Abstract
Since conventional sampling and laboratory soil analysis do not provide a cost effective capability for obtaining geo-referenced measurements with adequate frequency, different on-the-go sensing techniques have been attempted. One such recently commercialized sensing system combines mapping of soil electrical conductivity and pH. The concept of direct measurement of soil pH has allowed for a substantial increase in measurement density. In this publication, soil pH maps, developed using on-the-go technology and obtained for eight production fields in six US states, were compared with corresponding maps derived from grid sampling. It was shown that with certain field conditions, on-the-go mapping can significantly increase the accuracy of soil pH maps and therefore increase the potential profitability of variable rate liming. However, in many instances, these on-the-go measurements need to be calibrated to account for a field-specific bias. After calibration, the overall error estimate for soil pH maps produced using on-the-go measurements was less than 0.3 pH, while non-calibrated on-the-go and conventional field average and grid-sampling maps produced errors greater than 0.4 pH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Site-specific crop management allows growers to optimize the management of agricultural inputs while taking into consideration the variability of soil attributes within a field. Conventional geo-referenced soil sampling and laboratory analysis represent a popular technique to quantify the variability of important soil properties (Wollenhaupt et al. 1997). Sampling based on a pre-defined 1-ha grid pattern has become the most common strategy in many parts of the US and in other countries. To predict soil test values in locations between sampling points, various interpolation methods have been used. The values of interpolated surfaces in un-sampled locations can be valid only if a reliable spatial structure is present. A map interpolated from unrelated soil test values is frequently erroneous (Webster 2000).
Several studies have shown that the scale of soil pH spatial structure can be less than 100 m, which is equal to the length of a 1-ha square grid cell (Mulla and McBratney 2000). For example, in some instances, semivariogram analyses have revealed ranges as short as 20 m, indicating the maximum distance at which significant spatial dependency exists. In some fields, changes of up to 2 pH units have been observed over distances less than 12 m (Bianchini and Mallarino 2002).
Increased density conventional sampling has been shown to be impractical because of high sampling and analysis costs. Attempts have been made to improve spatial predictions from soil sampling using geostatistical methods (Laslett and McBratney 1990). However, even these methods frequently require a relatively large number of independent soil samples. Also, the currently evolving “management zone” strategy may not always allow proper delineation of field areas with different lime requirements, even when zone delineation is based on alternative high-density data layers (e.g., topography and yield maps, aerial imagery, etc.) (McBratney et al. 2003). To date, none of the existing approaches has been shown to be superior in every production field.
As an alternative, on-the-go soil sensors offer the potential to increase mapping density at a relatively low cost (Viscarra Rossel and McBratney 1997). Based on the experience gained during the development of a field prototype system for mapping soil nitrate content and pH (Loreto and Morgan 1996), a follow-up research study was undertaken to investigate the applicability of flat-surface combination ion-selective electrodes (ISEs) to measure soil properties (particularly pH) directly on moist soil samples (Adamchuk et al. 2005). The initial results illustrated a high correlation with conventional laboratory measurements (R² > 0.92), and a prototype automated system for mapping soil pH on-the-go was developed and tested (Adamchuk et al. 1999). Later, a commercial implement utilizing this approach was developed (Christy et al. 2004). Although most operation parameters are selected by users, this sensor can be operated at 8 km h−1 using 20-m transects (distance between passes) while conducting measurements every 10 s, which results in more than 20 measurements per hectare.
Since lime requirement does not always correlate with water-based soil pH measurements, buffer pH tests are typically performed to determine the amount of liming material needed to increase soil pH to a desired level. Implementation of buffer pH testing on-the-go, as accomplished by Viscarra Rossel et al. (2005), is one way to determine lime requirement. Alternatively, the simple direct soil measurement technique presented in this publication can be used to delineate acidic and alkaline areas of the field. In the past, conventional apparent electrical conductivity (EC) has been shown to correspond to changes in soil type and is therefore related to soil pH buffering characteristics (McBride et al. 1990). For that reason, the commercially available implement shown in Fig. 1 was developed to map soil pH as well as EC. The ability to acquire both data layers simultaneously provides the capability to incorporate them into a decision-making methodology that could provide the recommended liming rates.
The objective of this study was to evaluate soil pH maps produced using on-the-go measurements and compare them with maps produced using laboratory analysis of manual samples collected from the same production fields.
Materials and methods
On-the-go mapping of soil pH
During field operation, the soil pH mapping unit (Fig. 1) automatically collects a soil sample and records its geographic position while traveling across the field. The sampling depth is adjustable and is typically set approximately 10 cm below the surface. Measurements are conducted using two combination, gel-filled, epoxy-body, dome-glass membrane, ion-selective pH electrodes. Every recorded measurement represents an average of the outputs produced by two independent electrodes, which allows in-field cross-validation of electrode performance as well as filtering out erroneous readings. Extracted on-the-go soil cores are brought into direct contact with the electrodes and held in place for 7–25 s (depending on the electrode response). At the end of each measuring cycle, both electrodes are rinsed with water. Simultaneously, a new sample is obtained to replace the analyzed soil. The average cycle time is approximately 10 s, but may vary according to the selected electrode stabilization criterion and electrode performance.
All geo-referenced data are saved in two delimited text files (one for soil pH and one for EC). Both files can be retrieved from the recording device and imported into a geographic information system (GIS) for further analysis. While being downloaded, soil pH records are analyzed for consistency, estimates of steady-state electrode outputs are defined and mV to pH conversion is accomplished using pre-stored calibration data. Typically, each electrode is calibrated before and after field mapping using standard buffer solutions with pH equal to 7 and 4 (10 for alkaline fields). Otherwise, default calibration parameters are assumed.
Field data acquisition
For this study, data gathered from eight production fields obtained within a two-year period were used. These fields were located in six US states, including: Iowa, Illinois (two fields), Kansas (two fields), Nebraska, Oklahoma and Wisconsin. Each dataset consisted of soil pH measurements obtained on-the-go and denoted MSP pH and two soil test reports from a commercial analytical soil laboratory (Soil Testing Lab, Kansas State University, Manhattan, Kansas, USA). Each report contained soil pH (Lab pH) test values pertaining to analyses of the manually collected soil samples. The laboratory tests were conducted on soil samples obtained immediately after on-the-go mapping. Sampling locations were based on a predefined grid pattern (grid-based sampling) as well as established in arbitrary areas with relatively stable MSP pH (targeted sampling).
On-the-go soil mapping was performed using the distance between consecutive passes (swath width) of approximately 15–20 m, while the traveling speed was maintained between 8 km h−1 and 16 km h−1. Default electrode calibration parameters were used in every field. Manually extracted grid and targeted samples were composed of 8–10 soil cores collected from the 0–15 cm depth within a 3-m radius around the pre-defined location of each sampling point. Standard analytical procedures (Thomas 1996) were followed when preparing and analyzing manual soil samples in the commercial laboratory.
Data analysis
Comparison between maps generated using different data sources is typically done visually, using a linear regression analysis or by estimating errors associated with each map. The latter can be reported as the mean absolute error (MAE) when assuming Lab pH to represent true values. In each field, different mapping practices were compared based on the MAE estimates for five validation points. In addition, a simple linear regression approach was used to analyze the relationship between corresponding measured and predicted values for validation points pooled together from all fields.
Three different types of soil pH maps were compared: (1) interpolated grid sampling maps, (2) field average maps, and (3) maps produced using on-the-go sensor measurements. The sensor-based maps were generated using either unprocessed (raw) or calibrated data. According to current recommendations, a limited number of unbiased targeted laboratory pH measurements should be used to validate (and/or adjust) MSP pH measurements to account for possible field-specific systematic discrepancies between on-the-go and conventional laboratory measurements.
Interpolated grid sampling maps were produced using Lab pH measurements obtained for 1-ha grid sampling locations. The other ten Lab pH measurements from each field (targeted sampling) were randomly split in half. Five of these measurements were used to determine values of the field average maps and to calibrate MSP pH data. The other five measurements were used to validate different mapping approaches.
Since no significant differences were found among several interpolation methods and to maintain consistency, grid-based Lab pH and MSP pH measurements were interpolated using the inverse-distance weighting method with parameters automatically established by Geostatistical Analyst of ArcGIS 9.0 (ESRI, Redlands, California, USA). Values of these interpolated maps corresponding to the locations of targeted soil samples were used to calibrate sensor-based maps and to validate alternative mapping methods.
According to our previous experience, unprocessed (raw) MSP pH measurements may deviate significantly from Lab pH measurements. However, when this difference was found to be consistent, values of the MSP pH could be adjusted to match values of the local analytical soil lab (Lab pH). In this study, parameters of universal (all calibration points) and field-specific simple linear regressions were obtained to adjust MSP pH values. Also, a field-specific data shift (regression slope = 1) was applied as an alternative, which can be recommended in case the rate of response to change in soil acidity was similar for Lab pH and MSP pH measurements. Thus, MSP pH maps were processed using the following three equations:
As a result, the following six maps were compared in each field: (1) interpolated grid sampling map of Lab pH, (2) average soil pH map, (3) unprocessed MSP pH map, (4) Universal MSP pH map, (5) Adjusted MSP pH map, and (6) Shifted MSP pH map. Field-specific and overall MAE estimates were compared using a completely randomized block design with 0.05 level of significance.
Results and discussion
Field areas and the number of data points are summarized in Table 1. Based on this summary, the density of MSP pH data was between 8 and 26 measurements ha−1 while grid sampling resulted in 0.8–1.1 samples ha−1. It was also observed that the current eight fields represent fairly diverse soil conditions in terms of soil types, topography and levels of soil acidity (Table 2). Thus, the field average Lab pH (grid sampling) ranged from 5.2 to 6.7. Field average EC values also were quite different from field to field indicating the presence of soils with very coarse to fine textural characteristics. In addition, fields represented in this study varied substantially in terms of management practices, variability of soil pH, levels of spatial structure and conditions present during mapping. Although not addressed in our statistical analysis, each of these factors could significantly affect the results.
The field-specific coefficients of determination (R 2) of simple linear regression between Lab pH and corresponding values of interpolated maps are listed in Table 3. All but NE1 and WI1 fields had R 2 > 0.5 when relating interpolated MSP pH maps with calibration and validation Lab pH values. On the other hand, the interpolated grid-based Lab pH map did not exhibit this level of correlation (except for OK1 field).
Unlike interpolated grid-based maps, corresponding average values of MSP pH maps did not always agree with field average Lab pH values for calibration and validation points (Table 4). For example, the average difference between on-the-go measurements and corresponding Lab pH was −0.9 pH for OK1 field and +0.9 pH for IA1 field. Although the source of rinsing water was deemed to be a major contributor to such differences, the actual cause of the substantial field-to-field shift of MSP pH data remains unknown. Perhaps, lack of exact match between soil sample locations and corresponding MSP measurements could contribute to the observed disagreements in several sites.
When applying simple linear regression to calibrate MSP pH measurements against Lab pH in every field, regression slopes were not found to be significantly different from 1, and the intercept values were not significantly different from 0 (Table 5). The 1:1 relationship between Lab pH and MSP pH measurements also could not be rejected when defining Universal MSP pH. This indicates that our study found no evidence of a different rate of response to soil acidity between on-the-go and conventional laboratory methods (Fig. 2). Furthermore, the slopes determined for fields NE1, OK1, and WI1 were found to be insignificantly different from 0 (p-values equal to only 0.05 for WI1 and 0.06 for OK1 fields), indicating relatively poor correlations between laboratory and on-the-go measurements on these fields.
When validating different mapping methods, MAE was found to be field- as well as method-specific (Table 6). There was no significant difference among the methods for fields IL1, NE1 and WI1. For fields IL2, KS1 and KS2, several MSP pH-based maps produced MAE estimates significantly lower than those obtained for interpolated grid and field average maps. However, the need for sensor calibration was not apparent. Field-specific calibration of MSP pH data was found necessary for IA1 and OK1 fields. In the OK1 field, the interpolated grid-based Lab pH map also exhibited relatively low MAE.
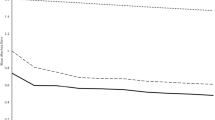
In overall comparison, each mapping approach resulted in significantly lower errors than the field average method. However, maps based on MSP pH values calibrated in every field revealed MAE estimates significantly lower than all other methods. The same conclusion was made when observing change of R 2 for linear regression between measured Lab pH and corresponding map values for all validation points pooled together (Fig. 3). The relatively poor correlation (R 2 < 0.5) revealed by grid sampling pH and field average pH methods was improved when Unprocessed MSP pH or Universal MSP pH were used (R 2 = 0.60). Involvement of the field-specific calibration process has further improved the overall correlation (R 2 = 0.81). Because there was no significant difference between errors associated with Adjusted MSP pH and Shifted MSP pH maps, a simple shift of MSP pH measurements to account for field-specific bias of on-the-go measurement appears to be the most favorable practice.
Based on the results observed, it has become evident that the benefits of on-the-go mapping are field-specific, and generally apparent when compared with conventional grid sampling and field average methods. However, a small number of calibration samples (5–10) should be obtained from each field. These samples can be used to assess the correlations between corresponding laboratory and MSP measurements as well as to shift MSP pH values if necessary. On the other hand, scaling MSP pH values to match Lab pH was shown to be unnecessary since these methods applied to five calibration points failed to reveal significantly different rates of response to soil acidity.
Conclusions
Based on the results of this study, commercially available on-the-go mapping of soil pH can be a successful alternative to conventional mapping strategies (grid sampling or field averaging). However, maps generated using raw MSP pH measurements did not produce MAE values lower than the interpolated grid sampling map. A universal calibration equation was also inappropriate when attempting to remove disagreements between Lab pH and MSP pH data. Field-specific calibration of the MSP pH map allowed for a significant reduction of MAE and can be recommended as a routine practice. It was also noted that shifting MSP pH measurements for the entire field is a more appealing technique than attempting to adjust values using simple linear regression.
References
Adamchuk VI, Morgan MT, Ess DR (1999) An automated sampling system for measuring soil pH. Trans ASAE 42(4):885–891
Adamchuk VI, Lund E, Sethuramasamyraja B, Morgan MT, Dobermann A (2005) Direct measurement of soil chemical properties on-the-go using ion selective electrodes. Comput Electron Agric 48(3):272–294
Bianchini AA, Mallarino AP (2002) Soil-sampling alternatives and variable-rate liming for a soybean-corn rotation. Agro J 94(6):1355–1366
Christy C, Collings KL, Drummond PD, Lund ED (2004) A mobile sensor platform for measurement of soil pH and buffering. Paper No. 041042, ASABE, St. Joseph, Michigan, USA
Laslett GM, McBratney AB (1990) Further comparison of spatial methods for predicting soil pH. Soil Sci Soc Am J 54(6):553–1558
Loreto AB, Morgan MT (1996) Development of an automated system for field measurement of soil nitrate. Paper No. 961087, ASABE, St. Joseph, Michigan, USA
McBride RA, Gordon AM, Shrive SC (1990) Estimating forest soil quality from terrain measurements of apparent electrical conductivity. Soil Sci Soc Am J 54(1):290–293
McBratney AB, Mendonca Santos ML, Minasny B (2003) On digital soil mapping. Geoderma 117:3–52
Mulla DJ, McBratney AB (2000) Soil spatial variability. In: Sumner ME (ed) Handbook of soil science. CRC Press, Boca Raton, Florida, USA, pp A321–A352
Thomas GW (1996) Soil pH and soil acidity. In: Bartels JM (ed) Methods of soil analysis. Part 3 chemical methods. SSSA-ASA, Madison, Wisconsin, USA, pp 475–490
Viscarra Rossel RA, McBratney AB (1997) Preliminary experiments towards the evaluation of a suitable soil sensor for continuous ‘on-the-go’ field pH measurements. In: Stafford JV (ed) Precision agriculture ‘97. Proceedings of the 1st European conference on precision agriculture. BIOS Scientific Publishers, Oxford, UK, pp 493–502
Viscarra Rossel RA, Gilbertson M, Thylen L, Hansen O, McVey S, McBratney AB (2005) Field measurements of soil pH and lime requirement using an on-the-go soil pH and lime requirement measurement system. In: Stafford J (ed) Precision agriculture ‘05: Proceedings of the 5th European conference on precision agriculture. Wageningen Academic Publishers, Wageningen, The Netherlands, pp 511–520
Webster R (2000) Is soil variation random? Geoderma 97:149–163
Wollenhaupt NC, Mulla DJ, Gotway Crawford CA (1997) Soil sampling and interpolation techniques for mapping spatial variability of soil properties. In: Pierce FT, Sadler EJ (eds) The state of site-specific management for agriculture. ASA-CSSA-SSSA, Madison, Wisconsin, USA, pp 19–53
Acknowledgments
This publication is a contribution of the University of Nebraska Agricultural Research Division, Lincoln, Nebraska, USA. This research was supported in part by funds provided through the Hatch Act and through the Small Business Innovation Research (SBIR) program of USDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adamchuk, V.I., Lund, E.D., Reed, T.M. et al. Evaluation of an on-the-go technology for soil pH mapping. Precision Agric 8, 139–149 (2007). https://doi.org/10.1007/s11119-007-9034-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11119-007-9034-0