Abstract
Background and Aims
The commonly observed trade-off between plant water use efficiency (WUE) and nitrogen use efficiency (NUE) has been attributed to physiological constraints in the leaf. We examined if a similar trade-off can occur between WUE and phosphorus use efficiency (PUE) and if changes in NUE and PUE in response to water and nutrient supply can be related to microbial N and P immobilisation.
Methods
We studied water and nutrient use efficiencies in leaves of four tree species (Eucalyptus sideroxylon, Eucalyptus tereticornis, Casuarina cunninghamiana, and Pinus radiata) that were grown under rainout shelters for 16 months at low and high levels of water and nutrient supply.
Results
Across all four species, WUE increased when water supply was low and nutrient supply was high, while NUE increased when water supply was high and nutrient supply was low. As a consequence, a trade-off was found between WUE and NUE for all four species. In contrast, no trade-off was found between WUE and PUE, likely because PUE and microbial P immobilisation in the soil unexpectedly increased with high nutrient supply.
Conclusions
With variable water and nutrient supply, physiological constraints generate a trade-off between WUE and NUE, but not between WUE and PUE; the latter may have been obscured by microbial control over P supply to plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When water and nutrients are scarce, efficient use of these resources may improve plant growth and chances of survival (Aerts and Chapin III 2000; Tilman 1988). Plants can respond to conditions of low water availability by transpiring less water per unit carbon fixed and biomass produced (i.e., they have a high water use efficiency, WUE, Ehleringer and Cooper 1988; Toft et al. 1989). Likewise, in environments with low nitrogen (N) availability plants often fix more carbon and produce more biomass per unit of N (i.e., they have a high N use efficiency, NUE, Aerts and Chapin III 2000; Vitousek 1982). However, plants often decrease WUE with increased NUE and vice versa, suggesting a trade-off between WUE and NUE (Field et al. 1983; Gong et al. 2011; Patterson et al. 1997). This trade-off can be explained by physiological constraints in the leaf: when plants increase leaf intercellular CO2 concentration by opening their stomata, this increases photosynthesis per unit of leaf N, but also increases transpiration thereby reducing WUE (or plant δ13C, an integrative index of leaf-scale WUE, Farquhar and Richards 1984). Therefore, reduced leaf WUE induced by greater water availability frequently increases leaf NUE (Li et al. 2003; Reich et al. 1989). Similarly, when N availability increases, leaf tissue N increases (and NUE decreases), which enhances the photosynthetic capacity in leaves thereby increasing WUE (Livingston et al. 1999; Ripullone et al. 2004).
The trade-off between WUE and NUE in leaves has also been explained by N availability in the soil (Cramer et al. 2009). It was argued that when N availability is low, plants may enhance transpiration rates to increase N uptake through mass flow in the soil. As a result, WUE may decrease with increased NUE induced by conditions of lower soil N availability, at least when plant growth is not limited by water.
In contrast, there is no clear evidence for a trade-off between leaf WUE and phosphorus (P) use efficiency (PUE) (but see Brown et al. 2011). Addition of P had no effect on WUE in the tropical tree Ficus insipida (Garrish et al. 2010), while leaf PUE was unrelated to WUE in Pinus pinaster (Warren et al. 2005), or was even positively related with WUE in Populus and grassland species (DesRochers et al. 2006; Zhou et al. 2013). Increased leaf P concentrations in trees and lianas were explained by enhanced transpiration rates increasing mass flow of P (Cernusak et al. 2010; Cernusak et al. 2011), so that variation in transpiration rates may actually result in positive relationships between WUE and PUE.
In all of these studies, plant-soil interactions have not been considered. However, changes in water and nutrient availability in the soil may affect microbial mineralisation and immobilisation of N and P (Austin et al. 2004; Bünemann et al. 2012; Manzoni and Porporato 2007), with consequences for the absolute and relative amounts of N and P that can be taken up by plants. Microbial requirement for P is usually much higher than for N (Cleveland and Liptzin 2007), which could potentially result in imbalanced supply of N and P to plants (Jonasson et al. 1996). Therefore, changes in microbial activity due to variation in water and nutrient availability could affect plant N and P uptake, and thereby influence relationships among WUE, NUE and PUE.
Here we examined leaf δ13C, C/N and C/P ratios (as indices of leaf-scale WUE, NUE and PUE, respectively) in four tree species in response to changes in soil water and nutrient supply. The species were grown in local native forest soil in large 75 L bags for 16 months under rainout shelters with two levels of water supply (constantly watered vs. drought periods) and two levels of N and P fertiliser additions in a full factorial design. We then examined relationships between leaf δ13C and C/N, and between leaf δ13C and leaf C/P, and determined whether relationships could be explained solely by physiological constraints in the leaf, or also by changes in microbial N and P immobilisation.
Materials and methods
Experimental design
This study was conducted at a rainout shelter facility at Western Sydney University, Richmond, NSW, Australia (long 150°44′23″E, lat 33°36′39″S). Mean monthly maximum temperatures at the site are 29.6 °C in January and 17.2 °C in July. Mean annual precipitation is 813 mm. Seedlings of Eucalyptus sideroxylon (EUSI), Eucalyptus tereticornis (EUTE), Casuarina cunninghamiana (CACU, N-fixer), and Pinus radiata (PIRA) were planted in 75 L bags filled with 90–100 kg of soil (one seedling per bag). The soil was a locally-sourced loamy sand with a pH of 6.2, 14 g kg−1 organic C, 1.2 g kg−1 total N, and 0.24 g kg−1 total P. All plants were planted on 10 November 2011 under 6 rainout shelters. Seedlings that did not survive during the first month were replanted with spare seedlings grown under the same conditions. Each shelter is made of aluminium and is 12 m long × 8 m wide × 7 m tall. Each shelter possesses a light-transparent retractable roof which closes during a rainfall event. When rainfall is detected, UV transparent curtains unfurl across the shelter roof, and along the sides of the shelter, preventing rain from falling into the shelter. A 1 m gap at the bottom of the shelter maintains air flow when the curtains are deployed to minimise impacts on ambient environmental conditions. After a rainfall event, the curtains return to their retracted position so that temperature and humidity conditions return to ambient field conditions.
For each species, there were 4 bags in each shelter where one bag was designated for each of the following treatments: low water and low nutrient supply (LW-LN), low water and high nutrient supply (LW-HN), high water and low nutrient supply (HW-LN), and high water and high nutrient supply (HW-HN). A total of 96 bags were included in the experiment (4 species × 2 water treatments × 2 nutrient treatments × 6 replicates). To prevent nutrient stress and insect damage, all bags received fertiliser on 2 May 2012. Bags with low nutrient supply received 0.37, 0.16, 0.43, and 0.25 g bag−1 of N, P, K, and S, respectively (corresponding to 2.2, 0.9, 2.6 and 1.5 g m−2), and bags with high nutrient supply received 0.62, 0.26, 0.73, and 0.42 g bag−1 of N, P, K, and S, respectively (3.8, 1.6, 4.4 and 2.5 g m−2) as Nitrophoska Special®. Bags with the high nutrient supply treatment received another 2.4, 1.0, 2.8, and 1.6 g m−2 of N, P, K, and S on 18 September 2012, and 2.6, 1.1, 3.0, and 1.7 g m−2 of N, P, K, and S on 21 December 2012. A total of 9.0, 3.7, 10.4, and 5.8 g m−2 of N, P, K, and S were added to the bags with the high nutrient supply treatment, or 4 times the amount added to the bags with the low nutrient supply treatment. Initially, soil moisture was maintained at field capacity in all bags by watering bags on a daily basis. Bags with the low water supply treatment received reduced water during the period 8 October – 2 November 2012 (first dry-down), 19 November – 21 December 2012 (second dry-down), and after 28 January 2013 until drought-induced plant mortality (third dry-down). On all other dates, soil moisture was maintained at conditions similar to the high water supply treatment. Volumetric soil moisture was monitored for each species in the low and high water supply treatments with high nutrient availability (n = 5) using TDR probes (Campbell Scientific, Townsville, QLD Australia, Fig. 1).
Volumetric soil moisture content between August 2012 and May 2013 in C. cunninghamiana (a), E. sideroxylon (b), E. tereticornis (c), and P. radiata (d). Soil moisture (average of 5 replicates for each species, error bars represent 95 % confidence intervals) is shown for high water supply (black circles) and low water supply (white circles). In November 2012 moisture probes were moved from the top of the bags to the base of the bags
Sampling and analyses
Green leaf samples from the mid-canopy of each tree were taken at the end of the second dry-down on 18 December 2012 and during the third dry-down on 25 March 2013. Leaf samples were dried (60 °C for 72 h), ground and analysed for C, 13C, and N on an isotope ratio mass spectrometer (IRMS, Thermo-Fisher Scientific, Bremen, Germany). Plant sub-samples were ashed (550 °C in a muffle furnace), dissolved in 6 N HCl, filtered (Whatman 42 filter paper), diluted with water, and analysed for P using the ammonium molybdate-vanadate reagent (Jackson 1958).
We used leaf δ13C values as a measure of leaf-scale WUE, where more negative values indicate lower WUE (Farquhar and Richards 1984). Green leaf C/N and C/P ratios were used as a measure of N and P use efficiencies (where high C/N and C/P reflect high NUE and PUE, respectively). The C/N and C/P ratios in senesced leaves would be a more accurate measure of plant nutrient use efficiency in perennial plants (Vitousek 1982). However, there was little leaf senescence during the period we sampled, and we therefore used green leaf C/N and C/P ratios as a proxy for leaf-scale NUE and PUE respectively. WUE, NUE and PUE in plants have been determined at different levels, including the whole plant, aboveground biomass, or photosynthetic (e.g., photosynthetic NUE). In our study we only measured the WUE, NUE and PUE at the leaf-scale, which may differ from the whole plant scale (Aerts and Chapin 2000).
Soil from each bag was sampled on the same days when leaf samples were taken. The top 20 cm of the soil was taken with a soil corer. Soils were sieved (4 mm) and roots that fell through were removed. Sub-samples were analysed for soil moisture (72 h oven drying at 60 °C). Microbial biomass N and P were measured using the fumigation-extraction technique (Brookes et al. 1985; Brookes et al. 1982). For microbial biomass N, 20 g of moist soil was fumigated with chloroform in a vacuum desiccator for 72 h. We added 40 mL of 0.05 M K2SO4 to 20 g of fumigated and non-fumigated soil, which was then shaken for an hour, and filtered (Whatman 42 filter paper). Extracts were analysed for total N on a total organic C analyser with an N measuring unit attached (TOC-VCSH, TNM-1, Shimadzu Scientific Instruments, Kyoto, Japan). Extracts from non-fumigated soils were also analysed for NH4 + and NO3 − on a Flow Injection Analyser (FIA automated ion analyzer, Lachat Instruments, Loveland, CO, USA). For microbial P, 5 g of moist soil was fumigated with chloroform in a vacuum desiccator for 24 h. Fumigated and non-fumigated samples were extracted with 0.03 M NH4F – 0.025 N HCl. The P concentration was measured colorimetrically using the ammonium molybdate–stannous chloride reagent (Olsen and Sommers 1982). Microbial N and P were calculated as the difference in N and P concentrations between fumigated and non-fumigated samples divided by extraction efficiencies of 0.54 and 0.4, respectively (Brookes et al. 1985; Brookes et al. 1982). To correct for P-sorption during fumigation and extraction, 100 μg P was added to five separate soil samples and recovery of P in the extracts was measured (Brookes et al. 1982). Microbial biomass N and P were expressed per kg dry soil.
We used the extractable NH4 +, NO3 − and P in the non-fumigated extracts as a measure of soil N and P availability at the time of sampling. We further used Plant Root Simulator resin probes (Western Ag Innovations, Saskatoon, CA, USA) as an integrative measure of soil N and P availability (PRS-N and PRS-P respectively) during the dry-rewetting cycles (Dijkstra et al. 2012). Probes were inserted at the start of the first dry-down (8 October 2012) at 2–7.6 cm soil depth (2 cation probes for NH4 + absorption and 2 anion probes for NO3 − and PO4 3− absorption in each soil bag) and retrieved on 25 March 2013, at the same time that the second set of soil samples were taken. Probes were washed with deionised water and extracted with 0.5 N HCl (2 cation and anion probes combined). The extractants were analysed for NH4 + and NO3 − colorimetrically on a flow injection analyser (Seal Analytical, Mequon, WI, USA) and for P using inductively coupled plasma emission spectroscopy (Perkin Elmer, Inc., Waltham, MA, USA).
Immediately after planting and on 25 March 2013 (during the third dry-down), tree height and stem diameter 5 cm above the soil surface were measured for each tree. Total plant biomass at both dates was then calculated using species-specific allometric equations. The equations were developed by harvesting a random sample of twelve trees from each species at four time points (19 April and 27 August 2012, 4 February, and 1 May 2013) over the course of the experiment. The first two harvests occurred before treatments were initiated. At each harvest, tree height (cm) and stem diameter (mm) 5 cm above the soil surface were measured, and each tree was cut at the soil surface. The aboveground portion was separated into leaves, stems, and branches, and the entire root system was washed free of soil. All components were dried at 70 °C for one week, and total tree biomass was calculated as the sum of the dry mass of each biomass component.
CACU, EUSI, and EUTE showed an exponential relationship between total biomass and stem volume index (cm3, stem diameter2 × tree height). For these species, allometric equations were developed by fitting a model of the natural logarithm of total tree biomass against the natural logarithm of stem volume index:
where Y is total tree biomass, X is stem volume index, a and k are intercept and slope regression coefficients, and ε is the random error. For each species, analysis of covariance was used to test for water supply effects on the allometric coefficients. In all cases, water supply had no significant effect (P ≤ 0.05) on the allometric coefficients, and data from both water treatments were combined into one model for each species. Corrections for logarithmic bias were made when back-transforming estimates of total tree biomass (Baskerville 1972). PIRA showed a linear relationship between total biomass and stem volume index instead of an exponential relationship. For this species, water supply significantly affected the slope parameter of the linear model (P = 0.01), but not the intercept parameter (P = 0.55). Thus, the allometric models for the low and high water treatments shared a common intercept, but possessed different slope parameters. All analyses were conducted in SAS v9.3. Total plant biomass gain during the experiment was calculated as the difference in total plant biomass at the start and end of the experiment.
We used ANOVA to test for main effects of species (EUSI, EUTE, CACU and PIRA), water supply (low and high water supply), nutrient supply (low and high nutrient supply), date (18 December 2012 and 25 March 2013), and their interactive effects on leaf C/N, C/P, and δ13C, soil moisture, soil extractable inorganic N and P, and microbial N and P. A nested model was used with the species, water and nutrient supply treatments as between-bags factors, and date as the within-bag factor. Rainout shelter was included as a random block effect. A similar ANOVA was used for PRS-N and -P, and total plant biomass gain, but with the main and interactive effects of date excluded. In some cases, data were log-transformed to fulfil assumptions of normality. When species effects were significant (P < 0.05), we examined how species differed from each other using Tukey’s HSD post-hoc test. While date effects were often significant, interactive effects with species, water and nutrient supply were rarely significant. We therefore present our results as averages across the two dates. All ANOVAs and post-hoc tests were performed with JMP (version 4.0.4; SAS Institute, Cary, NC, USA). Relationships between leaf δ13C and leaf C/N and C/P were investigated for each species, and across water and nutrient supply treatments using type II regressions. We used the standardised major axis method (sma function) in the SMATR package in R to examine whether the slope of the type II regressions differed among species and among the four water and nutrient supply treatment combinations (Warton et al. 2012). Relationships between total plant biomass gain and leaf C/N, C/P and δ13C and differences in slope among species and water and nutrient treatments were investigated in a similar way.
Results
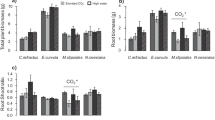
Water supply effects on leaf C/N were species-dependent (water supply × species interaction, P = 0.0005, Fig. 2a). In particular, high water supply increased leaf C/N in EUSI and PIRA, but not in EUTE and CACU. Water supply had no effect on leaf C/P (Fig. 2b), but high water supply decreased leaf N/P in EUSI, but not in the other species (water supply × species interaction, P = 0.02, Fig. 2c).
Mean leaf C/N (a), leaf C/P (b), and leaf N/P (c). Measurements on two dates were averaged. EUSI: E. sideroxylon, EUTE: E. tereticornis, CACU: C. cunninghamiana, PIRA: P. radiata. LW-LN: low water and low nutrient, LW-HN: low water and high nutrient, HW-LN: high water and low nutrient, HW-HN: high water and high nutrient. Error bars represent ±1 standard error. ANOVA P-values are shown when significant (P < 0.05, W: watering supply treatment, N: nutrient supply treatment, Sp: species treatment). Letters above bars indicate differences among species (Tukey’s HSD post-hoc test)
As expected, high nutrient supply caused a significant reduction in leaf C/N (on average by 9 %, P = 0.01, Fig. 2a), but increased leaf C/P (on average by 13 %, P = 0.01, Fig. 2b). As a consequence, leaf N/P significantly increased with high nutrient supply (on average by 20 %, P = 0.0002, Fig. 2c). Leaf C/N was on average lowest and leaf N/P highest for CACU (the only N-fixer), while leaf C/P was on average lowest for PIRA.
We observed a significant water supply × nutrient supply interaction (P = 0.006) on leaf δ13C (Fig. 3). Leaf δ13C increased strongly under high nutrient supply, but only when water supply was low (Fig. 3). Species varied in leaf δ13C, with EUSI showing the lowest values on average (this species also had the highest leaf C/N and C/P).
Mean leaf δ13C. Measurements on two dates were averaged. For treatment abbreviations, see Fig. 2. Error bars represent ±1 standard error. ANOVA P-values are shown when significant (P < 0.05). Letters above bars indicate differences among species (Tukey’s HSD post-hoc test)
As expected, we observed a trade-off between leaf δ13C and leaf C/N. Leaf δ13C values measured on two separate dates were negatively related to leaf C/N. This negative relationship was significant for all four species, where the slope for CACU was significantly steeper than for the other three species (Fig. 4a, P < 0.05). Likewise, the negative relationship was significant for all four water and nutrient supply treatments, where the LW-LN treatment showed a steeper slope compared to the HW-LN and HW-HN treatments (Fig. 4c, P < 0.05). In contrast, we observed no trade-off between leaf δ13C and leaf C/P (Fig. 4b, d). Instead, significant positive relationships between leaf δ13C and leaf C/P were observed for EUSI and CACU (Fig. 4b).
Relationships between leaf C/N and leaf δ13C (a, c) and between leaf C/P and leaf δ13C (b, d). Each data point represents one measurement on a specific date. Relationships are shown for the different species across the watering and nutrient supply treatments (a, b), and for the four water and nutrient supply treatment combinations across species (c, d). Only significant regression lines are shown (P < 0.05). For species and water and nutrient supply treatment abbreviations, see Fig. 2
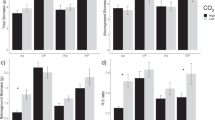
Gravimetric soil moisture content of the collected soil samples (average of two dates) was significantly higher in the high than in the low water supply treatment (P < 0.0001), and significantly lower in the high than in the low nutrient supply treatment (P < 0.0001, Table 1). Water supply had no effect on available N and P measured in the soil; extractable concentrations and PRS-N and PRS-P were unaffected by the watering treatment. High nutrient supply also had no effect on available N, but significantly increased available P in the soil (average increase of 86 % in extractable P and 98 % in PRS-P, P = 0.0002 and P = 0.002, respectively). It is noteworthy that the lowest values of PRS-N were observed for the N-fixing CACU, suggesting this species was very efficient at taking up available N from the soil. In contrast to soil available N, microbial N significantly increased with high water supply (on average by 56 %, P < 0.0001) and high nutrient supply (14 %, P = 0.04, Fig. 5). High nutrient supply also more than doubled microbial P (average increase of 116 %, P = 0.0009), so that microbial N/P declined with high nutrient supply (on average by 40 %, P = 0.02).
Mean microbial N (a), microbial P (b) and microbial N/P (c). Measurements on two dates were averaged. For treatment abbreviations, see Fig. 2. Error bars represent ±1 standard error. ANOVA P-values are shown when significant (P < 0.05). Letters above bars indicate differences among species (Tukey’s HSD post-hoc test)
Across species and nutrient supply treatments, total plant biomass gain was higher for trees maintained at high water supply compared to low water supply (on average by 17 %, P = 0.05), although for specific species-nutrient supply treatments this increase was not apparent (e.g., no increase for CACU under low nutrient supply, and no increase for PIRA). Total plant biomass gain was higher for trees growing under high nutrient supply compared to low nutrient supply (on average by 136 %, P < 0.0001, Fig. 6). Overall, the highest total plant biomass gains were observed for CACU. For this species, leaf C/N decreased in larger trees, while no relationships were observed for the other species (Fig. 7a). In contrast, leaf C/P showed a positive relationship with total plant biomass gain for EUTE and CACU (Fig. 7b), while leaf N/P increased with total plant biomass gain for EUSI and CACU (Fig. 7c). Leaf δ13C was also positively related with total plant biomass gain for EUSI and CACU (Fig. 7d).
Mean total plant biomass at the end of the experiment. For treatment abbreviations, see Fig. 2. Error bars represent ±1 standard error. ANOVA P-values are shown when significant (P < 0.05). Letters above bars indicate differences among species (Tukey’s HSD post-hoc test)
Relationships between total plant biomass and leaf C/N (a), leaf C/P (b), leaf N/P (c), and leaf δ13C (d) at the end of the experiment. Relationships are shown for the different species across the watering and nutrient supply treatments. Only significant regression lines are shown (P < 0.05). For species abbreviations, see Fig. 2
Discussion
By varying water and nutrient supply, we observed a trade-off between leaf δ13C and leaf C/N (indices of leaf-scale WUE and NUE, respectively) for all four tree species, and across the water and nutrient supply treatments. High water supply decreased leaf δ13C (and thus WUE) and increased leaf C/N (and thus NUE), while high nutrient supply increased leaf δ13C and decreased leaf C/N. This trade-off has been explained by leaf-level physiological constraints where high rates of photosynthesis per unit of leaf N (high NUE) can only occur when stomata are open and plants incur large water losses through transpiration (low WUE, Livingston et al. 1999; Patterson et al. 1997; Ripullone et al. 2004).
It is unlikely that the trade-off between NUE and WUE was regulated by plant transpiration rates in response to variable soil available N. Transpiration affects mass flow of nutrients (NO3 − in particular) through the soil to the roots. Cramer et al. (2009) suggested that plants can compensate for decreasing soil NO3 − concentrations by increasing transpiration to meet their N demand. A decrease in soil NO3 − availability will increase NUE, while an increase in transpiration will decrease WUE. We expected that the trade-off between NUE and WUE caused by this mechanism would most likely occur when transpiration was not limited by soil moisture, i.e., in the high water supply treatment. However, this was not observed as all four water and nutrient supply treatment combinations showed significant negative relationships between leaf C/N and leaf δ13C, while the slope was steepest for the LW-LN treatment (Fig. 4c). While plant regulation of transpiration in response to N supply may have occurred, its role for causing the NUE-WUE trade-off was most likely small.
We did not observe a trade-off between WUE and PUE across all treatments. Water supply did not affect C/P, while high nutrient supply increased leaf C/P and N/P, which caused the lack of a trade-off between WUE and PUE. The increase in leaf C/P and N/P with high nutrient supply was surprising, since the N/P ratio of the fertiliser added (2.4) was much lower than the N/P ratios measured in leaf tissue (ranging between 4 and 34), suggesting that for plant growth ample P was added compared to N. In two of the four species, leaf N/P increased with increased total plant biomass gain (Fig. 7c). The growth rate hypothesis predicts that faster growing plants should have lower N/P ratios because of a high requirement of P-rich RNA relative to N-rich proteins (Matzek and Vitousek 2009; Sterner and Elser 2002). Our observation of increased leaf N/P with increased total plant biomass gain for EUSI and CACU suggests that as plants grew bigger their growth became increasingly more constrained by P availability.
The increase in leaf C/P and N/P in response to high nutrient supply may have been a result of increased microbial P immobilisation with nutrient addition (Fig. 5b) that may have reduced P supply to plants compared to N supply. Microbial P increased more than microbial N in response to high nutrient supply resulting in significantly lower microbial N/P (Fig. 5c). Thus, high nutrient supply of N and P increased microbial P immobilisation more than N, thereby potentially reducing the supply of P to plants relative to the supply of N. Microbial N/P ratios were lower than plant N/P ratios across a wide range of ecosystems (Cleveland and Liptzin 2007), suggesting that the requirement of P relative to N is generally higher for soil microbes than for plants. Furthermore, the total amount of P stored in soil microbial biomass can be substantially higher than the P stored in plants in terrestrial ecosystems (Jonasson et al. 1999; Turner et al. 2013), suggesting that even small changes in microbial P storage can have large effects on P supply to plants. Microbial control over P supply to plants may therefore have resulted in the absence of a trade-off between PUE and WUE in response to the nutrient supply treatment. On the other hand, we observed that extractable P and PRS-P increased with high nutrient supply, particularly in the low water supply treatment (Table 1). However, these indices of soil available P may not reflect what plants experienced; plants may not have had access to the greater amount of ‘available’ P, particularly under dry conditions when soil P mobility is strongly reduced (Lambers et al. 2008).
Instead of a trade-off, we observed positive relationships between PUE and WUE for EUSI and CACU across watering and nutrient supply treatments (Fig. 4b). Cernusak et al. (2011) found positive relationships between leaf P/C (inverse of leaf C/P) and transpiration rates among four tropical tree seedlings, and suggested that these plants controlled the rate of P uptake from soil through transpiration. This could potentially help explain the positive relationship between PUE and WUE we observed for EUSI and CACU. These two species showed a positive relationship between leaf δ13C and total plant biomass (Fig. 7d), suggesting that they transpired less water per unit of leaf C with increased plant biomass, thereby taking up less P through mass flow as well. However, because of its low mobility in the soil, P is mostly taken up through diffusion rather than through mass flow (Lambers et al. 2008). Therefore, a low transpiration rate may not be the predominant cause of a low leaf P concentration. Rather, more C input by larger plants of EUSI and CACU may have increased the microbial demand for P (Helal and Sauerbeck 1984), which may have increased microbial P immobilisation, thereby reducing P supply to plants. Therefore, microbial control over P supply to plants may have played a larger role in driving the positive relationship between PUE and WUE observed for EUSI and CACU, rather than transpiration rates controlling P uptake.
We observed a trade-off between leaf-level NUE and WUE for all four tree species, including three angiosperms, a gymnosperm and a biological N-fixer, and under conditions of low and high water and nutrient supply. Our results support the microeconomics framework by Wright et al. (2003). They suggested that plants minimise costs associated with uptake and use of water and N for photosynthesis, so that in environments with low water availability, costs associated with N acquisition decrease compared to costs associated with water acquisition. Likewise, in environments with low N availability, water acquisition becomes less expensive compared to N acquisition. Because we measured NUE and WUE at the leaf level, we do not know if this trade-off is maintained at the whole plant level. Allocation of N and P may differ within the plant (Garrish et al. 2010; Güsewell 2004), and therefore the relationship between leaf-level NUE and WUE may not be the same at the whole plant level. Interestingly, the strongest WUE-NUE trade-off was observed for the N-fixing CACU (steepest slope, Fig. 4a), suggesting that the trade-off for this species was affected by costs associated with biological N fixation, and that these latter costs were not the same as costs associated with N uptake from the soil. We are not aware of other studies where a WUE-NUE trade-off was observed for an N-fixing species. The WUE-NUE trade-off could further imply that more moist conditions, where WUE is reduced, could slow down the N cycle. Plants with high NUE (induced by low WUE) and low tissue N concentration in litter tend to reduce N mineralisation in the soil and availability to plants (Hobbie 1992). Thus, an increase in NUE, induced by a decrease in WUE, could help explain the increased N limitation observed along a precipitation gradient in the Central Great Plains of North America (McCulley et al. 2009). However, it is unclear if the negative relationship between δ13C and C/N observed in green tissue persists in senesced plant tissue.
In contrast to NUE, WUE-PUE trade-offs were absent. In our nutrient-poor soil, leaf P concentrations and PUE may be more strongly controlled by soil microbial processes than by leaf-level processes. Subsequently, our results did not support the growth rate hypothesis, which predicts a decrease in leaf N/P with faster plant growth (Sterner and Elser 2002). The growth rate hypothesis assumes that leaf N and P concentrations are controlled by biochemical processes in the leaf that determine the relative amount of N- and P-containing compounds needed for growth. But, as Matzek and Vitousek (2009) have shown, other factors, such as the relative supply of N and P to plants may affect leaf N/P ratios that obscure relationships with plant growth. We conclude that with variable water and nutrient supply, leaf-level NUE-WUE trade-offs can be explained by physiological constraints in the leaf, whereas leaf P concentrations were affected by microbially-mediated P supply in the soil that may have obscured a PUE-WUE trade-off.
References
Aerts R, Chapin F III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329
Brown KR, Courtin PJ, Negrave RW (2011) Growth, foliar nutrition and δ13C responses of red alder (Alnus rubra) to phosphorus additions soon after planting on moist sites. For Ecol Manag 262:791–802
Bünemann EK, Oberson A, Liebisch F, Keller F, Annaheim KE, Huguenin-Elie O, Frossard E (2012) Rapid microbial phosphorus immobilization dominates gross phosphorus fluxes in a grassland soil with low inorganic phosphorus availability. Soil Biol Biochem 51:84–95
Cernusak LA, Winter K, Turner BL (2010) Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytol 185:770–779
Cernusak LA, Winter K, Turner BL (2011) Transpiration modulates phosphorus acquisition in tropical tree seedlings. Tree Physiol 31:878–885
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a "Redfield ratio" for the microbial biomass? Biogeochemistry 85:235–252
Cramer MD, Hawkins HJ, Verboom GA (2009) The importance of nutritional regulation of plant water flux. Oecologia 161:15–24
Delgado-Baquerizo et al (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502: 672–676
DesRochers A, van den Driessche R, Thomas BR (2006) NPK fertilization at planting of three hybrid poplar clones in the boreal region of Alberta. For Ecol Manag 232:216–225
Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM, Carrillo Y, LeCain DR, Follett RF, Williams DG (2012) Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol 196:807–815
Ehleringer JR, Cooper TA (1988) Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 76:562–566
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Field C, Merino J, Mooney HA (1983) Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384–389
Garrish V, Cernusak LA, Winter K, Turner BL (2010) Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree, Ficus insipida. J Exp Bot 61:3735–3748
Gong X, Chen Q, Lin S, Brueck H, Dittert K, Taube F, Schnyder H (2011) Tradeoffs between nitrogen- and water-use efficiency in dominant species of the semiarid steppe of Inner Mongolia. Plant Soil 340:227–238
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Helal HM, Sauerbeck DR (1984) Influence of plant roots on C and P metabolism in soil. Plant Soil 76:175–182
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339
Jackson ML (1958) Soil chemical analsysis. Prentice-Hall, Inc., Englewood Cliffs, NJ
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV, Callaghan TV (1996) Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar: implications for plant nutrient uptake. Oecologia 106:507–515
Jonasson S, Michelsen A, Schmidt IK (1999) Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Appl Soil Ecol 11:135–146
Lambers H, Chapin FS III, Pons TL (2008) Plant physiological ecology. Springer Science, New York, NY, USA
Li F, Kang S, Zhang J, Cohen S (2003) Effects of atmospheric CO2 enrichment, water status and applied nitrogen on water- and nitrogen-use efficiencies of wheat. Plant Soil 254:279–289
Livingston NJ, Guy RD, Sun ZJ, Ethier GJ (1999) The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ 22:281–289
Manzoni S, Porporato A (2007) A theoretical analysis of nonlinearities and feedbacks in soil carbon and nitrogen cycles. Soil Biol Biochem 39:1542–1556
Matzek V, Vitousek PM (2009) N:P stoichiometry and protein:RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis. Ecol Lett 12:765–771
McCulley RL, Burke IC, Lauenroth WK (2009) Conservation of nitrogen increases with precipitation across a major grassland gradient in the Central Great Plains of North America. Oecologia 159:571–581
Olsen SR, Sommers LE (1982) Phosphorus. In: Pace AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties. 2nd edition edn. American Society of Agronomy, Inc., Madison, WI, pp 403–430
Patterson TB, Guy RD, Dang QL (1997) Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 110:160–168
Reich PB, Walters MB, Tabone TJ (1989) Response of Ulmus americana seedlings to varying nitrogen and water status. 2 Water and nitrogen use efficiency in photosynthesis. Tree Physiol 5:173–184
Ripullone F, Lauteri M, Grassi G, Amato M, Borghetti M (2004) Variation in nitrogen supply changes water-use efficiency of Pseudotsuga menziesii and Populus x euroamericana; a comparison of three approaches to determine water-use efficiency. Tree Physiol 24:671–679
Sterner RW, Elser JJ (2002) Ecological stoichiometry. the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Tilman D (1988) Plant strategies and the dynamics and structure of plant communities. Princeton University Press, Princeton
Toft NL, Anderson JE, Nowak RS (1989) Water use efficiency and carbon isotope composition of plants in a cold desert environment. Oecologia 80:11–18
Turner B, Lambers H, Condron L, Cramer M, Leake J, Richardson A, Smith S (2013) Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 367:225–234
Vitousek PM (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119:553–572
Warren CR, McGrath JF, Adams MA (2005) Differential effects of N, P and K on photosynthesis and partitioning of N in Pinus pinaster needles. Ann For Sci 62:1–8
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) SMATR 3 - an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259
Wright IJ, Reich PB, Westoby M (2003) Least-cost input mixtures of water and nitrogen for photosynthesis. Am Nat 161:98–111
Zhou YC, Fan JW, Harris W, Zhong HP, Zhang WY, Cheng XL (2013) Relationships between C3 plant foliar carbon isotope composition and element contents of grassland species at high altitudes on the Qinghai-Tibet Plateau, China. PLoS One 8:e60794
Acknowledgments
This work was supported by a grant from the Australian Research Council (FT100100779) awarded to F.A.D. and ARC grants (DP0879531 and LP0989881) to D.T.T.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Harry Olde Venterink.
Rights and permissions
About this article
Cite this article
Dijkstra, F.A., Carrillo, Y., Aspinwall, M.J. et al. Water, nitrogen and phosphorus use efficiencies of four tree species in response to variable water and nutrient supply. Plant Soil 406, 187–199 (2016). https://doi.org/10.1007/s11104-016-2873-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2873-6