Abstract
Gorgonians are one of the most important benthic components of tropical and temperate areas, and play a fundamental role as ecosystem engineers. Although global warming and pollution increasingly threaten them, the acquisition of nutrients, which is a key process in fitness and stress resistance, has been poorly investigated in such species. This study has thus used an advanced in situ incubation chamber for the first time with gorgonians, to assess the daily acquisition of nutrients and the photophysiology of the Mediterranean symbiotic species, Eunicella singularis. The xanthophyll cycle was assessed in parallel. This work has revealed that E. singularis presents a different functioning than the Mediterranean symbiotic corals. This gorgonian indeed relies on both autotrophy and heterotrophy in summer to optimize its energetic budget, while corals mainly shift to autotrophy for their respiratory needs and tissue growth. In addition, although E. singularis lives in the same depths/locations, and harbours the same symbiont genotype than the corals, the photosynthetic performances of their respective symbionts are significantly different. Indeed, E. singularis acquired 2–3 times less autotrophic carbon from its symbionts than corals, but maintained a positive carbon budget by reducing respiration rates, and by presenting maximal photosynthetic rates throughout the day, suggesting a very efficient light utilization. Almost no photoinhibition was observed under very high light levels, because of the induction of a xanthophyll photoprotection process. These results help understanding why gorgonians often dominate many benthic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Octocorals and particularly gorgonians spread from intertidal to abyssal waters of the world’s oceans (Bayer 1961). They are one of the most important benthic components of many tropical reefs and temperate benthic ecosystems such as those from the Mediterranean Sea, often occupying 50 % or more of the available substrate (Fabricius and Alderslade 2001; Harmelin and Garrabou 2005; Tentori and Allemand 2006). They play a fundamental role as ecosystem engineer (Jones et al. 1994), because they build three-dimensional structures, which provide habitats for hundreds of associated organisms. They are thus key species for the maintenance of the biodiversity of these benthic communities. In addition, by releasing organic matter in the form of mucus, and capturing plankton and dissolved organic matter, they play a key role in the transfer of energy and nutrients between the pelagic and benthic systems (Gili and Coma 1998). Their conservation is thus essential to maintain the biodiversity of the communities they inhabit.
Gorgonians are, however, long-lived structural species with slow growth, late maturing and low fecundity, which render them particularly fragile to environmental disturbances (Coma et al. 2006; Garrabou et al. 2009). Since the last decades, many disturbances occurred at increasing frequencies, both in the tropics and temperate areas, leading either to mass bleaching or mass mortality events of these octocorals (Cerrano et al. 2000; Linares et al. 2008; Garrabou et al. 2009; Sammarco and Strychar 2013). Growing concern about the global degradation of these benthic communities of the world’s oceans has underscored the need to develop reliable conservation strategies.
Despite the abundance and ecological importance of gorgonians, their physiology has been poorly studied, and the acquisition of autotrophic carbon in species living in symbiosis with dinoflagellates of the genus Symbiodinium has attracted very little attention (Lasker et al. 1983). In scleractinian corals, it is generally admitted that autotrophic carbon can supply most of the host’s respiratory needs (Muscatine et al. 1981, 1984), except in deep or turbid environments (Falkowski et al. 1984; Palardy et al. 2008). Corals, thus, generally strongly depend on the symbiont’s photosynthetic activity for growth and survival can acclimate to different light levels by changing the symbiont’s physiology or population in favour of better-adapted clades (Rowan et al. 1997). Gorgonians, however, differ from corals with respect to their reliance on autotrophy, because they have different light scattering patterns and morphologies (fan, plume), which are better adapted to prey capture and heterotrophy (Lasker 1981; Ferrier-Pagès et al. 2013), and they often host a single symbiont clade within the same species (Goulet 2007), with a facultative symbiosis (Gori et al. 2012a). The main questions are thus to know whether gorgonians that can acquire large amounts of particulate food, have the same reliance on autotrophy than scleractinian corals, or whether the two modes of nutrition are largely dependent on each other. Also, how do specific and unique gorgonian symbionts cope with environmental changes? These questions are timely because the amount of nutrients acquired, and allocated to the different animal compartments, is a key determinant of gorgonian performance (i.e. growth, propagation, resilience to stress, survival…), and also influences ecosystem processes (Madin et al. 2012). Moreover, understanding the functioning of the gorgonian symbiodinium symbiosis will aid in deciphering why gorgonians dominate many coastal ecosystems. In temperate regions, such as the Mediterranean Sea, the role of symbionts in the nutritional needs of gorgonians is even more puzzling as light is rapidly attenuated with depth, and is very low in winter due to high seawater turbidity and short day length (Muller-Parker and Davy 2001). Rates of photosynthesis in such symbioses are thus highly variable during the year, but even vary from one day to another, or within the same day, depending on the irradiance received (Muller-Parker and Davy 2001). Symbionts can thus turn to be more parasitic than symbiotic (Ferrier-Pagès et al. 2013).
Amongst the 20 gorgonian species of the Mediterranean, E. singularis is the only one living in symbiosis with unicellular dinoflagellates of the genus Symbiodinium sp, belonging only to the temperate clade A (Forcioli et al. 2011). It is also found aposymbiotic in deep waters (Gori et al. 2012a), as this gorgonian spreads from the surface down to the edge of the continental shelf (Gili et al. 1989, 2011). Although its reproduction patterns (Gori et al. 2007; Ribes et al. 2007; Gori et al. 2012b) and spatial distribution before or after a stress event (Coma et al. 2006; Forcioli et al. 2011; Gori et al. 2011) have been investigated, the role of symbionts in the acquisition of nutrients has never been assessed. Two previous studies (Gori et al. 2012b; Cocito et al. 2013), based on the analysis of δ 13C and δ 15N of the gorgonian tissue, have however found a strong heterotrophic signature, even in summer during high light levels. To assess the summer primary production of the symbionts of E. singularis, we thus continuously monitored the rates of respiration and photosynthesis of the symbiotic association over several days, using an in situ chamber (Gevaert et al. 2011) equipped with sensors for temperature, salinity, photosynthetically active radiation (PAR), and an innovative oxygen sensor based on lifetime optical fluorescence technology. We also assessed, at different time points of the daily cycle, photoacclimation, by measuring the pigment profile, xanthophyll cycling and photosynthetic performance of the symbionts. Although such measurements are common in algae (Gevaert et al. 2011; Nitschke et al. 2012), and were performed few times with scleractinian corals (Patterson et al. 1991; Ferrier-Pagès et al. 2013), they have never been applied on gorgonians or octocorals in general. The final aims of this experiment were to (i) monitor for the first time the photosynthetic capacities and respiratory needs of the temperate symbionts associated to E. singularis over a daily cycle; (ii) assess whether these symbionts, in summer, can provide gorgonians with sufficient autotrophic carbon to contribute to the respiratory needs of the animal, and (iii) estimate whether they are able to photo-acclimate to the high irradiance levels that might occur in summer in this temperate area. The response of E. singularis symbionts to changes in irradiance levels will be compared to the response of those living in symbiosis with other Mediterranean scleractinian corals. As gorgonian symbionts belong to the same temperate clade A as most of the other Mediterranean symbionts, we hypothesize that they have the same photophysiological performances. A better knowledge of the functioning of the symbiosis in these animals will enable to better predict their distribution, their dominance in many coastal areas, as well as their response to thermal stress.
Materials and methods
Experimental set up
Experiments were performed in June in the coastal area of the Ligurian Sea (northwest Mediterranean Sea), where gorgonians are abundant. Summer conditions were selected to get saturating irradiance, long daylight periods and maximal production, especially in this temperate region where irradiance is particularly low in winter. Six large parent colonies of the symbiotic gorgonian E. singularis (Esper 1791) were randomly sampled by SCUBA diving (43°42′18′N, 7°18′45″E), and each divided into one large and one small colony. An automated closed-chamber, as described in Gevaert et al. (2011), was deployed on the bottom at 7 m depth during several cycles of two continuous days. The system was composed of a transparent Plexiglas incubation chamber, containing 3 of the large gorgonian colonies at each deployment, and connected to sealed PVC boxes containing batteries and the electronic system. The detector system was composed of an underwater quantum sensor (Ultra-miniature Light Intensity Recorder MDS MKV/L, JFE Advantech) to continuously monitor PAR, and a conductivity probe (TetraCon® 325, WTW®) to record salinity, connected to a Multi 350i WTW® logger. It also contained an oxygen sensor (Rugged Dissolved Oxygen for Multi-Parameter TROLL® 9000, In-Situ), which measured optically, every 10 s, the dissolved oxygen concentration in the chamber and the seawater temperature. The Perpex of the chamber does not alter the light properties in the visible area of the solar spectrum, but it absorbs ultra-violet radiations (UVR). UVR might affect organism’s photosynthetic performances by inducing photoinhibition (Hoegh-Guldberg and Jones 1999). However, in the Mediterranean Sea, the penetration depth of UVR is of few metres (Häder 1997; Piazena and Häder 2009; Durrieu de Madron et al. 2011), so that at 7–10 m depth, UVR are not a major component of the irradiance wavelengths. Inside the chamber, two pumps (Aussenpumpe Extra, Comet-Pumpen Systemtechnik, Kommanditgesellschaft) ensured continuous water mixing, with a constant flow of 12 L min−1 and the chamber was automatically and periodically flushed every 20 min and during the 20 min the incubation medium was renewed. A total of 36 renewals and 36 experimental measurements were thus achieved per 24 h cycle. The water was directly pumped from the surrounding water and automatically filtered on a 500 µm nylon membrane to exclude actively metabolizing organisms. Oxygen concentration measurements (μmol L−1) were transformed into net production or net consumption (during the night) using the volume of the chamber corrected by the volume of the gorgonians (measured by water displacement).
In parallel to each in situ deployment of the chamber, the three small colonies, duplicated from the large colonies incubated inside the chamber, were positioned outside but near the chamber. The effective quantum yield (ΔF:F′ m) of the photosystem II (PSII) was assessed on these small colonies, at regular intervals during the day, using a diving PAM (Waltz, ®). After this measurement, colonies were enclosed with a black plastic cover for 10–15 min, after which the maximal quantum yield (Fv:Fm) was measured by applying a saturation pulse (2500 µmol photons m−2 s−1, 0.8 s). The black plastic was then removed, so that colonies remained in the ambient light until the next measurement. The relative electron transport rate (rETR) was calculated by multiplying ΔF:F′ m by PAR and 0.5, and the non-photochemical quenching (NPQ) was calculated according to: NPQ = (Fm−Fm’)/Fm’. After each PSII measurements, few polyps of the above colonies were sampled and immediately frozen in liquid nitrogen, for pigment determination according to the protocol used in Ferrier-Pagès et al. (2013). After pigment extraction, 20 μL from each sample was injected into a high-performance liquid chromatography (HPLC, 32 Karat Gold system equipped with a diode array detector, Beckman Coulter) with a reverse phase column (C18 Allure, Restek) and was separated following the method of Arsalane et al. (1994). Pigments were detected and quantified close to their absorption maxima by peak area calculations using the integrator of the photodiode array detector. A preliminary calibration of the apparatus was made by injecting known amounts of standards, estimated with the molar extinction coefficient cited by Berkaloff et al. (1990). The de-epoxidation ratio (DR), i.e. de-epoxidation of diadinoxanthin to diatoxanthin, pigments of the xanthophyll cycle (Hoegh-Guldberg and Jones 1999; Lavaud et al. 2004) was calculated as in Warner and Berry-Lowe (2006):
where Dt diatoxanthin, Dd diadinoxanthin and cisDd cis-diadinoxanthin amounts.
Characteristics of the gorgonian colonies
The total surface area of the colonies was determined by measuring the length of each gorgonian branch and the width using a calliper. For each colony, three samples were collected to assess chlorophyll, zooxanthellae and protein concentrations as described below. For zooxanthellae and chlorophyll concentrations, tissue was separated from the main axis using an air-pick, homogenized using a Potter tissue grinder, and the zooxanthellae collected by centrifugation at 2000g for 5 min. They were re-suspended in 5 mL filtered seawater. One mL was sampled for the determination of the zooxanthellae density using an inverse microscope (Leica®) and an improved version of the Histolab 5.2.3 image analysis software (Microvision®). Chlorophyll a (Chl a) concentration was determined according to the equations of Jeffrey and Humphrey (1975) using a spectrophotometer, after extraction in acetone. Protein concentrations were measured using a bicinchonic acid protein assay (Uptima®, Interchim, Montluçon), after extraction in a sodium hydroxide solution (1 mol L−1) for 30 min at 90 °C. Protein standards across a concentration range from 0 to 2000 mg mL−1 were also prepared using bovine serum albumin (BSA, Interchim®). Absorbance of each sample was measured at 560 nm, and protein content was determined using the GENESIS® programme (Kontron Instruments, Bletchley) with reference to the standards. Results were used to express daily carbon production per protein content, to be compared with literature data.
Daily autotrophic carbon acquisition
Rates of gross photosynthesis (Pg), calculated by adding respiration rates (R) measured during the night to net photosynthetic rates (Pn) measured during the day (ca. 20 to 24 measurements) were used to calculate the total daily autotrophic acquisition of carbon (PC). For this purpose, Pg (in µmol O2 cm−2 h−1) was transformed into carbon equivalents according to Muscatine et al. (1981):
where PQ is the photosynthetic quotient equal to 1.1 mol O2:mol C and RQ is the respiratory quotient equal to 0.8 mol C:mol O2.
Results
Temperature in the chamber ranged from 18 to 21 °C depending on the time of the day and salinity remained constant at 37.6 ± 0.1. During the incubations, oxygen concentration varied from 220 to 250 µmoles O2 L−1, depending on the activity of the gorgonian colonies, but the chamber was never oversaturated. Colonies contained between 17.1 and 20.2 µg Chl a cm−2 for a zooxanthellae density of 3.5 ± 0.52 × 106 cells cm−2, and a protein content of ca. 1.02 ± 0.20 mg cm−2. The day length in summer ran from 6:00 am to 9:00 pm.
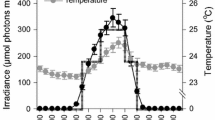
Figure 1 is an example of net oxygen production and consumption cycles obtained for E. singularis on three consecutive days. Irradiance at 7 m depth in summer days was high and, in these conditions, net photosynthesis (Pn) followed irradiance levels. On the first day, Pn rapidly increased to 0.15 µmoles O2 cm−2 h−1 around 10:30 am, then decreased to 0.09 µmole O2 cm−2 h−1 at noon due to a slight photoinhibition, before re-increasing and reaching a maximal value of 0.18 µmole O2 cm−2 h−1 at the end of the afternoon. The same pattern was observed the day after, with a high Pn (0.25 µmole O2 cm−2 h−1) at the beginning of the morning and a maximal value (0.26 µmole O2 cm−2 h−1) at the end of the afternoon. It has to be noticed that Pn was positive and high from the early morning to late in the afternoon. The mean (over the two cycles) daily autotrophic carbon production was equal to 48.4 ± 0.5 µg C cm−2 d−1 or 44.0 ± 0.4 µg C (mg protein)−1 d−1. During the night, gorgonian colonies respired at a constant rate varying between 0.12 and 0.17 µmoles O2 cm−2 h−1. Mean daily respiration thus consumed between 34.5 ± 1.2 and 45.0 ± 2.3 µg C cm−2 d−1 (or 31.4–41.0 µg C (mg protein)−1 d−1). Overall, P:R ratio was always above 1 during the length of the experiment. The relationship between gross production (Pg) and PAR followed a least square fit model as described in Eilers and Peeters (1988), with an adjustment coefficient R 2 > 0.95 (Fig. 2). In some of the experiments, there was no photoinhibition, even at 1000 µmole photons m−2 s−1 whereas in some other days, a slight photoinhibition occurred above 600 µmole photons m−2 s−1. Maximal Pg varied between 0.36 and 0.42 µmol O2 cm−2 h−1 and the sub-saturation irradiance (I k) was low, at ca. 50–90 µmoles photons m−2 s−1 depending on the day considered. Finally, the initial slope of the curve (α) was also low, between 0.004 and 0.009, suggesting an efficient light utilization.
Concerning the photosynthetic efficiency of the PSII of zooxanthellae in hospite, (Fv:Fm) measured in situ, it remained low during the whole day (Fig. 3a, b), for the two cycles. In the first cycle, there was a slight photoinhibition around 15 h (Wilkoxon test, p = 0.001), which corresponded to the concurrent decrease in oxygen production. The relative electron transport rate presented large variations over the two cycles and no real tendency with the irradiance could be observed (Fig. 3c, d). The non-photochemical quenching (NPQ, not shown) followed the irradiance level, with increasing values during the morning up to 3.4 between 11:00 am and 03:00 pm and a rapid decrease in the afternoon, down to 0.1–0.2 at 18:00 pm. The de-epoxidation ratio of diadinoxanthin into diathoxanthin (DR) followed the irradiance level (Fig. 4) and reached values of 0.20 ± 0.04 to 0.22 ± 0.08 for the first and second day, respectively. Moreover, the total amount of diatoxanthin + diadinoxanthin remained stable during the incubations, with 35–44 mol per 100 mol of chl a (and 3–10 mol per 100 mol chl a of diatoxanthin). This result suggests that there isn’t any synthesis of pigments during the day, but rather a conversion from one pigment to the other one.
Changes in (a, b) the maximal quantum yield (Fv:F m, dark lozenges) and (c, d) the relative electron transport rate (rETR, dark lozenges) following changes in PAR (dashed lines, and µmol photons m−2 s−1) during two cycles, represented by real hours on the x-axis. These data are mean ± standard deviations of 3 replicates
Discussion
In situ diel fluctuations in photosynthesis, PSII photochemistry and carbon acquisition have mostly been investigated in tropical scleractinian corals (Brown et al. 1999; Gorbunov et al. 2001; Lesser and Gorbunov 2001; Warner et al. 2002), but only once, to our knowledge, in temperate corals (Ferrier-Pagès et al. 2013) and never in octocorals such as gorgonians. This work has revealed significant differences between temperate scleractinian corals and gorgonians such as E. singularis regarding nutrient acquisition, although the two groups host the same symbiont clade and live in the same locations. Contrary to corals, which shifted in summer from heterotrophy to autotrophy, E. singularis kept the two nutrition modes in parallel to optimize its nutrient input. In addition, it presented lower rates of photosynthesis compared to corals, but maintained a positive autotrophic carbon budget, by achieving maximal rates of photosynthesis throughout the day and down-regulating respiration rates, which were 2–3 times lower than those measured in corals. Finally, very few photoinhibition was observed at high irradiances, thanks to the induction of an active xanthophyll photoprotection.
Previous measurements of the carbon and nitrogen isotopic signature of the tissue of E. singularis sampled in the North Mediterranean Sea clearly showed that zooplankton capture contributed for a large share of the diet of this temperate gorgonian species in summer (Cocito et al. 2013). The δ 13C signature of the gorgonian tissue was indeed very negative and close to the zooplankton signature, suggesting both a very limited autotrophic input and symbiont behaviour closer to parasitism than to symbiosis regarding carbon acquisition. The results obtained in this study challenge the above hypothesis by showing that gorgonian symbionts actually achieved high rates of photosynthesis and carbon acquisition in summer, in the same range as many temperate symbiotic anemones (Muller-Parker 1987; Davy et al. 1996; Verde and McCloskey 1996; 2007), although the animal kept high rates of heterotrophic feeding. It spreads the light on the functioning of this symbiotic association, in which the autotrophically acquired carbon is not fixed in the tissue, but mainly covers the immediate respiratory needs of the gorgonian, whilst carbon acquired through heterotrophic feeding is used to build tissue biomass. Such carbon partitioning explains the very low δ 13C signature of the gorgonian tissue in summer. E. singularis thus presents a different functioning than the one observed in temperate corals. Indeed, corals shift from a dominance of heterotrophy in winter to autotrophy in summer, and mainly rely on the autotrophically acquired carbon for both respiration and tissue growth under high irradiances (Ferrier-Pagès et al. 2011). Conversely, E. singularis presents two independent and complementary nutrition modes, certainly because it has a facultative symbiosis and can be found asymbiotic in deep waters (Gori et al. 2012a). The same pattern was observed in the facultative symbiotic coral Oculina arbuscula (Piniak 2002), in which per-polyp capture rate and feeding efficiency were independent of the symbiotic condition. Combining autotrophy to heterotrophy is a way to maximize nutrient acquisition and ecological success, in environments where light, but not plankton concentration, is often limiting (Muller-Parker and Davy 2001).
Although E. singularis lives in the same depths/locations, and harbours the same symbiont genotype (temperate clade A) than the other Mediterranean corals such as Cladocora caespitosa (Visram et al. 2006), the photosynthetic performances of their respective symbionts are significantly different. Such difference might be due to fine level genetic variability within this clade A that has never been investigated yet. It has been demonstrated with tropical corals that the use of broad cladal designations may not be suitable to describe differences in clade physiology, only detectable at the sub-cladal level (Sampayo et al. 2008; LaJeunesse et al. 2014). On the other side, the host can also change the photosynthetic performances of its symbionts (Mieog et al. 2009), thus more studies are needed to better understand, which, from the host or the symbionts, are responsible for the differences observed. Thus, compared to Mediterranean corals, E. singularis presented photosynthetic rates much lower than C. caespitosa or O. patagonica (Rodolpho-Metalpa et al. 2006, 2008, 2014), but it implemented several processes for a better maximization of the capture of low irradiance levels, such as a value of the light limited photosynthesis (α = 0.0064–0.009), and the onset of light saturation (E k = 50–90 µmole photons m−2 s−1) in the lowest ranges reported (Enriquez et al. 1995; Anthony and Hoegh-Guldberg, 2003a, b). The maintenance of high rates of photosynthesis throughout the day, even in the early mornings or late afternoons, together with almost no decrease at high irradiances, allowed E. singularis to optimize its autotrophic nutrient acquisition. E. singularis, limited photoinhibition via the induction of photoprotective pigments (increase in the DR ratio) and non-photochemical quenching (Brown et al. 1999; Warner and Berry-Lowe 2006; Middlebrook et al. 2008). Such quenching is initiated by a proton gradient across the thylakoid membrane, leading to the acidification of the thylakoid lumen, and the catalysis of the de-epoxidation of diadinoxanthin to diatoxanthin, pigments of the xanthophyll cycle (Hoegh-Guldberg and Jones 1999; Lavaud et al. 2004). Overall, the de-epoxidation ratio was comparable to those measured for tropical (Brown et al. 1999; Warner and Berry-Lowe 2006) and temperate corals (Ferrier-Pagès et al. 2013). The maintenance of high photosynthetic rates at midday was previously observed in the coral C. caespitosa (Ferrier-Pagès et al. 2013), and highlights the great plasticity of the temperate clade A symbionts towards the large range of irradiance levels occurring in their environment. Conversely, tropical corals change their symbiont genotypes according to the environmental conditions (Berkelmans and van Oppen 2006; Frade et al. 2008) and can experience a large photoinhibition at midday, with a significant drop in rates of photosynthesis (Brown et al. 1999; Hoegh-Guldberg and Jones 1999; Gorbunov et al. 2001; Lesser and Gorbunov 2001).
Reduced dark respiration rate was the last adaptation of E. singularis to optimize its energetic budget (Enriquez et al. 1995; Anthony and Hoegh-Guldberg 2003a, b). Indeed, although autotrophic carbon acquisition in E. singularis (48 µg C cm−2 d−1) was much lower than those measured for the Mediterranean coral C. caespitosa (150 µg C cm−2 d−1, Ferrier-Pagès et al. 2013), and other tropical corals (>150 µg C cm−2 d−1, Atkinson and Grigg 1984; Anthony and Hoegh-Guldberg 2003b; Yakovleva and Hidaka 2004), its expenditure through respiration was also very limited. E. singularis consumed only 34 µg C cm−2 d−1, against 2–3 times more in scleractinian corals (Anthony and Hoegh-Guldberg 2003a; Ferrier-Pagès et al. 2013). The strategy used by gorgonians to optimize their energetic budget, i.e. photosynthetic efficiency maximization and down-regulation of respiration, was thus fundamentally different to the one used by most scleractinian corals, i.e. maximization of photosynthetic and respiration rates, these latter representing more than 80 % of the photosynthetically-acquired carbon (Tremblay et al. 2012). Factors that might explain the lower respiration rates in gorgonians compared to corals include their low growth rates, their lack of hard skeleton, and a possibly different respiratory substrate, but this last point remains to be investigated. CZAR (contribution of symbiont translocated carbon to animal respiration, Muscatine et al., 1981) values for E. singularis at 7 m depth were thus always above 100 %. Although these CZAR values have to be interpreted with caution as a result of several assumptions, in summer, with ideal sunny days, E. singularis can survive autotrophically, despite its high heterotrophic capacity.
All together, these results reveal that symbionts associated to E. singularis presented different photophysiological performances than those associated to Mediterranean scleractinian corals, although they both belong to the temperate clade A. In addition, in summer, E. singularis relied on autotrophy to sustain its energetic needs and on heterotrophy to build its tissue biomass. This dual nutrition mode allows gorgonians to optimize their acquisition of nutrients, especially in summer when particulate food is rare in the surrounding waters, due to water-column stratification. This dual nutrition also helps understanding why gorgonians dominate many coastal ecosystems.
References
Anthony KRN, Hoegh-Guldberg O (2003a) Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys? Funct Ecol 17:246–259
Anthony KRN, Hoegh-Guldberg O (2003b) Kinetics of photoacclimation in corals. Oecologia 134:23–31
Arsalane W, Rousseau B, Duval J-C (1994) Influence of the pool size of the xanthophyll cycle on the effects of light stress in a diatom: competition between photoprotection and photoinhibition. Photochem Photobiol 60:237–243
Atkinson MJ, Grigg R (1984) Model of a coral reef ecosystem: Part II Gross and net primary production at French Frigate Shoals, Hawaii. Coral Reefs 3:13–22
Bayer FM (1961) The shallow water octocorallia of the West Indian region. Martinus Nijhoff, Netherlands
Berkaloff C, Caron L, Rousseau B (1990) Subunit organization of PSI particles from brown algae and diatoms: polypeptide and pigments analysis. Photosynth Res 23:181–193
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc B 273:2305–2312
Brown B, Ambarsari I, Warner ME, Fitt WK, Dunne RP, Gibb SW, Cummings DG (1999) Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs 18:99–105
Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S et al (2000) A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (NW Mediterranean), summer 1999. Ecol Letters 3:284–293
Cocito S, Ferrier-Pagès C, Cupido R, Rottier C, Meier-Augenstein W, Kemp H, Reynaud S, Peirano A (2013) Nutrient acquisition in four Mediterranean gorgonian species. Mar Ecol Prog Ser 473:179–188
Coma R, Linares C, Ribes M, Diaz D, Garrabou J, Ballesteros E, Zabala M (2006) Consequences of a mass mortality in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean). Mar Ecol Progr Ser 327:51–60
Davy SK, Lucas IAN, Turner JR (1996) Carbon budgets in temperate anthozoan-dinoflagellate symbioses. Mar Biol 126:773–783
Durrieu de Madron X, Guieu C, Sempéré R, Conan P, Cossa D, D’Ortenzi F, Estournel C, Gazeau F, Rabouille C, Stemmann L et al (2011) Marine ecosystems’ responses to climatic and anthropogenic forcings in the Mediterranean. Prog Oceanogr 91:97–166
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Enriquez S, Darte CM, Sand-Jensen K (1995) Patterns in the photosynthetic metabolism of Mediterranean macrophytes. Mar Ecol Prog Ser 119(1):243–252
Fabricius K, Alderslade P (2001) Soft corals and sea fans: a comprehensive guide to the tropical shallow water genera of the Central West Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science, Townsville
Falkowski PG, Dubinsky Z, Muscatine L, Porter JW (1984) Light and the bioenergetics of a symbiotic coral. BioSci 34:705–709
Ferrier-Pagès C, Peirano A, Abbate M, Cocito S, Negri A, Rottier C, Riera P, Rodolfo Metalpa R, Reynaud S (2011) Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol Oceanogr 56(4):1429–1438
Ferrier-Pagès C, Gevaert F, Reynaud S, Beraud E, Menu D, Janquin M-A, Cocito S, Peirano A (2013) In situ assessment of the daily primary production of the temperate symbiotic coral Cladocora caespitosa. Limnol Oceanogr 58(4):1409–1418
Forcioli D, Merle P-L, Caligara C, Ciosi M, Muti C, Francour P, Cerrano C, Allemand D (2011) Symbiont diversity is not involved in depth acclimation in the Mediterranean sea whip Eunicella singularis. Mar Ecol Prog Ser 439:57–71
Frade PRF, De Jongh F, Vermeulen F, Van Bleijswijk J, Bak RPM (2008) Variation in symbiont distribution between closely related coral species over large depth ranges. Mol Ecol 17:691–703
Garrabou J, Coma R, Chevaldonné P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, Lejeune C, Linares C et al (2009) Mass mortality in NW Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Change Biol 15:1090–1103
Gevaert F, Delebecq G, Menu D, Brutier L (2011) A fully automated system for measurements of photosynthetic oxygen exchange under immersed conditions: an example of its use in Laminaria digitata (Heterokontophyta: Phaeophyceae). Limnol Oceanogr Methods 9:361–379
Gili JM, Coma R (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol 13:316–321
Gili JM, Murillo J, Ros JD (1989) The distribution pattern of benthic Cnidarians in the Western Mediterranean. Cienc Mar 53:19–35
Gorbunov MY, Kolber ZS, Lesser MP, Falkowski PG (2001) Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr 46:75–85
Gori A, Linares C, Rossi S, Coma R, Gili JM (2007) Spatial variability in reproductive cycle of the gorgonians Paramuricea clavata and Eunicella singularis (Anthozoa, Octocorallia) in the Western Mediterranean Sea. Mar Biol 151:1571–1584
Gori A, Rossi S, Berganzo E, Pretus LJ, Dale MRT, Gili JM (2011) Spatial distribution patterns of the gorgonians Eunicella singularis, Paramuricea clavata, and Leptogorgia sarmentosa (Cape of Creus, Northwestern Mediterranean Sea). Mar Biol 158:143–158
Gori A, Bramanti L, Lopez-Gonzalez P, Thoma JN, Gili JM, Grinyo J, Uceira V, Rossi S (2012a) Characterization of the zooxanthellate and azooxanthellate morphotypes of the Mediterranean gorgonian Eunicella singularis. Mar Biol 159:1485–1496
Gori A, Viladrich N, Gili JM, Kotta M, Cucio C, Magni L, Bramanti L, Rossi S (2012b) Reproductive cycle and trophic ecology in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Coral Reefs 31:823–837
Goulet TL (2007) Most scleractinian corals and octocorals host a single symbiotic zooxanthella clade. Mar Ecol Prog Ser 335:243–248
Häder DP (1997) Vertical migration and distribution of primary producers in aquatic ecosystems. The effects of enhanced solar UVB. Photochem Photobiol 65:263–264
Harmelin JG, Garrabou J (2005) Suivi d’une population de Paramuricea clavata (Risso, 1826) (Cnidaria, Octocorallia, Gorgonacea) dans le parc national de Port-Cros (Mediterranée, France): comparaison des états 1992 et 2004 sur le site de la Galère. Sci Rep Port-Cros Natl Park 21:175–191
Hoegh-Guldberg O, Jones R (1999) Diurnal patterns of photoinhibition and photoprotection. Mar Ecol Pro Ser 183:76–86
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pfl 167:191–194
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA (2014) Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 53:305–319
Lasker HR (1981) A comparison of the particulate feeding abilities of three species of gorgonian soft coral. Mar Ecol Prog Ser 5(6):61–67
Lasker HR, Gottfried MD, Coffroth MA (1983) Effects of depth on the feeding capabilities of two octocorals. Mar Biol 73:73–78
Lavaud J, Rousseau B, Etienne A (2004) General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae). J Phycol 40:130–137
Lesser MP, Gorbunov MY (2001) Diurnal and bathymetric changes in chlorophyll fluorescence yields of reef corals measured in situ with a fast repetition rate fluorometer. Mar Ecol Prog Ser 212:69–77
Linares C, Coma R, Garrabou J, Díaz D, Zabala M (2008) Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J Appl Ecol 45:688–699
Madin JS, Hoogenboom MO, Connolly SR (2012) Integrating physiological and biomechanical drivers of population growth over environmental gradients on coral reefs. J Exp Biol 215:968–976
Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211:1050–1056
Mieog JC, Olsen JL, Berkelmans R, Bleuler-Martinez SA, Willis BL et al (2009) The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS ONE 4(7):e6364
Muller-Parker G (1987) Seasonal variation in light-shade adaptation of natural populations of the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943) in Hawaii. J Exp Mar Biol Ecol 112(2):165–183
Muller-Parker G, Davy SK (2001) Temperate and tropical algal–sea-anemone symbiosis. Invertebr Biol 120:104–123
Muscatine L, McCloskey R, Marian RE (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26:601–611
Muscatine L, Falkowski PG, Porter JW, Dubinsky Z (1984) Fate of photosynthetic fixed carbon in light- and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B 222:181–202
Nitschke U, Connan S, Stengel DB (2012) Chlorophyll a fluorescence responses of temperate Phaeophyceae under submersion and emersion regimes: a comparison of rapid and steady-state light curves. Photosynth Res 114:29–42
Palardy JE, Rodrigues LJ, Grottoli AG (2008) The importance of zooplankton to the daily metabolic carbon requirements of healthy and bleached corals at two depths. J Exp Mar Biol Ecol 367:180–188
Patterson MR, Sebens KP, Olson RR (1991) In situ measurements of flow effects on primary production and dark respiration in reef corals. Limnol Oceanogr 36:936–948
Piazena H, Häder DP (2009) Solar UV-B and UV-A irradiance in arid high-mountain regions: measurements on the island of Tenerife as compared to previous tropical Andes data. J Geophys Res 114:GO4024
Piniak G (2002) Effects of symbiotic status, flow speed, and prey type on prey capture by the facultatively symbiotic temperate coral Oculina arbuscula. Mar Biol 141:449–455
Ribes M, Coma R, Rossi S, Micheli M (2007) Cycle of gonadal development in Eunicella singularis (Cnidaria: Octocorallia): trends in sexual reproduction in gorgonians. Invertebr Biol 126:307–317
Rodolfo Metalpa R, Hoogenboom MO, Rottier C, Ramos Esplá A, Baker AC, Fine M, Ferrier Pagès C (2014) Thermally tolerant corals have limited capacity to acclimatize to future warming. Glob Change Biol 20(10):3036−3049
Rodolfo-Metalpa R, Richard C, Allemand D, Ferrier-Pagès C (2006) Growth and photosynthesis of two Mediterranean corals, Cladocora caespitosa and Oculina patagonica, under normal and elevated temperatures. J Exp Biol 209(22):4546–4556
Rodolfo-Metalpa R, Huot Y, Ferrier-Pagès C (2008) Photosynthetic response of the Mediterranean zooxanthellate coral Cladocora caespitosa to the natural range of light and temperature. J Exp Biol 211(10):1579–1586
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388(6639):265–269
Sammarco PW, Strychar KB (2013) Responses to high seawater temperatures in zooxanthellate octocorals. PLoS ONE 8(2):e54989
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Nat Acad Sci 105:10444–10449
Tentori E, Allemand D (2006) Light-enhanced calcification and dark decalcification in isolates of the soft coral Cladiella sp. during tissue recovery. Biol Bull 211:193–202
Tremblay P, Grover R, Maguer J-F, Legendre L, Ferrier-Pagès C (2012) A new model of photosynthate translocation and carbon budget in the coral-zooxanthellae symbiosis. J Exp Biol 215:1384–1393
Verde EA, McCloskey LR (1996) Photosynthesis and respiration of two species of algal symbionts in the anemone Anthopleura elegantissima (Brandt)(Cnidaria; Anthozoa). J Exp Mar Biol Ecol 195(2):187–202
Verde EA, McCloskey LR (2007) A comparative analysis of the photobiology of zooxanthellae and zoochlorellae symbiotic with the temperate clonal anemone Anthopleura elegantissima (Brandt). III. Seasonal effects of natural light and temperature on photosynthesis and respiration. Mar Biol 152(4):775–792
Visram S, Wiedenmann J, Douglas AE (2006) Molecular diversity of symbiotic algae of the genus Symbiodinium (Zooxanthellae) in cnidarians of the Mediterranean Sea. J Mar Biol Assoc U. K 86:1281–1283
Warner ME, Berry-Lowe S (2006) Differential xanthophyll cycling and photochemical activity in symbiotic dinoflagellates in multiple locations of three species of Caribbean coral. J Exp Mar Biol Ecol 336:86–95
Warner M, Chilcoat G, McFarland F, Fitt W (2002) Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar Biol 141:31–38
Yakovleva I, Hidaka M (2004) Different effects of high temperature acclimation on bleaching-susceptible and tolerant corals. Symbiosis 37:87–105
Acknowledgments
We thank D. Allemand, Director of the Scientific Centre of Monaco. Thanks are due to the Marine Police of Monaco for assistance and to Séverine Sikorski for her logistic organization. Financial support was provided by funds from the Centre Scientifique de Monaco. The in situ incubation chamber was built up at the Laboratoire d’Océanologie et de Géosciences (LOG), Unité Mixte de Recherche 8187, who also contributed to the financial support of this field trip.
Conflict of interest
The authors declare no competing interests
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrier-Pagès, C., Reynaud, S., Béraud, E. et al. Photophysiology and daily primary production of a temperate symbiotic gorgonian. Photosynth Res 123, 95–104 (2015). https://doi.org/10.1007/s11120-014-0042-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0042-4