Abstract
Background
Mixed plantations utilize resources through specific strategies. However, the impact mechanism of mixed plantations on the water and nutrient use efficiency of different tree species remains unclear.
Methods
Robinia pseudoacacia mixed (R. pseudoacacia and Amygdalus davidiana, RPAD; R. pseudoacacia and Armeniaca sibirica, RPAS) and monoculture stands (R. pseudoacacia, RP; A. davidiana, AD; and A. sibirica, AS) were used to determine mixing species relative to monoculture effects on leaf water (WUE), nitrogen (NUE), and phosphorus (PUE) use efficiencies and their potential drivers in the Loess Hilly Region.
Results
Mixing R. pseudoacacia and A. sibirica is a good strategy to maximize soil nutrient resources. Compared to monocultures of all species, R. pseudoacacia and A. davidiana in RPAD and A. sibirica in RPAS had higher WUE and lower NUE and PUE; P limitation of R. pseudoacacia in mixed stands was lower than that in RP. Furthermore, R. pseudoacacia WUE was positively correlated with stand density and crown area, A. davidiana and A. sibirica were the opposite. Regarding all tree species, WUE was influenced by leaf dry matter content, leaf tissue density, and soil available P, while NUE, PUE, and N:P ratio were by average tree height and specific leaf area. Notably, irrespective of tree species, WUE exhibited a negative correlation with NUE and PUE, but a positive correlation with N:P ratio.
Conclusions
Mixed plantations affect water and nutrient use efficiency by altering the above- and below-ground niche through tree structure, and there is a degree of trade-off between WUE, and NUE, PUE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Chinese Loess Plateau (CLP) has an area of about 6.35 × 105 km2, and the destruction of the original vegetation has resulted in one of the most severe water and soil erosion in the world (Fu et al. 2004). To improve the ecological environment of the CLP, the Grain for Green Project was launched in 1999 (Cao et al. 2011). The plantation coverage on the CLP increased from 1.42 Mha in 2000 to 7.13 Mha in 2021 (Meng et al. 2023). More importantly, the afforestation project changed the cycle of water and nutrients in the plant-soil system (Su and Shangguan 2021). In particular, plant growth is severely constrained by a deficient water supply and weak nutrient uptake (Wei et al. 2018). Previous studies used carbon stable isotope (δ13C) analysis and chemical stoichiometry (C:N:P) in plant organs and soil at the stand or regional scale to study the water and nutrient status of plants on the CLP (Tanaka-Oda et al. 2010; Cao and Chen 2017). However, because of the use of various resource acquisition methods, plant growth in this region with different forms of expressions in water and nutrient use strategies (Su and Shangguan 2020; Wang et al. 2020).
Adaptation of different tree species to resource changes can be reflected through water and nutrient use efficiency (Luo et al. 2004; Liu et al. 2015). More specifically, plants responding to water and nutrient stress by changing their physiological activities, such as water (WUE), nitrogen (NUE), and phosphorus (PUE), use efficiencies (Forrester et al. 2005). Generally, plants indirectly reduce their WUE by opening leaf stomata to increase intercellular carbon dioxide (CO2) concentration, photosynthetic rate per unit nitrogen, and transpiration water consumption (Farquhar and Richards 1984; Garrish et al. 2010). With the increase in soil available nitrogen (AN), leaf nitrogen concentration also frequently increases while NUE decreases, increasing WUE (Ripullone et al. 2004). Guo et al. (2016) found that when soil AN was unchanged, interspecific competition significantly increased the leaf nitrogen concentration but decreased its WUE. Garrish et al. (2010) found that WUE of the tropical plant Ficus insipida Willd. (Moraceae) varied as a function of soil AN but not AP. Moreover, according to Dijkstra et al. (2016), WUE increased when the water supply was low while the nutrient supply was high, whereas NUE showed the absolutely opposite trend. Thus, plant physiological constraints generated a trade-off between WUE and NUE. Additionally, strong correlations have been observed between WUE and PUE that is determined by the plant genotype (Meier et al. 2022).
As previous studies focused on the changes in WUE and NUE of monoculture stands (Su and Shangguan 2021), it remains unclear whether mixed-species plantations can attenuate regional water and nutrient stress by increasing both WUE and nutrient use efficiency (i.e. NUE and PUE) of the overall community. A mixed-species plantation is a stand composed of at least two tree species, where the proportion of the main tree species is equal to or greater than 10% (Coll et al. 2018). Because of niche differentiation, positive interactions, or both, the mixed stand can improve its use efficiency of resources, such as light, water, and nutrients, compared with the monoculture stand (Anderegg et al. 2018). For instance, mixed stands containing N-fixing species can increase soil total nitrogen (TN) stocks by biological N fixation and improve the growth of neighboring species in N-limited ecosystems (Taylor et al. 2017). Moreover, the interaction of species with the same growth cycle or leaf phenology or belonging to the same general ecological niche may lead to increased competition for water and N and P among them (Drössler et al. 2018). However, coexisting species in a community can avoid direct interspecific competition, to some extent, when differences in root depth and density allow each species to exploit different underground niches, resulting in more efficient water and nutrient consumption (Dimitrakopoulos and Schmid 2004).
Robinia pseudoacacia L. is a representative N-fixing species in a wide range of habitats spanning from Europe to China (Tsunekawa et al. 2014). On the CLP, unreasonable afforestation measures can cause the ecological degradation of an R. pseudoacacia monoculture stand, frequently leading to dead branches and even death (Wei et al. 2018). To guide suitable management and conservation of R. pseudoacacia monoculture stands, numerous studies have explored their water use and nutrient strategies, as well as potential drivers. For example, Tanaka-Oda et al. (2010) found that WUE and leaf TN concentration of R. pseudoacacia decreased with increasing tree height, indicating that water and nutrients are crucial factors for its growth. Apart from water limitation (Wang et al. 2021), the growth of R. pseudoacacia is limited by the availability of P (Cao and Chen 2017). However, few studies have reported water and nutrient use strategies of R. pseudoacacia in mixed stands on the CLP. Two common native tree species in this area are Amygdalus davidiana (Carr.) C. de Vos ex Henry var. davidiana and Armeniaca sibirica (L.) Lam., and they are planted widely mixed with R. pseudoacacia. However, it is unclear whether these mixed patterns can improve the water and nutrient suitability of R. pseudoacacia, A. davidiana, and A. sibirica.
To address this knowledge gap, we analyzed water and nutrient use efficiencies of R. pseudoacacia and A. davidiana with A. sibirica in both monoculture and mixed stands and analyzed their correlations and potential drivers. We hypothesize that (1) the mixed stand can increase WUE, NUE, and PUE and alleviate nutrient restriction; (2) WUE, NUE, and PUE are affected by many factors, including stand characteristics and soil physicochemical properties; (3) there is a trade-off between WUE, and NUE, PUE, nutrient limitation for these tree species. The results offer valuable insights into tree mixtures and their management by elucidating the factors driving water and nutrient use efficiency in mixed stands, as well as the associated trade-offs.
Materials and methods
Study site
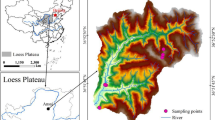
The study site was located in Ansai County (36°35′–36°36′ N, 109°13′″–109°16′ E, 1195–1212 m a.s.l), Shaanxi Province, China. This region belongs to a typical forest-steppe ecotone with a warm temperate semi-humid continental monsoon climate. The mean annual temperature is 8.95 °C, and the mean annual precipitation is 500 mm, with most precipitation occurring from July to September. The soils are classified as Calcic Cambisols (FAO 2020). After 20 years of R. pseudoacacia afforestation in this region, the plant has become the leading tree species.
Experimental design
This study was conducted in five stand types with three replicates (15 plots): mixed stands of R. pseudoacacia with A. davidiana (RPAD) and with A. sibirica (RPAS), and monoculture stands of R. pseudoacacia (RP), A. davidiana (AD), and A. sibirica (AS). These plots share similar altitude, slope, soil condition, stand age (16–25 years), and climatic conditions (Table S1). For each stand type, three 20 m × 20 m quadrats were used for the plot survey, which included calculating stand density (SD, trees·ha−1), diameter at breast height (DBH, cm), average tree height (AH, m), and crown area (CA, m2). To avoid edge effects, a 25 m buffer zone was established around each plot and the distance between two plots did not exceed 3 km.
Plot survey and sampling
In August 2021, green leaves from each plot were sampled from lower, middle, and upper canopies of five model trees with similar height and DBH and then divided into two parts. One part was placed in an ice box and brought back to the laboratory to analyze leaf functional traits. The other was heated at 105 °C for 15 min and then heated to a constant weight at 70 °C in an oven to analyze leaf chemical components. To analyze soil chemical properties, 0–20 cm soil was sampled using a soil drill (40 mm in diameter). Soil samples of mixed stands were composed of soil collected near R. pseudoacacia and A. davidiana, and R. pseudoacacia and A. sibirica in mixed stands. Near R. pseudoacacia and A. davidiana (RPAD), and near R. pseudoacacia and A. sibirica (RPAS), soils were collected and mixed into a soil sample, respectively. To analyze soil physical properties, undisturbed soil was sampled using a ring cutter (volume 100 cm3) at 0–20 cm as above.
Sample measurement
Five to ten intact, disease-free leaves of the three tree species were sampled in monoculture and mixed stands to measure and calculate leaf functional traits. Specific leaf area (SLA, cm2·g–1), leaf dry matter content (LDMC, g·g–1), and leaf tissue density (LD, g·cm–3) were calculated by the following equation:
where fresh (FW) and dry (DW) leaf weights are determined using Electronic balance (accuracy 0.001 g), leaf thickness (LT, mm) is determined using a Digital vernier caliper, and leaf area (LA, cm2) is determined using Epson Perfection V850 Pro Scanner, ImageJ software.
Soil pH was determined using an automatic acid–base titrator (PB-10 standard pH meter; Sartorius, Göttingen, Germany) with water:soil ratio of 2.5:1. Soil bulk density (BD, g·cm–3) was determined using the ring knife method (Bao 2000). Soil particle composition, e.g., soil sand content (SA, %), soil silt content (SI, %), and soil clay content (CL, %) were determined using a Mastersizer 2000 Laser Particle Size Analyzer (Malvern Panalytical, Malvern, UK).
Leaf total carbon (TC, g·kg–1) and soil organic carbon (SOC, g·kg−1) concentrations were determined using the potassium dichromate volumetric method (Nelson and Sommers 1982). Additionally, Leaves and soil total nitrogen (TN, g·kg–1) concentrations were determined using the Kjeldahl method (Bremner and Mulvaney 1982), while soil available nitrogen (AN, mg·kg–1) concentration was determined using the alkaline hydrolysis diffusion method (Bao 2000). Finally, both soil total phosphorus (TP, g·kg–1) and available phosphorus (AP, mg·kg–1) concentrations were determined using the molybdenum blue method (Murphy and Riley 1962).
The stable carbon isotope value δ13C (‰) was determined by isotope mass spectrometry (MAT 253; Thermo Fisher Scientific, Waltham, MA, USA) using the equation:
where Rsample and Rstandard are the carbon isotope ratio (13C/12C) of the samples and the international carbon isotope standard (Pee Dee Belemnite (PDB), RPDB = 0.0112372, δ13CPDB = 0‰), respectively (Farquhar et al. 1989).
Calculation of water and nutrient use efficiency
There is a significant correlation between leaf δ13C and the ratio of intercellular CO2 concentration (Ci) to atmospheric CO2 concentration (Ca, http://www.esrl.noaa.gov) (Farquhar et al. 1982), and the equation is as follows:
where Δ13C (‰) is the carbon isotope discrimination during photosynthesis, δ13Ca (− 8‰) is the 13C abundance in the atmosphere, and a (4.4‰) and b (27‰) are the stable carbon isotope fractionation produced by the diffusion and carboxylation reactions, respectively (Farquhar and Richards 1984).
The leaf conductance to water vapor \(\left({\mathrm g}_{{\mathrm H}_2\mathrm O}\right)\) is 1.6 times the CO2 conductance rate \(\left({\mathrm g}_{{\mathrm{CO}}_2}\right)\), and the net photosynthetic rate (A) is significantly correlated with \({\mathrm{g}}_{{\mathrm{H}}_{2}\mathrm{O}}\) (Peñuelas et al. 2011). Thus, A can be calculated with the following equation:
Δ13C can be related to \(\mathrm{A}/{\mathrm{g}}_{{\mathrm{H}}_{2}\mathrm{O}}\), which is long-term water use efficiency (WUE, μmol·mmol–1) (Osmond et al. 1980):
Leaf C:N and C:P ratios as a measure of nitrogen use efficiency (NUE, gC·g–1·N) and phosphorus use efficiency (PUE, gC·g–1·P), respectively (Vitousek 1982; Dijkstra et al. 2016). Leaf N:P ratio to determine plant nutrient limitation and leaf N:P ratios of < 10, 10–20, and > 20 indicate N limitation, N and P co-limitation, and P limitation, respectively (Güsewell 2004). Leaf C:N, C:P, and N:P mass ratios from leaf TC, TN, and TP concentrations.
Statistical analysis
The data were transformed to meet the assumptions of normality and homogeneity when needed. One-way analysis of variance (ANOVA) with Tukey’s honest significant difference (HSD) test was used to analyze the differences in R. pseudoacacia, and t-test in A. davidiana and A. sibirica, for WUE, NUE, PUE, and N:P ratio, in different stand types (ST). A linear mixed-effects model (LMM) was performed to verify the interactive effects of ST and tree species (TS) on WUE, NUE, PUE, and N:P ratio. Pearson’s correlation and partial correlation coefficients were used to quantify the correlations among WUE, NUE, PUE, N:P ratio, and their potential drivers. Principal component analysis (PCA) was performed to identify the primary axes of covariation among the potential drivers using Canoco 5.0 (ter Braak and Smilauer 2012). A linear regression analysis was performed to examine the relationship between WUE, and NUE, PUE, N:P ratio, respectively. Statistical analysis was performed using SPSS Statistics 23.0 (IBM Corp., Armonk, NY, USA) at a significance level of 0.05 (***P < 0.001; **P < 0.01; *P < 0.05). Figures were created using Origin 2017 software (Originlab Inc., USA).
Results
Stand characteristics and soil physicochemical properties
As shown in Table 1, diameter at breast height (DBH), average tree height (AH), and specific leaf area (SLA) were lower for R. pseudoacacia growing in mixed stands than when growing in the monoculture stand (P < 0.05). Crown area (CA), and SLA were higher, and stand density (SD), leaf dry matter content (LDMC), and leaf tissue density (LD) were lower for A. davidiana growing in mixed stands than they were for the same species growing in monoculture stands (P < 0.05). AH, CA and SLA were higher, and SD, LDMC, and LD were lower for A. sibirica growing in mixed stands than in the monoculture stand (P < 0.05).
The values of soil bulk density (BD), soil sand content (SA), and soil clay content (CL) were higher in the mixed stand of R. pseudoacacia and A. davidiana (RPAD) than in monoculture stands of R. pseudoacacia (RP) and A. davidiana (AD), whereas their values in the mixed stand of R. pseudoacacia and A. sibirica (RPAS) were between those in RP and monoculture stand of A. sibirica (AS) (P > 0.05). Soil pH was lower in the RPAD than in RP and AD, and it was lower in the RPAS than in RP and AS (P > 0.05). The soil total nitrogen (TN) value in the RPAD was between that in RP and AD, and it was between that in RP and AS in the RPAS (P > 0.05). The values of soil available nitrogen (AN) and available phosphorus (AP) were lower in the RPAS than in RP and AS, whereas their values in the RPAD were between those in RP and AD (P > 0.05) (Table 2).
Water and nutrient use efficiency
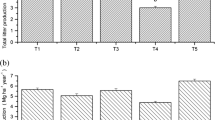
Compared to the RP, water use efficiency (WUE) of R. pseudoacacia was higher in the RPAD (P < 0.05) (Fig. 1a), and nitrogen use efficiency (NUE) of R. pseudoacacia was higher in the RPAS (P < 0.05) (Fig. 1b). WUE values of A. davidiana and A. sibirica in mixed stands were higher than they were in monoculture stands (P < 0.05) (Fig. 1a), while the values of NUE and phosphorus use efficiency (PUE) of A. davidiana and A. sibirica in monoculture stands were higher than in mixed stands (P < 0.05) (Fig. 1b-c). The N:P ratio of R. pseudoacacia was lower in mixed stands compared to monoculture stands, while that of A. davidiana and A. sibirica were higher in the mixed stands (P > 0.05) (Fig. 1d). Additionally, tree species (TS) and stand types (ST) had significant effects on WUE, NUE, and PUE (P < 0.05) (Fig. 1a-c); TS had significant effect on N:P ratio (P < 0.001) (Fig. 1d).
Changes in water (WUE, a), nitrogen (NUE, b), and phosphorus (PUE, c) use efficiencies, and N:P ratios (d) of three tree species in monoculture and mixed stands, and as affected by stand types (ST), tree species (TS), and their interaction (ST × TS). RPAD, R. pseudoacacia and A. davidiana; RPAS, R. pseudoacacia and A. sibirica; RP, R. pseudoacacia; AD: A. davidiana; AS, A. sibirica. Values are means ± SE (n = 3). Different lowercase letters (e.g., a and b) above the bars indicate significant differences among the same tree species for the different stands, while different uppercase letters (e.g., A–C) indicate significant differences among different tree species for the same stands. The effects of TS and ST on nutrition use efficiency are shown with degree of freedom (df), F, and P values. ***P < 0.001; **P < 0.01; *P < 0.05
WUE of R. pseudoacacia and A. davidiana in the RPAD and that of A. sibirica in the RPAS was 19.20%, 19.74%, and 6.86% higher than that of tree species in the monoculture stand, respectively. NUE of R. pseudoacacia and A. davidiana in the RPAD and A. sibirica in the RPAS were 4.23%, 33.39%, and 29.56% lower, respectively. PUE of R. pseudoacacia and A. davidiana in the RPAD and that of R. pseudoacacia and A. sibirica in the RPAS was 16.19%, 26.32%, 4.56%, 21.71% lower than that of tree species in the monoculture stands. The N:P ratio of R. pseudoacacia in RPAD and RPAS was 12.33% and 7.89% lower, while that of A. davidiana in RPAD and A. sibirica in RPAS was 10.38% and 10.99% higher than that of tree species in monoculture stands, respectively (Fig. 2).
Relationship between potential drivers and water and nutrient use efficiency
In both monoculture and mixed stands, WUE was positively correlated with SD for R. pseudoacacia (P < 0.05) (Fig. 3a), while it was the opposite for A. davidiana and A. sibirica (P < 0.05) (Fig. 3b-c); WUE was negatively correlated with CA for R. pseudoacacia (P < 0.05) (Fig. 3a), while it was the opposite for A. davidiana and A. sibirica (P < 0.05) (Fig. 3b-c). NUE and PUE were negatively correlated with SD for R. pseudoacacia (P < 0.05) (Fig. 3a), while it was the opposite for A. davidiana (P < 0.05) (Fig. 3b) and A. sibirica (partly P < 0.05) (Fig. 3c); NUE and PUE were positively correlated with CA for R. pseudoacacia (P > 0.05) (Fig. 3a), while it was the opposite for A. davidiana and A. sibirica (P < 0.05) (Fig. 3b-c).
Correlation matrix of water (WUE), nitrogen (NUE), and phosphorus (PUE) use efficiencies, and N:P ratios of (a) R. pseudoacacia, (b) A. davidiana, (c) A. sibirica and (d) all species, with stand characteristics and soil physicochemical properties in monoculture and mixed stands. SD, stand density; DBH, diameter at breast height; AH, average tree height; CA, crown area; SLA, specific leaf area; LDMC, leaf dry matter content; LD, leaf tissue density; pH, soil pH; BD, soil bulk density; SA, soil sand content; SI, soil silt content; CL, soil clay content; SOC, soil organic carbon; TN, soil total nitrogen; TP, soil total phosphorus; AN, soil available nitrogen; AP, soil available phosphorus. ***P < 0.001; **P < 0.01; *P < 0.05
All in all, WUE was positively correlated with soil AP and negatively correlated with LDMC and LD (P < 0.05). NUE and PUE were positively correlated with LDMC and LD, and negatively correlated with AH, SLA, soil total phosphorus (TP), soil AN, and AP (P < 0.05). The N:P ratio was positively correlated with SD, AH, and SLA (P < 0.05) (Fig. 3d).
Combining the partial correlation coefficients of all potential drivers showed that AH had the greatest effect on WUE, NUE, PUE, and N:P ratio, SD and CA on WUE and N:P ratio, DBH on NUE, PUE, and N:P ratio. SLA had the biggest impact on NUE and N:P ratio. SA and CL had an important effect on WUE, NUE, and PUE, SI on WUE and PUE, and soil pH on NUE and PUE. Soil AP had the greatest influence on WUE, and SOC and soil TN on PUE (P < 0.05) (Table 3).
Potential drivers modulate water and nutrient use efficiency
Principal component analysis (PCA) results of potential drivers of water and nutrient use efficiency showed that PC1 and PC2 accounted for 37.02% and 22.17% of the total variance, respectively (Fig. 4). PC1 had loads mainly from AH (0.775), CA (0.693), SLA (0.844), LDMC (−0.788), LD (−0.733), CL (−0.625), TP (0.846), and AN (0.708), which could be identified as the “leaf functional trait component”. PC2 could be defined as the “soil particle and chemical component” because it had loads from soil pH (−0.653), SI (−0.708), CL (0.714), SOC (0.862), and TN (0.77). PC3 was defined as the “tree structure component” because it had explained 13.60% of the total variance and load mainly from SD (0.669) and DBH (0.703) (Table S2).
Principal component analysis of stand characteristics and soil physicochemical properties in monoculture and mixed stands. SD, stand density; DBH, diameter at breast height; AH, average tree height; CA, crown area; SLA, specific leaf area; LDMC, leaf dry matter content; LD, leaf tissue density; pH, soil pH; BD, soil bulk density; SA, soil sand content; SI, soil silt content; CL, soil clay content; SOC, soil organic carbon; TN, soil total nitrogen; TP, soil total phosphorus; AN, soil available nitrogen; AP, soil available phosphorus
Additionally, PCA analysis results also showed that the dispersion within the same species depending on the community was lower for R. pseudoacacia than in the other two native non-N-fixing species (Fig. 4).
Further, a forward regression analysis was performed to determine the potential drivers of WUE, NUE, PUE, and N:P ratio in monoculture and mixed stands. WUE was mainly determined by LD; NUE was mainly determined by DBH, AH, SLA, soil pH, and AP; PUE was mainly determined by DBH, SLA, and soil pH; N:P ratio was mainly determined by SD, DBH, AH, and CA (Table 4).
Relationship between water and nutrient use efficiency
For individual trees or all species, there were negative correlations between WUE and NUE (Fig. 5a), and between WUE and PUE (Fig. 5b), while positive correlations between WUE and N:P ratio (Fig. 5c). Among them, WUE, was negatively correlated with NUE for R. pseudoacacia and A. davidiana (P < 0.05) (Fig. 5a), and with PUE for R. pseudoacacia (P < 0.05) (Fig. 5b); WUE and N:P ratio were positively correlated for R. pseudoacacia (P < 0.05) (Fig. 5c).
Regression analyses of water (WUE), nitrogen (NUE), and phosphorus (PUE) use efficiencies, and N:P ratios on specific and all tree species in monoculture and mixed stands; subfigures show the relationship between WUE, and NUE (a), PUE (b), N:P ratio (c), respectively. The R2 and P values are shown. *P < 0.05
Discussion
Potential drivers of water and nutrient use efficiency
Generally, tree structure directly affects the morphological and physiological characteristics of the constituent species (Dawud et al. 2016). Compared to the monoculture stand, mixed stands can alter plants’ water and nutrient use efficiencies (Fig. 1) by improving physiological functions and mitigating competition through niche segregation (Danescu et al. 2016; Coll et al. 2018). For instance, trees in mixed stands had lower DBH than those in monoculture stands (Table 1), indicating a reduction in productivity to adapt to the complex environment. Moreover, A. davidiana and A. sibirica exhibited higher AH and SLA in mixed stands compared to monoculture stands, while R. pseudoacacia was the opposite (Table 1). Thus, A. davidiana and A. sibirica in mixed stands tend to be more competitive, potentially decreasing the growth of R. pseudoacacia. However, the dispersion of R. pseudoacacia in terms of species composition, between monoculture and mixed-species communities, was relatively lower than that of A. davidiana and A. sibirica (Fig. 4). This suggests that R. pseudoacacia exhibits partial independence from biotic and abiotic factors and a competitive advantage over coexisting species.
In plantations, nutrients in plants and soil are mainly from rock weathering, litter decomposition, and N-fixing bacteria. Consistent with previous studies, soil TN, and AN concentrations were higher in mixed stands than in monoculture stands of R. pseudoacacia and non-N-fixing tree species (Table 2) (Manzoni et al. 2010; Coll et al. 2018). The potential explanations are as follows: 1) the microenvironment created by coexisting species in mixed stands accelerating litter decomposition and facilitating the rapid release of nutrients into the soil; 2) mixed stands containing R. pseudoacacia could enhance rhizobium N fixation capacity, resulting in higher soil AN concentrations (Forrester et al. 2006; Cao and Chen 2017); 3) coexisting species with varying root depths secrete extracellular enzymes that promote rock weathering (Dimitrakopoulos and Schmid 2004; Stubbs and Wilson 2004).
Water use efficiency and its correlation with potential drivers
Effect of mixed stand on water use efficiency
Plants growing in comparable environments tend to adopt similar resource utilization strategies, regardless of their structural features, species composition, and functional traits. For example, water supply plays a crucial role in plants’ adaptation to drought stress, making high WUE an important trait for plant growth in semi-arid regions (Gong et al. 2011). Additionally, increased N supply did not affect the diurnal pattern of photosynthetic parameters in response to soil water content, but enhanced plant WUE by reducing transpiration rates. For these reasons, R. pseudoacacia exhibited a high WUE in response to water stress and increased soil TN (Fig. 1a, Table 2). However, A. sibirica demonstrated a higher WUE than R. pseudoacacia and A. davidiana, in monoculture stands, indicating that tree species affect plant-level WUE (Fig. 1a) (Wang et al. 2020). Thus, A. sibirica was more negatively affected by drought stress, showing higher sensitivity to stomatal conductance under water-deficit conditions (Du et al. 2011; Wei et al. 2018). Edwards et al. (2012) and Meier et al. (2022) reported that plant genotypes with high WUE are less susceptible to drought stress than those with low WUE. Consequently, A. davidiana exhibited the lowest WUE in monoculture stands (Fig. 1a), indicating a profligate water use strategy in response to drought stress (Garrish et al. 2010; Su and Shangguan 2020).
However, the WUE of R. pseudoacacia and A. davidiana in the RPAD were higher than in RP and AD (Figs. 1a and 2), which may be attributed to various factors (Wang et al. 2020). First, trees tend to develop superior morphological structures and physiological functions to adapt to drought stress. For instance, A. davidiana growing below the main canopy of R. pseudoacacia experiences less coupling with the atmosphere, resulting in a higher ratio of boundary layer conductance to canopy stomatal conductance (Wullschleger et al. 2000; Forrester et al. 2006). This leads to less CO2 (lower δ13C) assimilated within the canopy and closure of leaf stomata, thereby reducing mesophyll conductance (gm) while increasing A. davidiana WUE (Fig. 1a). Additionally, coexisting species with lateral and deep roots adopt different strategies for water uptake, potentially leading to high WUE (Hooper and Dukes 2004). Similar results were found in mixtures of Eucalyptus globulus Labill. and Acacia mearnsii De Wilde compared to the respective monocultures (Forrester et al. 2010). Second, increased soil TN from N-fixing species can alleviate N restriction for coexisting species, stimulate N accumulation in leaves, and improve photosynthesis, thereby enhancing WUE (Batterman et al. 2018). Thus, increased WUE of A. davidiana when mixed with R. pseudoacacia may be related to enhanced leaf photosynthesis caused by increased soil TN concentration (Jennings et al. 2016; Battipaglia et al. 2017).
What is more, mixed planting decreased the WUE of R. pseudoacacia, while the WUE of A. sibirica increased, compared to their respective monocultures (Figs. 1a and 2). This suggests that coexisting species exhibit different sensitivities to changing soil water reserves. Jian et al. (2015) reported that A. sibirica has a wider lateral root distribution than R. pseudoacacia. In the mixed stands, A. sibirica obtained a greater percentage of water from the topsoil (60.96%) than R. pseudoacacia (43.72%) during the growing season (Wang et al. 2020). Hence, there exists species-specific niche segregation in ecophysiology between R. pseudoacacia and A. sibirica within the RPAD community (Moreno-Gutiérrez et al. 2012). Moreover, topsoil did not meet the water requirements of A. sibirica, while that of deeper soil layers partially alleviated drought stress for R. pseudoacacia.
Influence of potential drivers on water use efficiency
In arid and semi-arid areas, when the air temperature rises or soil water content decreases, increased plant WUE can be directly attributed to reduced stomatal conductance (Jia et al. 2022). For example, in response to the gradual depletion of soil water reserves (Ouyang et al. 2022), R. pseudoacacia enhances WUE through tight stomatal control and limited transpiration rates (Fu et al. 2020). Tanaka-Oda et al. (2010) reported that R. pseudoacacia plantations with high SD tend to consume more soil water stores than the same stand with low SD. Moreover, the larger the SD, the smaller the CA of individual tree species. As a result, R. pseudoacacia WUE was positively correlated with SD, but negatively correlated with CA (Fig. 3a) (Brookshire et al. 2020). In contrast, small-sized trees growing in mixed stands with high SD and large canopy closures may receive less photosynthetically active radiation (PAR), resulting in lower air temperatures within the stand than in open ground (Forrester et al. 2006; Khanna et al. 2008; Nygren and Leblanc 2015). Meanwhile, high CA induced the leaves to close the stoma and reduce transpiration rates, thus increasing WUE. Hence, A. davidiana and A. sibirica WUE were negatively correlated with SD and positively correlated with CA (Fig. 3b-c), indicating the negative association of low temperature with WUE of understory tree species. Therefore, soil water content and air temperature jointly regulate leaf stomatal conductance and further regulate the WUE of understory coexisting species.
When soil nutrient availability increases, plants tend to accumulate nutrients in their leaves, while reducing nutrient utilization efficiency, resulting in an elevated WUE (Ripullone et al. 2004), partly consistent with our results (Table 2, Fig. 1a). However, R. pseudoacacia in RPAS shows an opposite trend than expected compared to the RP, as higher soil TN, TP, and AN concentrations imply lower rather than higher WUE (Table 2, Figs. 1a and 2). One possible explanation is that soil particle composition greatly impacts the N-fixing capacity of rhizobia (Table 2), leading to increased N supply for R. pseudoacacia, causing fine roots to uptake more water to absorb N, and synchronize absorb large amounts of soil P. To reduce leaf transpiration and adapt to low soil water supplies, R. pseudoacacia tends to maintain WUE by strictly controlling stomatal closure. As a result, R. pseudoacacia WUE was negatively correlated with soil TP concentration (Fig. 3a). Thus, R. pseudoacacia WUE was not dependent on soil TN concentration, while closely correlated with the N-fixing capacity of rhizobia. Similarly, WUE of A. davidiana and A. sibirica showed no significant correlations with soil TN concentrations (Fig. 3b-c), consistent with previous studies, such as Eucalyptus saligna Sm. (Hubbard et al. 2004), Picea abies (L.) H. Karst. and Eucalyptus grandis W. Hill ex Maiden stands (Phillips et al. 2001; du Toit and Dovey 2005). In brief, the impact of altered soil nutrients and their interactions with other factors on coexisting species (i.e. A. davidiana, and A. sibirica) WUE vary by tree species and site-specific.
Nutrient use efficiency and its correlation with potential drivers
The NUE of different tree species was affected by various factors. For instance, R. pseudoacacia NUE was the lowest in RP compared to AD and AS (Fig. 1b), while soil AN concentration in RP was the highest (Table 2), suggesting that R. pseudoacacia NUE was closely correlated with soil AN (Dijkstra et al. 2016). However, in addition to R. pseudoacacia, the NUE of A. davidiana and A. sibirica were less associated with soil AN (Fig. 3a-b). The reason may be that litter decomposition of both tree species releases N primarily into the topsoil (Voigtlaender et al. 2012); R. pseudoacacia growth also absorbed N fixed by rhizobia, resulting in R. pseudoacacia NUE being partially correlated with soil AN (Fig. 3a) (Turner and Lambert 2014; Nygren and Leblanc 2015). Relatively, NUE of all species was negatively correlated with soil AN (Fig. 3c-d), indicating that R. pseudoacacia in mixed stands facilitates N cycling in the plant-soil system (Forrester et al. 2006; Su and Shangguan 2020). Additionally, NUE of A. sibirica and all tree species were negatively correlated with soil TP (Fig. 3c-d); soil TP concentrations were higher in RP than in AD and AS, and they were higher in RPAS than in RP and AS (Table 2). This suggests that leaf functional traits of A. sibirica may promote P cycling, particularly in RPAS. Thus, lower A.sibirica NUE in RPAS than in AS (Figs. 1b and 2) is likely associated with plants high water demand (Wang et al. 2020) promoting root uptake of water and nutrients (Du et al. 2011; Sardans and Peñuelas 2012). Moreover, plantation stands with high density may exhibit rich roots, which benefits rhizobia in fixing N; increased soil AN concentration leads to higher levels of leaf TN concentration. Hence, R. pseudoacacia NUE was negatively correlated with SD (Fig. 3a). In contrast, NUE of A. davidiana and A.sibirica were positively correlated with SD (Fig. 3b-c), possibly due to high-density stands consuming huge soil water, and thus reducing access of non-N-fixing species to soil N (Tanaka-Oda et al. 2010; Dijkstra et al. 2016).
Factors affecting the PUE of different tree species are more complex. For example, the PUE of R. pseudoacacia was negatively correlated with SD (Fig. 3a), while that of A. davidiana and A. sibirica were the opposite (Fig. 3b-c). This is because high-density stands exhibit abundant root systems, which benefits rhizobia in fixing N, and promotes plants that are limited by soil P to allocate more resources to synthesize phosphatase (Treseder and Vitousek 2001). Meanwhile, high-density stands consume huge soil water, thus reducing access of non-N-fixing species root to soil P. Moreover, the PUE of A. davidiana and A. sibirica was negatively correlated with CA (Fig. 3b), suggesting that large CA may limit P transport to leaves (Johnson 2010). Additionally, low light leads to a large SLA (Xu et al. 2022). Thus, A. davidiana PUE was positively correlated with PAR; lower A. davidiana PUE in RPAD compared to AS (Figs. 1c and 2) may be attributed to the canopy shade of R. pseudoacacia. In comparison, A. sibirica PUE in RPAS was less affected by the canopy shade of R. pseudoacacia, but increased chlorophyll to improve the rate of leaf photosynthesis. Interestingly, mixed planting of A. sibirica and R. pseudoacacia enhanced soil P mineralization and leaching processes (Table 2). Consequently, A. sibirica PUE was negatively correlated with soil TP (Fig. 3c) and lower in RPAS than in AS (Figs. 1c and 2) (Vitousek et al. 2010; Turner and Lambert 2014). This was consistent with previous studies that mixed species with comparable growth cycles or leaf phenology typically exhibit intense competition for resources (Feller et al. 1999; de-Dios-García et al. 2018). Hence, A. sibirica in the RPAS compared to AS may show greater competition for soil AP.
The N:P ratio of all tree species was > 20 (20.67, Fig. 1d) (Güsewell 2004), suggesting that the growth of all species was limited by soil P (Cao and Chen 2017). Moreover, tree species with high SD, AH, and SLA tend to be more susceptible to soil P constraints, due to the positive correlations between the N:P ratio and SD, AH, and SLA (Fig. 3d). Specifically, large tree structures indicate rich root systems, which benefit N fixation by rhizobia but lead to increased soil P restriction (Treseder and Vitousek 2001). Another study found that high-density stands with sufficient soil N sources tend to allocate more P to synthesize biomass in plant crowns, roots, and mycorrhizas, thereby reducing P transport to leaves (Johnson 2010). Additionally, SLA was larger for R. pseudoacacia growing in mixed stands than in monoculture stands, and A. davidiana and A. sibirica were the opposite (Table 1). Based on a previous study, low PAR leads to large SLA and leaf photosynthetic intensity was positively correlated with LDMC (Xu et al. 2022). Therefore, R. pseudoacacia growth was less restricted by soil P, in mixed stands compared to monoculture stands, while that of which was more for A. davidiana and A. sibirica. Predictably, mixed species, especially these large sizes, need to assimilate amounts of soil P to support physiological and biochemical reactions.
Trade-off between water, and nutrient use efficiency
Water and nutrients are vital resources for plant growth, and their efficient utilization significantly influences plant functional traits (Huang et al. 2015). Inefficient WUE may indirectly lead to higher transpiration rates, favoring the uptake of more soluble P and overall N. Consequently, there may be an inverse relationship between WUE, and NUE, PUE (Patterson et al. 1997; Dijkstra et al. 2016). Thus, WUE was negatively correlated with NUE, for individual trees or all species (Fig. 5a) (Gong et al. 2011; Dijkstra et al. 2016; Su and Shangguan 2020). Additionally, R. pseudoacacia exhibited a greater AH and CA than A. sibirica and A. davidiana (Table 1), indicating a higher water demand and WUE for R. pseudoacacia than A. sibirica and A. davidiana. Furthermore, achieving high WUE is only possible if the photosynthetic machinery and energy transfer capacity are robust, which necessitates higher N concentrations or a preference for low NUE (Dijkstra et al. 2016). Accordingly, the decreasing order of the adaptability of these three trees (R2) to water stress is as follows: A. davidiana (0.674), R. pseudoacacia (0.616), and A. sibirica (0.461) (Fig. 5a). Furthermore, understory tree species are often influenced by lower air temperatures and reduced PAR, resulting in lower transpiration rates but increased WUE (Garrish et al. 2010). Thus, a trade-off between WUE and NUE is expected to be more pronounced for A. davidiana than A. sibirica, in mixed stands compared to monoculture stands.
Few studies have confirmed a stable trade-off between WUE and PUE, as overall P uptake depends not only on soil water migration and transpiration, but also on root structure, distribution, and its exudates (e.g., extracellular enzyme) (Brown et al. 2011; Huang et al. 2015). For N-fixing species, the process of water uptake and nutrient absorption is less connected, because rhizobia can independently fix N and mobilize more soil P to synthesize phosphatase, thereby enhancing protein production capacity (Cregger et al. 2014). Therefore, R. pseudoacacia WUE showed a significant negative correlation with PUE (P < 0.05) (Fig. 5b), probably due to the effect of microbial regulation on P supply leading to a decrease in PUE and an increase in WUE (Ripullone et al. 2004; Cleveland and Liptzin 2007). Additionally, plants may not have access to larger amounts of soil AP, especially under water stress conditions where the mobility of soil P is strongly reduced (Lambers et al. 2008; Dijkstra et al. 2016). Thus, the trade-off between WUE and PUE was lower for A. davidiana and A. sibirica than for R. pseudoacacia (Fig. 5b).
There was a positive correlation between WUE and N:P ratios for individual trees or all species (Fig. 5c), consistent with various shrubs and trees on the CLP (Yan et al. 2016). A closer examination reveals that WUE increases with higher N concentrations in plant biomass, all other variables being equal (Cernusak et al. 2007). Additionally, increased soil AP or alleviation of P deficiency tends to improve WUE (Raven et al. 2004). This correlation is likely widespread because it links the N:P ratio of plants to transpiration, thereby integrating nutrient and hydrological cycles (Dijkstra et al. 2016; Su and Shangguan 2020). Furthermore, the correlation between WUE and leaves TN and TP concentrations may differ depending on restricted soil nutrients. For instance, under N-limited conditions, WUE showed a positive correlation with the N:P ratio and a negative correlation with P concentration, suggesting that WUE increased in response to higher leaf TN concentrations (Cernusak et al. 2007). However, when soil P is limited, leaf TN concentrations may increase significantly compared to soil N shortages, and excessive N leads to reduced transpiration rate and then decreases soil water availability and P uptake through reduced soil solution mass flow (Wilkinson et al. 2007; Cernusak et al. 2010). As such, the correlation between WUE and N:P ratio is particularly pronounced for R. pseudoacacia compared to other tree species (Fig. 5c). This implies that R. pseudoacacia exhibits elevated leaf TN concentrations when faced with limited soil P. Finally, it is worth noting that in this water-limited region, mixing different tree species can alleviate nutrient limitations, resulting in improved WUE but potentially reduced NUE and PUE. These can be advantageous for plant growth in environments where water availability is severely limited.
Conclusions
Compared to the respective monocultures, mixing R. pseudoacacia and A. sibirica was a good strategy to maximize soil nutrient resources.
R. pseudoacacia and A. davidiana showed higher WUE in RPAD than in RP and AD, which were attributed to leaf photosynthesis influenced by canopy shade and lateral and deep roots. Mixed planting decreased R. pseudoacacia WUE while A. sibirica WUE increased in RPAS, compared to monoculture stands, possibly due to water niche segregation caused by different root distributions. R. pseudoacacia WUE was closely correlated with rhizobia N fixation capacity, while soil nutrient effects and interactions with other factors on WUE of A. davidiana and A. sibirica are species and site-specific.
A trade-off between WUE and NUE is expected to be more pronounced for A. davidiana than A. sibirica, in mixed stands compared to monoculture stands, as smaller trees adapt to low temperature and less PAR with reduced transpiration rates but increased WUE. A trade-off between WUE and PUE was weak in A. davidiana and A. sibirica, possibly because soil P mobility was reduced under water-scared conditions. The correlation between WUE and N:P ratio suggests that R. pseudoacacia exhibits elevated leaf TN concentration when faced with limited soil P.
Data availability
Data and materials can be obtained by contacting the corresponding authors.
References
Anderegg WRL, Konings AG, Trugman AT, Yu K-L, Bowling DR, Gabbitas R, Karp DS, Pacala S, Sperry JS, Sulman BN, Zenes N (2018) Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 561:538–541
Bao S-D (2000) Soil and agriculture chemistry analysis, 3rd edn. China Agriculture Press, Beijing (in Chinese)
Batterman SA, Hall JS, Turner BL, Hedin LO, Walter JKL, Sheldon P, van Breugel M (2018) Phosphatase activity and nitrogen fixation reflect species differences, not nutrient trading or nutrient balance, across tropical rainforest trees. Ecol Lett 21:1486–1495
Battipaglia G, Pelleri F, Lombardi F, Altieri S, Vitone A, Conte E, Tognetti R (2017) Effects of associating Quercus robur L. and Alnus cordata Loisel. on plantation productivity and water use efficiency. For Ecol Manag 391:106–114
Bremner JM, Mulvaney CS (1982) Nitrogen–total. In: Page AL (ed) Methods of soil analysis, part 2, chemical and microbiological properties, agronomy monograph. ASA and SSSA, Madison, vol 9
Brookshire ENJ, Stoy PC, Currey B, Finney B (2020) The greening of the Northern Great Plains and its biogeochemical precursors. Global Change Biol 26(10):5404–5413
Brown KR, Courtin PJ, Negrave RW (2011) Growth, foliar nutrition and δ13C responses of red alder (Alnus rubra) to phosphorus additions soon after planting on moist sites. For Ecol Manag 262:791–802
Cao Y, Chen Y-M (2017) Coupling of plant and soil C:N: P stoichiometry in black locust (Robinia pseudoacacia) plantations on the Loess Plateau, China. Trees 31:1559–1570
Cao S-X, Chen L, Shankman D, Wang C-M, Wang X-B, Zhang H (2011) Excessive reliance on afforestation in China’s arid and semiarid regions: lessons in ecological restoration. Earth-Sci Rev 104:240–245
Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD (2007) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J Exp Bot 58:3549–3566
Cernusak LA, Winter K, Turner BL (2010) Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytol 185:770–779
Cleveland CC, Liptzin D (2007) C:N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Coll L, Ameztegui A, Collet C, Lof M, Mason B, Pach M, Verheyen K, Abrudan I, Barbati A, Barreiro S, Bielak K, Bravo-Oviedo A, Ferrari B, Govedar Z, Kulhavy J, Lazdina D, Metslaid M, Mohren F, Pereira M, Peric S, Rasztovits E, Short I, Spathelf P, Sterba H, Stojanovic D, Valsta L, Zlatanov T, Ponette Q (2018) Knowledge gaps about mixed forests: what do European forest managers want to know and what answers can science provide? For Ecol Manag 407:106–115
Cregger MA, McDowell NG, Fangle RE, Pockman WT, Classen AT (2014) The impact of precipitation change on nitrogen cycling in a semi-arid ecosystem. Funct Ecol 28:1534–1544
Danescu A, Albrecht AT, Bauhus J (2016) Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 182:319–333
Dawud SM, Raulund-Rasmussen K, Domisch T, Finér L, Jaroszewicz B, Vesterdal L (2016) Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 19:645–660
de-Dios-García J, Manso R, Calama R, Fortin M, Pardos M (2018) A new multifactorial approach for studying intra-annual secondary growth dynamics in Mediterranean mixed forests: integrating biotic and abiotic interactions. Can J Forest Res 48(4):333–344
Dijkstra FA, Carrillo Y, Aspinwall MJ, Maier C, Canarini A, Tahaei H, Choat B, Tissue DT (2016) Water, nitrogen and phosphorus use efficiencies of four tree species in response to variable water and nutrient supply. Plant Soil 406(1–2):187–199
Dimitrakopoulos PG, Schmid B (2004) Biodiversity effects increase linearly with biotope space. Ecol Lett 7(7):574–583
Drössler L, Agestam E, Bielak K, Dudzinska M, Koricheva J, Liziniewicz M, Löf M, Mason B, Pretzsch H, Valkonen S, Wellhausen K (2018) Over- and underyielding in time and space in experiments with mixed stands of Scots Pine and Norway Spruce. Forests 9(8):18
Du S, Wang YL, Kume T, Zhang JG, Otsuki K, Yamanaka N, Liu GB (2011) Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agr Forest Meteorol 151(1):1–10
du Toit B, Dovey SB (2005) Effect of site management on leaf area, early biomass development, and stand growth efficiency of a Eucalyptus grandis plantation in South Africa. Can J For Res 35(4):891–900
Edwards CE, Ewers BE, McClung CR, Lou P, Weinig C (2012) Quantitative variation in water-use efficiency across water regimes and its relationship with circadian, vegetative, reproductive, and leaf gas-exchange traits. Mol Plant 5(30):653–668
FAO ISRIC (2020) Revised legend of the FAO-UNESCO SoilMap of the world. ISRIC Report. ISRIC-World Soil Information
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct Plant Biol 9(2):121–137
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol 40:503–537
Feller IC, Whigham DF, O’Neill JP, McKee KL (1999) Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 80(7):2193–2205
Forrester DI, Bauhus J, Cowie AL (2005) On the success and failure of mixed -species tree plantations: lessons learned from a model system of Eucalyptus globulus and Acacia mearnsii. For Ecol Manag 209(1–2):147–155
Forrester DI, Bauhus J, Cowie AL, Vanclay JK (2006) Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: a review. For Ecol Manag 233(2–3):211–230
Forrester DI, Theiveyanathan S, Collopy JJ, Marcar NE (2010) Enhanced water use efficiency in a mixed Eucalyptus globulus and Acacia mearnsii plantation. For Ecol Manag 259(9):1761–1770
Fu B-J, Meng Q-H, Qiu Y (2004) Effects of land use on soil erosion and nitrogen loss in the hilly area of the Loess Plateau. China Land Degrad Dev 15(1):87–96
Fu L, Xu Y, Xu Z, Wu B, Zhao D (2020) Tree water-use efficiency and growth dynamics in response to climatic and environmental changes in a temperate forest in Beijing. China Environ Int 134:105209
Garrish V, Cernusak LA, Winter K, Turner BL (2010) Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree. Ficus Insipida J Exp Bot 61(13):3735–3748
Gong X-Y, Chen Q, Lin S, Brueck H, Dittert K, Taube F, Schnyder H (2011) Tradeoffs between nitrogen- and water-use efficiency in dominant species of the semiarid steppe of InnerMongolia. Plant Soil 340:227–238
Guo R-Q, Sun S-C, Liu B (2016) Difference in leaf water use efficiency/ photosynthetic nitrogen use efficiency of Bt-cotton and its conventional peer. Sci Rep 6:33539
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hooper DU, Dukes JS (2004) Overyielding among plant functional groups in a long-term experiment. Ecol Lett 7(2):95–105
Huang Z-Q, Liu B, Davis M, Sardans J, Peñuelas J, Billings S (2015) Long-term nitrogen deposition linked to reduced water use efficiency in forests with low phosphorus availability. New Phytol 210:431–442
Hubbard RM, Ryan MG, Giardina CP, Barnard H (2004) The effect of fertilization on sap flux and canopy conductance in a Eucalyptus saligna experimental forest. Glob Chang Biol 10:427–436
Jennings KA, Guerrieri R, Vadeboncoeur MA, Asbjornsen H (2016) Response of Quercus velutina growth and water use efficiency to climate variability and nitrogen fertilization in a temperate deciduous forest in the northeastern USA. Tree Physiol 36(4):428–443
Jia G-D, Chen L-X, Yu X-X, Liu Z-Q (2022) Soil water stress overrides the benefit of water-use efficiency from rising CO2 and temperature in a cold semi-arid poplar plantation. Plant Cell Environ 45:1172–1186
Jian S-Q, Zhao C-Y, Fang S-M, Yu K (2015) The distribution of fine root length density for six artificial afforestation tree species in Loess Plateau of Northwest China. Forest Syst 24(1):e003
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Khanna M, Dhungana B, Clifton-Brown J (2008) Costs of producing Miscanthus and switchgrass for bioenergy in Illinois. Biomass Bioenerg 32:482–493
Lambers H, Chapin FS, Pons TL (2008) Plant physiological ecology. Springer Science, New York
Liu Y-B, Xiao J-F, Ju W-M, Zhou Y-L, Wang S-Q, Wu X-C (2015) Water use efficiency of China’s terrestrial ecosystems and responses to drought. Sci Rep 5:13799
Luo Y-Q, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate BA, McMutrie RE, Oren R, Parton WJ, Pataki DE, Shaw RM, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54(8):731–739
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80(1):89–106
Meier S, Morales A, López-Olivari R, Matus I, Aponte H, Campos PS, Khan N, Cartes P, Meriño-Gergichevich C, Castillo D, Seguel A (2022) Synergistic role between phosphorus and water use efficiency in spring wheat genotypes. Agric Water Manag 263:107481
Meng Y-Y, Hou B-W, Ding C, Huang L, Guo Y-P, Tang Z-Y (2023) Spatiotemporal patterns of planted forests on the Loess Plateau between 1986 and 2021 based on Landsat NDVI time-series analysis. GIsci Remote Sens 60(1):2185980
Moreno-Gutiérrez C, Dawson TE, Nicolás E, Querejeta JI (2012) Isotopes reveal contrasting water use strategies among coexisting plant species in a Mediterranean ecosystem. New Phytol 196(2):489–496
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nelson D, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2, chemical and microbiological properties, agronomy monograph. ASA and SSSA, Madison, vol 9
Nygren P, Leblanc HA (2015) Dinitrogen fixation by legume shade trees and direct transfer of fixed N to associated cacao in a tropical agroforestry system. Tree Physiol 35(2):134–147
Osmond CB, Björkman O, Anderson DJ (1980) Physiological processes in plant ecology: toward a synthesis with Atriplex. Ecological studies 36, Springer-Verlag Berlin, Heidelberg, 468
Ouyang L, Lu L-W, Wang C-L, Li Y-Q, Wang J-Y, Zhao X-H, Gao L, Zhu L-W, Ni G-Y, Zhao P (2022) A 14-year experiment emphasizes the important role of heat factors in regulating tree transpiration, growth, and water use efficiency of Schima superba in South China. Agric Water Manag 273:107902
Patterson TB, Guy RD, Dang Q-L (1997) Whole-plant nitrogen- and water- relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 110:160–168
Peñuelas J, Canadell JG, Ogaya R (2011) Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob Ecol Biogeogr 20(4):597–608
Phillips N, Bergh J, Oren R, Linder S (2001) Effects of nutrition and soil water availability on water use in a Norway spruce stand. Tree Physiol 21(12–13):851–860
Raven JA, Handley LL, Wollenweber B (2004) Plant nutrition and water use efficiency. In: Bacon MA (ed) Water use efficiency in plant biology. Blackwell Publishing Ltd, Oxford, pp 171–197
Ripullone F, Lauteri M, Grassi G, Amato M, Borghetti M (2004) Pseudotsuga menziesii and Populus x euroamericana: a comparison of three approaches to determine water-use efficiency. Tree Physiol 24(6):671–679
Sardans J, Peñuelas J (2012) The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol 160:1741–1761
Tsunekawa A, Liu G-B, Yamanaka N, Du S (2014) Restoration and Development ofthe Degraded Loess Plateau. Springer Science, China
Stubbs WJ, Wilson JB (2004) Evidence for limiting similarity in a sand dune community. J Ecol 92(4):557–567
Su B-Q, Shangguan Z-P (2020) Patterns and driving factors of water and nitrogen use efficiency in Robinia pseudoacacia L. on the Loess Plateau in China. CATENA 195:104790
Su B-Q, Shangguan Z-P (2021) Response of water use efficiency and plant-soil C:N: P stoichiometry to stand quality in Robinia pseudoacacia on the Loess Plateau of China. CATENA 206:105571
Tanaka-Oda A, Kenzo T, Koretsune S, Sasaki H, Fukuda K (2010) Ontogenetic changes in water-use efficiency (δ13C) and leaf traits differ among tree species growing in a semiarid region of the Loess Plateau, China. For Ecol Manag 259(5):953–957
Taylor BN, Chazdon RL, Bachelot B, Menge DNL (2017) Nitrogen-fixing trees inhibit growth of regenerating Costa Rican rainforests. Proc Natl Acad Sci U S A 114(33):8817–8822
ter Braak T, Smilauer P (2012) Canoco reference manual and user's guide: software for ordination, version 5.0. Microcomputer Power, Ithaca, 496
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82(4):946–954
Turner J, Lambert MJ (2014) Analysis of nutrient use efficiency (NUE) in Eucalyptus pilularisforests. Aust J Bot 62(7):558–569
Vitousek PM (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119(4):553–572
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20(1):5–15
Voigtlaender M, Laclau JP, Gonçalves JLM, Piccolo MC, Moreira MZ, Nouvellon Y, Ranger JP, Bouillet JP (2012) Introducing Acacia mangium trees in Eucalyptus grandis plantations: consequences for soil organic matter stocks and nitrogen mineralization. Plant Soil 352:99–111
Wang J, Fu B-J, Wang L-X, Lu N, Li J-Y (2020) Water use characteristics of the common tree species in different plantation types in the Loess Plateau of China. Agric For Meteorol 288–289:108020
Wang J, Fu B-J, Jiao L, Lu N, Li J-Y, Chen W-L, Wang L-X (2021) Age-related water use characteristics of Robinia pseudoacacia on the Loess Plateau. Agric for Meteorol 301–302:108344
Wei J-S, Li Z-S, Feng X-Y, Zhang Y, Chen W-L, Wu X, Jiao L, Wang X-C (2018) Ecological and physiological mechanisms of growth decline of Robinia pseudoacacia plantations in the Loess Plateau of China: a review. Chin J Appl Ecol 29(7):2433–2444
Wilkinson S, Bacon MA, Davies WJ (2007) Nitrate signalling to stomata and growing leaves: interactions with soil drying. ABA, and xylem sap pH in maize. J Exp Bot 58(7):1705–1716
Wullschleger SD, Wilson KB, Hanson PJ (2000) Environmental control of whole-plant transpiration, canopy conductance and estimates of the decoupling coefficient for large red maple trees. Agric for Meteorol 104(2):157–168
Xu R, Wang L-Y, Zhang J, Zhou J, Cheng S-D, Tigabu M, Ma X-Q, Wu P-F, Li M (2022) Growth rate and leaf functional traits of four broad-leaved species underplanted in Chinese fir plantations with different tree density levels. Forests 13(2):308
Yan W-M, Zhong Y-Q-W, Zheng S-X, Shangguan Z-P (2016) Linking plant leaf nutrients/stoichiometry to water use efficiency on the Loess Plateau in China. Ecol Eng 87:124–131
Funding
This study was funded by the National Nature Science Foundation of China (No. 41771556). JP and JS were supported by the Spanish Government Grants PID2020-115770RB-I00 and TED2021-132627B-I00 funded by the Spanish MCIN, AEI/10.13039/501100011033 and the European Union Next Generation EU/PRTR, the Catalan government grant SGR2021-1333 and the Fundación Ramón Areces grant CIVP20A6621.
Author information
Authors and Affiliations
Contributions
SBL and YMC conceived and designed this study. SBL performed the fieldwork, analyzed the data, and wrote the original manuscript. YMC guided the paper. JS and JP provided technical and theoretical support and constructive suggestions. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Mian Gu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, S., Chen, Y., Sardans, J. et al. Water and nutrient use efficiency of three tree species in monoculture and mixed stands and potential drivers in the Loess Hilly Region, China. Plant Soil 496, 657–675 (2024). https://doi.org/10.1007/s11104-023-06389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06389-6