity was significantly greater at low than at high CO2 for both juvenile (1.65-fold, SD = 0.61) and mature leaves (2.34-fold, SD = 0.79) (Fig. 4a; Table 2). At dusk, the amount of starch was significantly greater at high than at low CO2 in both juvenile (1.63-fold, SD = 2.06) and mature leaves (1.75-fold, SD = 2.42) (Fig. 4b; Table 2). The amount of starch left at the beginning of the next day in juvenile and mature leaves was both significantly lower at low, compared to high CO2. Compared to dusk, the amount of starch present at dawn was reduced by 75 and 67% for mature and juvenile leaves, respectively, at low CO2, but at high CO2 this reduction was only 31 and 42% for mature and juvenile leaves, respectively (Fig. 4b).
Overnight changes in leaves detached from plants grown at high and low CO2
The overnight incubation experiments and acclimation experiments were performed on the same plants material collected at dusk. The measurements, the next morning, were made on leaves attached to the plant in the acclimation experiments or detached from the plant in the overnight incubation experiment. This provided an opportunity to compare responses of attached and detached juvenile or mature leaves from plants grown at low or high CO2.
Enzyme activity
The pattern of enzyme activity in detached leaves was very similar to that found in attached leaves. Irrespective of the leaf type, the activities of Rubisco, PEPC and PPDK in leaves detached from plants grown at low CO2 were significantly higher than those in leaves detached from plants grown at high CO2, at either the start or the end of the overnight incubation. In contrast, the activities of NAD-ME and NADP-ME were the same in both types of leaf irrespective of CO2 concentration (Fig. S1). In detached mature leaves from plants grown at low CO2 at dawn, the activity of PPDK was significantly higher in detached than in attached leaves, but there were no other significant differences for any of the other enzyme activities (Table 3).
Rates of gas exchange in the dark
Rates of respiratory O2 consumption were similar in detached juvenile and mature leaves from high CO2- or low CO2-treated plants (Fig. 5a). For the mature leaves detached from plants grown at high CO2, there was a net release of CO2 and the molar ratio of CO2 released to O2 consumed was 0.13 (SD = 0.02). For the juvenile leaves detached from plants grown at high CO2, there was a net uptake of CO2 and the ratio of CO2 consumed to O2 consumed was 0.83 (SD = 0.13). In contrast, at low CO2, there was a net uptake of CO2 for both types of detached leaves. The CO2 uptake was 120% (SD = 11) and 280% (SD = 20) of O2 uptake for mature and juvenile leaves, respectively (Fig. 5a).
Responses of detached leaves from O. alismoides during an overnight incubation in the dark at high CO2. a gas exchange rate, b acidity and c starch content. There are four treatments: detached mature leaves grown at high CO2 (HC, 302–604 µmol L−1); detached juvenile leaves grown at high CO2 (HC, 302–604 µmol L−1); detached mature leaves grown at low CO2 (LC, 0.1–5 µmol L−1); detached juvenile leaves grown at low CO2 (LC, 0.1–5 µmol L−1). Mean values with their SD (n = 3) are presented and differences among means were tested using one-way ANOVA with Duncan’s and Tukey’s post hoc tests. Data with different letters are significantly different within the four treatments (P < 0.05); NS not significant
Acidity and starch
The pattern of acidity changes in attached and detached leaves was not significantly different (Figs. 4a, 5b; Table 3). Like in the previous experiment with attached leaves (see Fig. 4a), the diel change in acidity was not significantly different in mature leaves detached from plants grown at high CO2. However, for plants grown at low CO2 the change in acidity in detached juvenile and mature leaves was significantly different at 17 and 25 µequiv g−1 FW, respectively (Fig. 5b).
There were no significant differences for starch variation between attached and detached leaves at dusk (Table 3). Like in the acclimation experiments using attached leaves, the amount of starch decreased after overnight incubation in all detached leaves from plants grown at either low or high CO2 (Fig. 5c). Moreover, after the overnight incubation, when compared to the corresponding attached leaves, the starch content was significantly higher in juvenile leaves detached from plants grown at high CO2 and mature leaves detached from plants grown at low CO2 (Table 3).
Capture of respired CO2 by malic acid
Using the assumptions that 2 moles of H+ are equivalent to 1 mol of malic acid and that the respiratory quotient (CO2/O2) is 1, the percent of dark respiration that could be trapped as malic acid was 6.3% for juvenile leaves and 4.9% for mature leaves detached from plants grown at high CO2. The equivalent value for leaves detached from plants grown at low CO2, was 8.4 and 6.6% for juvenile and mature leaves, respectively.
Relationships between acidity, starch and enzyme activities for plants grown at different concentrations of CO2
We combined data from juvenile and mature leaves to analyse the relationships between enzyme activities and change in acidity. For plants grown at low CO2, there were no significant relationships between acidity and PEPC activity for attached leaves grown under low CO2 neither at dusk (r = 0.688, P = 0.131) nor at dawn (r = 0.719, P = 0.107). However, there was a significant positive relationship between the change in acidity and PEPC activity (with combined data from dawn and dusk) for attached leaves grown at low CO2 (r = 0.624, P = 0.03, Fig. 6a). In contrast, the activities of Rubisco, PPDK, NAD-ME and NADP-ME were not correlated to change in acidity (P > 0.05). Combining data from mature leaves grown at both CO2 concentrations, a positive correlation between change in acidity and consumption of starch was observed (r = 0.885, P = 0.019, Fig. 6b); this relationship was absent in juvenile leaves.
Relationship between PEPC activity or starch content and CAM activity. a Relationship between PEPC and CAM activity for mature and juvenile leaves of O. alismoides grown at low CO2 (0.1–5 µmol L−1); Pearson correlation for PEPC activity (y) versus CAM activity (x): y = 1.06x + 14.27, P < 0.05, R2 = 0.39. b Relationship between change in starch content and CAM activity for mature leaves of O. alismoides grown at low and high CO2 concentration (low CO2, 0.1–5 µmol L−1; high CO2, 302–604 µmol L−1); Pearson correlation for change in starch (y) versus CAM activity (x): y = 0.59x + 15.26, P < 0.05, R2 = 0.78
pH-drift
The pH-drift experiments provided clear evidence for bicarbonate use in both types of leaf grown at different CO2 concentrations since final CO2 concentrations were substantially less than 1 µmol L−1. Both the mature and juvenile leaves grown at high CO2 were able to raise the pH to over 9.20, at which point the [CO2] and [HCO3−] were 0.113 µmol L−1 and 0.11 mmol L−1 for mature leaves, 0.282 µmol L−1 and 0.373 mmol L−1 for juvenile leaves, respectively (Table 4). Both the mature and juvenile leaves grown at low CO2 were able to raise the pH to over 10.0, at which point the [CO2] and [HCO3−] were 0.023 µmol L−1 and 0.18 mmol L−1 for mature leaves and 0.093 µmol L−1 and 0.387 mmol L−1 for juvenile leaves, respectively (Table 4). The alkalinity for mature leaves grown at high CO2 was substantially reduced at the end of the drift probably because of carbonate precipitation or release of organic acid or both. The carbon uptake ability estimated using the quotient of final [CT] to alkalinity (CT/Alk) is shown in Table 4. The CT/Alk for mature and juvenile leaves grown at high CO2 were both significantly higher than at low CO2. Moreover, the ratio for mature leaves grown at low CO2 was statistically different and lower than for juvenile leaves at low CO2 (Table 4).
Discussion
The results presented here are concordant with earlier work showing that mature leaves of O. alismoides have a constitutive use of bicarbonate and C4 metabolism, and operate CAM facultatively at low CO2 (Zhang et al. 2014; Yin et al. 2017; Shao et al. 2017) and confirmed our hypotheses that (i) juvenile leaves behave differently to mature leaves, and (ii) that developmental stage and CO2 concentration both have an effect on CCM activity. Although the conclusion that O. alismoides possesses C4 metabolism relies mainly on enzyme activity, it is consistent with studies performed with other species such as Hydrilla where interpretation of enzyme activities has been confirmed by other approaches such as radiocarbon pulse-chase experiments (Salvucci and Bowes 1983; Bowes et al. 2002). Although CAM was induced at low CO2 in both types of leaf, the extent of CAM was lower in juvenile leaves. Even in mature leaves, the magnitude of titratable acid change (maximum of 34 µequiv g−1 FW; Zhang et al. 2014) is substantially lower than in other freshwater plants such as Isoetes (Pedersen et al. 2011). The smaller diel change in acidity measured in O. alismoides is not the result of the different pH end-points used here (pH 8.3) and in Pedersen et al. (2011) (pH 6.4), since Keeley et al. (1983) showed that the difference between acidity measured at these two pH end-points was only 8%. However, similar low CAM activity has been found in two species of the terrestrial plant Portulaca that operate C4 photosynthesis and CAM when droughted. Values of up to 9 and 54 µequiv g−1 FW in P. cyclophylla and P. digyna (Holtum et al. 2017) are in the same range as for O. alismoides. The low CAM activity in plants with an additional C4 metabolism may therefore result from an anatomical and/or biochemical limitation.
Leaf maturity seems to be a factor determining the magnitude of induced CAM activity in O. alismoides. A similar developmental effect on CAM activity can occur in terrestrial plants. Gas exchange measurements showed that under drought conditions CAM was only present in mature leaves of Clusia rosea (Winter et al. 2008). PEPC is a highly regulated enzyme (Winter 1980) that plays a key role in C4 and CAM. In O. alismoides, the activity of PEPC during day and night increased with leaf maturity, especially at low CO2, concurrent with an increase in CAM activity. Similarly, in terrestrial plants, PEPC activity was higher in mature than in young leaves in Mesembryanthemum crystallinum (von Willert et al. 1976), Bryophyllum calycinum (Nishida 1978) and in Peperomia camptotricha (Ting et al. 1993). Therefore, the possibility that environmental signals, in concert with developmental processes, affects an accelerated shift towards CAM is certainly plausible in O. alismoides.
During the day, CO2 is fixed and partly converted into transitory starch that is degraded during the following night (Huber 1983). The diurnal turnover of transitory starch is an important characteristic of plant metabolism and its alteration by environmental conditions may affect both plant growth and plant habit (Schulze et al. 1990). In several CAM-induced plants, the metabolic products of nocturnal starch degradation are mainly converted into the primary CO2 acceptor PEP (Smith and Bryce 1992). For example, in M. crystallinum, the induction of CAM by exposure to salinity was accompanied by increased activities of a range of starch-degrading enzymes (Paul et al. 1993). Therefore, the large increase in starch turnover when CAM was induced in both juvenile and mature leaves of O. alismoides is consistent with the important role of starch metabolism in CAM plants. Especially in mature leaves, there was a positive correlation between change in starch and CAM activity. The higher starch content at the end of the night in detached leaves compared to attached leaves implies that there are alternative fates for starch in attached leaves including export for growth of young leaves and the plant as a whole as also found in M. crystallinum by Borland and Dodd (2002).
In the present study, at high and low CO2 the PEPC-to-Rubisco ratio in mature leaves is in a range typical of terrestrial C4 plants and consistent with previous reports showing that C4 metabolism is constitutive in O. alismoides (Zhang et al. 2014; Shao et al. 2017; Yin et al. 2017). Similarly, in juvenile leaves grown at low CO2, the PEPC-to-Rubisco ratio was between 1.4 and 2.2, that is also consistent with C4 metabolism. In contrast, in juvenile leaves grown at high CO2, the PEPC-to-Rubisco ratio (0.9–1.1), was significantly lower than that of mature leaves and implies that C4 metabolism is absent. Although juvenile leaves are produced at the base of the plants, the low light is unlikely to be responsible for the lack of C4 metabolism since Shao et al. (2017) showed that the PEPC-to-Rubisco ratio of mature leaves at high CO2 and low light was between 2 and 3. This down-regulation of C4 metabolism at high CO2 is the pattern also found in other C4 freshwater plants such as H. verticillata (Bowes et al. 2002) and E. densa (Casati et al. 2000). Further studies are ongoing to characterise C4 photosynthesis in leaves of O. alismoides in the light.
Our data showed that photosynthetic carbon uptake was able to drive the concentration of CO2 in the solution well below 1 µM (i.e. about 7% of air-equilibrium) with both types of leaf irrespective of CO2. This indicates that carbon uptake was not relying solely on passive diffusion of CO2, since freshwater macrophytes restricted to CO2 typically have CO2 compensation point ranging from 2 to 6 µM (Maberly and Spence 1983). Our results suggest that O. alismoides can use bicarbonate like many other macrophytes (Maberly and Gontero 2017). The markedly higher value of CT/Alk in juvenile leaves compared with mature leaves when grown at low CO2 indicate that the mature leaves have a greater ability for carbon extracting from the solution, especially at low CO2.
Hitherto, O. alismoides, is the only known species with three CCMs: bicarbonate use, C4 metabolism and CAM. These CCMs are differentially regulated in response to high and low CO2 and in the two types of leaf. In juvenile leaves, C4 metabolism was not induced at high CO2, CAM activity was 1.7-fold lower, while bicarbonate use was the least down-regulated by 1.3-fold. In mature leaves, CAM was the most strongly down-regulated by 2.3-fold at high CO2, bicarbonate use was down-regulated by 1.6-fold while C4 metabolism was not affected. Moreover, CAM in O. alismoides is induced by a combination of factors including low CO2 and leaf ageing. High light and temperature are also likely to promote CAM in this species because Zhang et al. (2014) found greater changes in acidity (34 compared to 19 µequiv g−1 FW) in mature leaves grown at natural light and temperatures up to 31 °C. These conditions also produced a greater PEPC-to-Rubisco ratio of about 6.0 (compared to 3.4 here) and a lower CT/Alk of 0.27 (compared to 0.47 here). Therefore, the regulation of CCMs in O. alismoides is controlled by a combination of external environmental factors and internal ontogeny. More work is required to understand better the molecular mechanisms underlying the regulation of these CCMs.
Conclusions and outlook
Mature leaves of O. alismoides possess bicarbonate use and C4 metabolism constitutively and operate low-level CAM at low CO2, while juvenile leaves only have bicarbonate use as a constitutive CCM and operate CAM and C4 facultatively. The magnitude of CAM activity in juvenile leaves is lower than in mature leaves but both are in a similar range to two species of terrestrial C4-CAM from the genus Portulaca. The costs and benefits of operating multiple CCMs in parallel in aquatic and terrestrial plants and the mechanisms involved in regulating them require further study. There might be value in research that combines studies on aquatic and terrestrial plants since the environmental stresses (CO2 or water) differ while the responses are similar.
Abbreviations
- Alk:
-
Alkalinity
- CAM:
-
Crassulacean acid metabolism
- CCM:
-
Carbon dioxide-concentrating mechanism
- CT :
-
Concentration of total inorganic carbon
- FW:
-
Fresh weight
- HC:
-
High CO2
- LC:
-
Low CO2
- NAD(P)-ME:
-
NAD(P)-malic enzyme
- PEP:
-
Phosphoenol pyruvate
- PEPC:
-
PEP carboxylase
- PPDK:
-
Pyruvate phosphate dikinase
- Rubisco:
-
Ribulose 1,5-bisphosphate carboxylase-oxygenase
- SD:
-
Standard deviation
References
Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138:171–190
Aulio K (1985) Differential expression of diel acid metabolism in two life forms of Littorella uniflora (L.) Aschers. New Phytol 100:533–536
Borland AM, Dodd AN (2002) Carbohydrate partitioning in crassulacean acid metabolism plants: reconciling potential conflicts of interest. Funct Plant Biol 29:707–716
Bowes G, Salvucci ME (1989) Plasticity in the photosynthetic carbon metabolism of submerged aquatic macrophytes. Aquat Bot 34:233–266
Bowes G, Rao SK, Estavillo GM, Reiskind JB (2002) C4 mechanisms in aquatic angiosperms: comparisons with terrestrial C4 systems. Funct Plant Biol 29:379–392
Brain RA, Solomon KR (2007) A protocol for conducting 7-day daily renewal tests with Lemna gibba. Nat Protoc 2:979–987
Casati P, Lara MV, Andreo CS (2000) Induction of a C4-like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiol 123:1611–1621
Ceusters J, Borland AM, Taybi T, Frans M, Godts C, De Proft MP (2014) Light quality modulates metabolic synchronization over the diel phases of crassulacean acid metabolism. J Exp Bot 65:3705–3714
Cook CDK, Urmi-König K (1984) A revision of the genus Ottelia (Hydrocharitaceae). 2. The species of Eurasia, Australasia and America. Aquat Bot 20:131–177
Guralnick LJ, Edwards GE, Ku MSB, Hockema B, Franceschi VR (2002) Photosynthetic and anatomical characteristics in the C4-crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Funct Plant Biol 29:763–773
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Holtum JAM, Hancock LP, Edwards EJ, Winter K (2017) Optional use of CAM photosynthesis in two C4 species, Portulaca cyclophylla and Portulaca digyna. J Plant Physiol 214:91–96
Hostrup O, Wiegleb G (1991) The influence of different CO2 concentrations in the light and the dark on diurnal malate rhythm and phosphoenolpyruvate carboxylase activities in leaves of Littorella uniflora (L.) Aschers. Aquat Bot 40:91–100
Huber SC (1983) Relation between photosynthetic starch formation and dry-weight partitioning between the shoot and root. Can J Bot 61:2709–2716
Jones MB (1975) The effect of leaf age on leaf resistance and CO2 exchange of the CAM plant Bryophyllum fedtschenkoi. Planta 123:91–96
Keeley JE (1981) Isoetes howelli—a submerged aquatic CAM plant. Am J Bot 68:420–424
Keeley JE, Rundel PW (2003) Evolution of CAM and C4 carbon concentrating mechanisms. Int J Plant Sci 164:S55–S77
Keeley JE, Walker CM, Mathews RP (1983) Crassulacean acid metabolism in Isoetes bolanderi in high elevation oligotrophic lakes. Oecologia 58:63–69
Kitajima M, Butler WL (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376:105–115
Klavsen SK, Maberly SC (2010) Effect of light and CO2 on inorganic carbon uptake in the invasive aquatic CAM plant Crassula helmsii. Funct Plant Biol 37:737–747
Klavsen SK, Madsen TV (2008) Effect of leaf age on CAM activity in Littorella uniflora. Aquat Bot 89:50–56
Klavsen SK, Madsen TV, Maberly SC (2011) Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: a review. Photosynth Res 109:269–279
Koch K, Kennedy RA (1980) Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiol 65:193–197
Maberly SC (1996) Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshw Biol 35:579–598
Maberly SC, Gontero B (2017) Ecological imperatives for aquatic CO2-concentrating mechanisms. J Exp Bot 68:3797–3814
Maberly SC, Madsen TV (2002) Use of bicarbonate ions as a source of carbon in photosynthesis by Callitriche hermaphroditica L. Aquat Bot 73:1–7
Maberly SC, Spence DHN (1983) Photosynthetic inorganic carbon use by freshwater plants. J Ecol 71:705–724
Madsen TV (1987) The effect of different growth conditions on dark and light carbon assimilation in Littorella uniflora. Physiol Plant 70:183–188
Madsen TV, Maberly SC, Bowes G (1996) Photosynthetic acclimation of submersed angiosperms to CO2 and HCO3 –. Aquat Bot 53:15–30
Neuhaus HE, Schulte N (1996) Starch degradation in chloroplasts isolated from C3 or CAM (crassulacean acid metabolism)-induced Mesembryanthemum crystallinum L. Biochem J 318:945–953
Nishida K (1978) Effect of leaf age on light and dark 14CO2 fixation in a CAM plant, Bryophyllum calycinum. Plant Cell Physiol 19:935–941
Nobel PS, Hartsok TL (1983) Relationships between photosynthetically active raditation, nocturnal acid accumulation, and CO2 uptake for a crassulacean acid metabolism plant, Opuntia ficus-indica. Plant Phys 71:71–75
Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Ann Rev Plant Physiol 29:379–414
Paul MJ, Loos K, Stitt M, Ziegler P (1993) Starch-degrading enzymes during the induction of CAM in Mesembryanthemum crystallinum. Plant Cell Environ 16:531–538
Pedersen O, Rich SM, Pulido C, Cawthray GR, Colmer TD (2011) Crassulacean acid metabolism enhances underwater photosynthesis and diminishes photorespiration in the aquatic plant Isoetes australis. New Phytol 190:332–339
Robe WE, Griffiths H (1990) Photosynthesis of Littorella uniflora grown under two PAR regimes: C3 and CAM gas exchange and the regulation of internal CO2 and O2 concentrations. Oecologia 85:128–136
Sage RF (2016) A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. J Exp Bot 67:4039–4056
Salvucci ME, Bowes G (1983) Two photosynthetic mechanisms mediating the low photorespiratory state in submersed aquatic angiosperms. Plant Physiol 73:488–496
Sand-Jensen K, Gordon DM (1986) Variable HCO3 – affinity of Elodea canadensis Michaux in response to different HCO3 – and CO2 concentrations during growth. Oecologia 70:426–432
Schulze W, Stitt M, Schulze ED, Neuhaus HE, Fichtner K (1990) A quantification of the significance of assimilatory starch for growth of Arabidopsis thaliana L. Heynh. Plant Physiol 95:890–895
Shao H, Gontero B, Maberly SC, Jiang HS, Cao Y, Li W, Huang WM (2017) Responses of Ottelia alismoides, an aquatic plant with three CCMs, to variable CO2 and light. J Exp Bot 68:3985–3995
Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC (2010) Evolution along the crassulacean acid metabolism continuum. Funct Plant Biol 37:995–1010
Smith JAC, Bryce JH (1992) Metabolite compartmentation and transport in CAM plants. In: Tobin AK (ed) Plant organelles. Cambridge University Press, Cambridge, pp 141–167
Smith AM, Zeeman SC (2006) Quantification of starch in plant tissues. Nat Protoc 1:1342–1345
Taybi T, Cushman JC, Borland AM (2002) Environmental, hormonal and circadian regulation of crassulacean acid metabolism expression. Funct Plant Biol 29:669–678
Ting IP, Hann J, Sipes D, Patel A, Walling LL (1993) Expression of P-enolpyruvate carboxylase and other aspects of CAM during the development of Peperomia camptotricha leaves. Bot Acta 106:313–319
Ting IP, Patel A, Kaur S, Hann J, Walling L (1996) Ontogenetic development of crassulacean acid metabolism as modified by water stress in Peperomia. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism. Biochemistry, ecophysiology and evolution. Springer, Heidelberg, pp 204–215
Vadstrup M, Madsen TV (1995) Growth limitation of submerged aquatic macrophytes by inorganic carbon. Freshw Biol 34:411–419
von Willert DJ, Kirst GO, Treichel S, von Willert K (1976) The effect of leaf age and salt stress on malate accumulation and phosphoenolpyruvate carboxylase activity in Mesembryanthemum crystallinum. Plant Sci Lett 7:341–346
Wen H, Wagner J, Larcher W (1997) Growth and nocturnal acid accumulation during early ontogeny of Agave attenuate grown in nutrient solution and in vitro culture. Biol Plant 39:1–11
Winter K (1980) Day/night changes in the sensitivity of phosphoenolpyruvate carboxylase to malate during crassulacean acid metabolism. Plant Physiol 65:792–796
Winter K, von Willert DJ (1972) NaCl-induzierter crassulaceen säurestoffwechsel bei Mesembryanthemum crystallinum. Z Pflanzenphysiol 67:166–170
Winter K, Garcia M, Holtum JAM (2008) On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoё, and Opuntia. J Exp Bot 59:1829–1840
Yin LY, Li W, Madsen TV, Maberly SC, Bowes G (2017) Photosynthetic inorganic carbon acquisition in 30 freshwater macrophytes. Aquat Bot 140:48–54
Yu LF, Yu D (2009) Responses of the threatened aquatic plant Ottelia alismoides to water level fluctuations. Fund Appl Limnol 175:295–300
Zhang YZ, Yin LY, Jiang HS, Li W, Gontero B, Maberly SC (2014) Biochemical and biophysical CO2 concentrating mechanisms in two species of freshwater macrophyte within the genus Ottelia (Hydrocharitaceae). Photosynth Res 121:285–297
Acknowledgements
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000), the Chinese Academy of Sciences President’s International Fellowship Initiative to SCM and BG (2015VBA023, 2016VBA006) and the National Key Research and Development Program of China (2016YFA0601000). We thank the two reviewers for their helpfull comments.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, W.M., Shao, H., Zhou, S.N. et al. Different CO2 acclimation strategies in juvenile and mature leaves of Ottelia alismoides. Photosynth Res 138, 219–232 (2018). https://doi.org/10.1007/s11120-018-0568-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0568-y