Abstract
Background and aims
Spatial heterogeneity in soil can influence the distribution of plant communities because plants differ in their ability to acquire resources. In nutrient-poor soils, plants should bear traits that prioritize conservation of resources over growth, whereas the opposite is expected in nutrient-rich soils. A coordinated response to soil fertility, in which leaves and roots converge into the same strategy, would lead to a community-level plant economics spectrum (PEScom). Here, we examine how nutrient availability across different soil types shapes the functional structure of desert shrub communities and how much of this variation is explained by species turnover or intraspecific variability (ITV).
Methods
We measured leaf and root traits of 16 shrub species in four sites with contrasting soil nutrient availability in the southern edge of the Atacama Desert.
Results
In leaves, changes in soil nutrient availability were associated with a leaf economics spectrum, shifting from communities with more acquisitive strategies to communities with more conservative strategies with increasing nutrient limitation. No such pattern of co-variation was observed for roots; thus, the relationship between above and below-ground traits did not fit the PEScom. Variation of leaf traits was mainly determined by species turnover, while for root traits it was determined equally by species turnover and ITV.
Conclusion
The lack of a PEScom appears to be related to the co-limitation of multiple resources, which promotes different responses in root traits. We highlight the importance of incorporating leaf and root traits to understand how multiple resource limitation shapes plant ecological strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding patterns of variation in soil nutrients across landscapes and their relationship with patterns of plant distribution has become the focal point of much ecological research (Fu et al. 2004; Stevens and Carson 2002; El-keblawy et al. 2015). Landscape-level variation in soil resource availability can change due to variation in soil properties resulting from the nature of the parent material (Yavitt 2000; Augusto et al. 2017), or from variation in edaphic factors that occur during soil development (Walker and Syers 1976; Vitousek and Howarth (1991) ; Coomes et al. 2013). Such spatial heterogeneity in soil nutrients affects the distribution of plant communities (Maestre and Reynolds 2006) because plants differ in their ability to acquire and exploit soil resources (Grime 1977; Aerts and Chapin 2000a). In the context of the trade-off between acquisition and conservation of resources, contrasting nutrient availabilities can lead to changes in plant resource acquisition strategies, which are reflected by relationships among functional traits in both leaves (leaf economics spectrum [LES], Wright et al. 2004) and roots (root economics spectrum [RES], Roumet et al. 2016). Plant species growing in nutrient-poor soils are expected to bear leaf and root traits that allow them to prioritize conservation of resources over growth (e.g., low SLA, low SRL and low nutrient concentrations, with low uptake capacity, high nutrient-use efficiency and longer-lived organs); in contrast, in nutrient-rich soils plants should favor traits that enable faster resource acquisition and growth at the cost of a lower resource conservation (e.g., high SLA, high nutrient concentrations and short-lived organs, Chapin et al. 1993; Aerts and Chapin 2000b; Westoby et al. 2002; Comas and Eissenstat 2004). A coordinated response between leaf and root traits to changes in soil resources should thus lead to a “plant economics spectrum” (PES), in which all plant organs converge into the same strategy (Freschet et al. 2010; Reich 2014).
When the dominant species in a community have similar strategies for their resource acquisition, the resource economics spectrum at the species level can scale up to the community level for leaves (LEScom; Ordoñez et al. 2009; Fernandez-Going et al. 2013; Jager et al. 2015; Carvajal et al. 2019), roots (REScom; Prieto et al. 2015; de la Riva et al. 2018; Hou et al. 2019), and the whole plant (PEScom; Liu et al. 2010; de la Riva et al. 2016), respectively. Although some studies have demonstrated the existence of a PEScom in response to contrasting soil resource availabilities (e.g., Holdaway et al. 2011; Pérez-Ramos et al. 2012), very few have examined whether leaf and root traits converge at the community level in resource-poor environments (Liu et al. 2010). One of such studies found no consistent integrated whole-plant trait coordination among communities distributed along a strong water availability gradient in the Atacama Desert (i.e., no PEScom), probably because the LEScom and REScom were responding to different environmental factors (Carvajal et al. 2019). However, whether variation in soil nutrient availability in this desert can lead to integrated changes in leaf and root traits of plant communities (PEScom) or not still needs to be tested comprehensively. Evidence from more temperate ecosystems revealed a weak or lack of coordination between leaf and their analogue root traits (e.g., between SLA and SRL) in a soil fertility gradient, suggesting that soil resources may not lead to a PEScom (Kramer-Walter et al. 2016). This weak or lack of coordination may occur because leaves and roots are exposed to different selective pressures (e.g., leaf growth and development may be constrained by light and water availability, whereas root development may be more constrained by soil nutrients, water availability and/or by soil structure) or because they have dissimilar responses to the same selective pressure (Kembel and Cahill 2011; Weemstra et al. 2016; Carvajal et al. 2019). In desert ecosystems aridity not only leads to a strong limitation in water availability, but also promotes slow biogeochemical cycles, which ultimately results in low soil nutrient P and K availability in plant communities (Noy-Meir 1973, Schlesinger et al. 1996, Hartley et al. 2007). Particularly soil P and K largely constrain plant growth and development in these systems because their limited availability reduces plant metabolic activity and photosynthesis (Lambers et al. 2010 ; Sardans and Peñuelas 2015). Therefore, plants in these ecosystems are typically exposed to multiple selective pressures.
Changes in the functional structure of plant communities along resource gradients (e.g., water or nutrient) are driven by changes in species turnover and intraspecific variability (ITV) (Lepš et al. 2011; Siefert et al. 2015; Read et al. 2017). Most studies thus far have focused on changes in leaf traits, revealing that, along edaphic or nutrient gradients, traits are mainly determined by changes in species turnover (Pérez-Ramos et al. 2012; de la Riva et al. 2016a. Nonetheless, there is also empirical evidence showing that SLA, LDMC and LPC express high ITV in response to changes in environmental conditions (e.g., Lepš et al. 2011; Siefert et al. 2015; Fajardo and Siefert 2018). The role of ITV in shaping root traits has rarely been addressed; however, the few studies that have examined this revealed that root traits have comparatively higher ITV than do leaf traits (Freschet et al. 2015; Kramer-Walter and Laughlin 2017; Read et al. 2017). This may be because changes in soil resources could be more limiting for roots than for leaves, and hence promote higher ITV in root that in leaf traits.
In this study, we used 11 leaf and root traits that have a functional significance for woody shrub species to determine how nutrient availability shapes the functional structure of plant communities in the southern edge of the Atacama Desert. We hypothesize that under similar climatic conditions (i.e., precipitation and temperature), differences in soil nutrient availability shape the resource acquisition strategies of the shrub communities. Specifically, we predict that: 1) resource acquisition strategies of shrub communities will shift from more conservative to more acquisitive with increasing soil nutrient availability, promoting a LEScom and a REScom; that 2) community-level leaf and root traits will be coordinated and that 3) changes in the community functional structure via ITV will be higher for root than for leaf traits.
Material and methods
We conducted this study during 2016 in the southern edge of the Atacama Desert, within a shrubland located 21 km N of La Serena, Chile (El Romeral, 29° 43’ S, 71°14’ W, 150 m.a.s.l.). Mean annual precipitation in this area is 82.9 mm and mean annual temperature is 11.4 °C (1956–2003; Squeo et al. 2006). According to De Martonne’s aridity index (De Martonne, 1926, DEMAI = MAP/[MAT+10)]), the region is classified as hyper-arid (DEMAI = 3.87). Most of the rainfall falls in few pulses during the winter (May to September), interspersed with drought periods and a long summer drought with little, or no precipitation from December to March (Squeo et al. 2006).
Sampling design
Using the geological map developed by the Chilean National Geology and Mining Service (SERNAGEOMIN), we selected four sites within an area of approximately 10 km2, differing in soil age and/or geological origins (i.e., rock type) (Table 1, Fig. S1) (Emparán and Pineda 2000). At each site, we randomly established ten 50 × 2 m plots separated by at least 50 m. Within each plot, we recorded shrub species composition, the number of individual plants per species, and species-specific plant cover to estimate the relative abundance of each species (see Table S1 for species identity and cover data per site).
Soil variables
To collect soil samples, within each site we randomly selected five points separated by at least 100 m from each other. At each point, we collected five soil cores at a depth of 0–20 cm from bare ground areas to avoid any potential influence of the vegetation. These cores were combined into a single composite sample per point (N = 5 points per site). Soil samples were analyzed in the Soil Analysis Laboratory of the Technological Center of Soil and Crops at Universidad de Talca (Talca, Chile) using standard procedures (INIA 2006). For each sample, we determined the proportion of groove sand (Ø 2–1 mm), fine sand (Ø 1–0.5 mm) and lime/clay (Ø < 0.5 mm), as well as total soil organic matter (%, wet digestion method), available N (mg kg−1, 2 M KCl extraction method), available P (mg kg−1, Olsen method), total K (mg kg−1, Flame spectroscopy method) and pH (1:2.5 soil: water suspension).
Plant trait measurements
Following standardized protocols (Pérez-Harguindeguy et al. 2013), in spring of 2016 we measured a set of 11 morphological and physiological leaf and fine root (diameter < 2 mm) traits that are linked with ecological strategies related to the plant economics spectrum (see Table S2 for the description of traits and their functional roles). In each of the sites, we selected 10 individuals of each of the dominant species (i.e., those that comprised 90% of the species community abundance; Pakeman and Quested 2007) for trait measurements. These individuals were selected outside the plots where the relative abundance of each species was measured but in interspaces between plots to have a more representative measure of mean trait values of these communities.
Morphological traits
We collected between 1 and 20 fully expanded sun-exposed leaves per individual (depending on the species) to measure specific leaf area (SLA) and leaf dry matter content (LDMC). To measure morphological root traits, we excavated roots under the crown of the same individuals in which we collected leaves and collected fine root samples (< 2 mm diameter, corresponding to the diameter threshold for the first- and second- order roots for most shrub species at our site) from a depth between 10 and 20 cm. We chose this depth interval because shrub roots are very scarce in the top 10 cm; thus, not enough material can be collected from this layer. Leaf and root samples were placed in a sealed bag immediately after collection in the field, stored in a cooler and taken to the laboratory within 8 h of sampling. Once in the laboratory, we recorded their fresh weight (FW, g), scanned them (600 dpi resolution, HP Scanjet 200) and oven-dried all samples at 60 °C for 48 h to determine their dry weight (DW, g). The resulting images were processed with ImageJ (Schneider et al. 2012) to determine leaf area (LA, cm2) and root length (RL, mm). We then calculated specific leaf area (SLA, m2 kg−1) and specific root length (SRL, m g−1) as LA/DW × 1000 and RL/(DW/1000), respectively, and LDMC (mg g−1) and RDMC (mg g−1) were calculated as DW/(FW × 1000).
We measured the root depth distribution (β index) in three out of the ten selected individuals per species and site. In these individuals, we excavated a trench 1.6 m long × 1 m width × 1 m deep starting at the center of each shrub. Beginning in the top left corner of the trench, we then collected 100 blocks of soil (20 × 20 × 10 cm) from the trench walls, both down- (1 m) and sideways (1.6 m) (see Fig. S2). We collected all roots within each block and classified them according to their diameter into very fine (Ø <1 mm), fine (1–2 mm) and coarse roots (>2 mm). We then oven dried root samples at 60 °C for 48 h and determined the biomass of each diameter class. We estimated the total root mass of each individual shrub according to Morales et al. (2014). The β index was calculated using the asymptotic equation: Y = 1 – βd, where Y is the accumulated proportion of root biomass from the soil surface down to a d depth (Gale & Grigal 1987). Higher values of β indicate a greater proportion of roots are allocated deeper in the soil (Gale & Grigal 1987).
Chemical traits
We measured leaf nitrogen (LNC), phosphorus (LPC) and potassium concentrations (LKC), as well as leaf carbon to nitrogen ratio (LC:N). In roots, we measured nitrogen concentration (RNC) and carbon to nitrogen ratio (RC:N). Leaf and root C and N were measured in the Laboratory for Biogeochemistry and Applied Stable Isotopes (LABASI) at the Pontificia Universidad Católica de Chile (Santiago, Chile) using a Thermo Delta V Advantage IRMS coupled with a Flash 2000 Elemental Analyzer. Foliar P and K concentrations were measured using inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo Elemental Iris Intrepid II XDL, Franklin, TN, USA) after a microwave-assisted digestion with HNO3:H2O2 (4:1, v:v) at the Ionomics facility of CEBAS-CSIC (Murcia, Spain). Leaf and root chemical traits were measured in five of the ten individuals selected per species per site for the morphological measurements. For some species, in each site (PH = 4 sp., QR = 5 sp., S1 = 7 sp., S2 = 3 sp.), LPC and LKC were measured in only one individual.
Statistical analysis
To examine differences among sites for each of the soil variables, we conducted a general linear model (GLM), using a Gaussian error distribution (link function “identity”) and Tukey HSD post-hoc tests. For proportional data (i.e., soil texture), values were square root-arcsine transformed prior to analysis.
Within each of the four sites and for each leaf and root trait we calculated the community-weighted mean (CWM) in each community (i.e., plot). The CMW was calculated following Garnier et al. (2004) as: CWM \( ={\sum}_{i=1}^s{p}_{ik}{x}_{ik} \), where pik is the relative abundance of species i at site k and xik is the trait value of species i at site k. We used plant cover as a measure of the relative abundance of each species in each community. Following Carvajal et al. (2019), we searched patterns of covariation in leaf traits and in root traits at the community level according to the economics spectrum by conducting two independent principal component analyses (PCA): one for leaves (PCAleaf) using CWM values of the six leaf traits measured (SLA, LDMC, LC:N, LNC, LPC and LKC) and one for roots (PCAroot) using CWM values of the five root traits measured (SRL, RDMC, RNC, RC:N and β index). We used the first PCAs axis because it captured a high proportion of the CWM variation; consequently, its scores can be used as a proxy of the economics spectrum given that they represent gradients of trait variation across sites. We extracted the scores of the first axis from the PCAleaf and PCAroot of each community at each site and used them as a continuous variable defining the LEScom or REScom. To assess differences in the functional structure of shrub assemblages in plant communities across sites (i.e., differences in resource use strategies along the plant economics spectrum), we performed one-way ANOVAs using the first axis of the PCAleaf and PCAroot as dependent variables and site as a factor, followed by Tukey HSD post-hoc tests to assess differences among sites.
The degree of coordination between leaf and root covariation was assessed using standardized major axis regressions (SMA) (Warton et al. 2012). Because we observed a clear differentiation within the resource economics spectrum only for leaf traits, we regressed the scores of the first PCAleaf axis against the CWM of each individual root trait. To explore relationships between the LEScom and soil nutrient variables, we performed linear regressions with the scores of the first axis of the PCAleaf as the dependent variable and each of the soil variables as the independent variable.
Following Lepš et al. (2011), we quantified the relative contribution of species turnover and intraspecific trait variation (ITV) in the variation of the functional structure of shrub assemblages by calculating three parameters for each plant organ: 1) “fixed” community trait values \( ={\sum}_{i=1}^s{p}_i{x}_i \), where pi is the relative abundance of each species in a given community, and xi is the mean trait values of each species across all sites (i.e., site-independent trait values), and vary only due to changes in species turnover; 2) “specific” community trait values \( ={\sum}_{i=1}^s{p}_i{x}_{i\_ site} \), where xi _ site is the mean trait values of each species within each site, which include both species turnover and ITV and; 3) “intraspecific variability” community trait values, which are calculated from the differences between the “specific” and “fixed” average traits and allow an estimation of the pure effects of ITV. We then performed individual PERMANOVAs for each parameter using sites as the independent factors to extract the sum of squares (SS) from each model (SSspecific, SSfixed, and SSintraspecific variability). Because species turnover and ITV could be responding to environmental factors in the same (= positive covariation) or opposite directions (= negative covariation), we calculated the covariation component (SScov) by subtracting SSfixed and SSintraspecific variability from SSfixed. All statistical analyses were performed using the R statistical environment (R Core Development Core, 2014).
Results
Sites differed in their soil physical and chemical properties, revealing differences in N, P and K availability across our study area. Our results show that P availability was lowest at the oldest site (PH) compared to the other three sites (χ2 = 231, P < 0.001, Table 2). Total N was higher in PH and QR compared to S1 and S2 (χ2 = 288.68, P < 0.001). This resulted in differences in N:P ratios that were highest in PH ≥ QR ≥ S1 ≥ S2 (χ2 = 4.05, P < 0.001). Sites also differed in their total K, which was significantly lower in PH and QR than in S2 (χ2 = 2.08, P < 0.001).
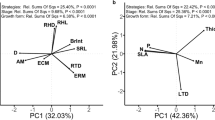
The first axis of the PCAleaf accounted for 85.3% of the total variation in CWM traits (Fig. 1a). Coupled with significant correlations amongst traits, this suggests the existence of a LEScom (Table S5 and S6). Traits associated with an acquisitive strategy (e.g., high SLA, LNC, LPC and LKC) had positive loadings on the first PCAleaf axis. Conversely, traits linked with a conservative strategy (e.g., high LDMC and high LC:N) had negative loadings on the first PCAleaf axis (Table S5). Soil properties (i.e., site) had a strong effect on leaf trait attributes; sites with the lower P and K and higher N:P ratios (PH and QR) were associated with the conservative side of the PCAleaf (i.e. high LDMC and LC:N) and thus had the lowest score values (Fig. 1b). In contrast, sites with higher P and K and lower N:P ratios (S1 and S2) were associated with the acquisitive side of the PCAleaf (i.e., high SLA, LNC, LPC and LKC) and had higher score values (Fig. 1b; site effect F3,36 = 291.8, P < 0.001).
a) Principal components analysis (PCA) for six leaf traits at the community level (community-weighted mean values) and b) mean (± SE) scores of the first PCA axis for leaves for the four different sites. Vectors represent plant traits and symbols denote plant communities sampled in each site. Different lowercase letters above bars indicate significant differences among sites. Trait abbreviations are as follows: SLA = Specific leaf area, LDMC = Leaf dry matter content, LNC = Leaf nitrogen concentration, LPC = Leaf phosphorus concentration, LKC = Leaf potassium concentration and LC:N = leaf carbon to nitrogen ratio
The covariation pattern for root traits was less clear (Fig. 2a, Table S5) and CWM traits for fine roots showed multiple associations along two axes of variation in the PCAroot (Fig. 2a). The first axis accounted for 59.4% of the total variability and was related to changes in root morphology (SRL and RDMC) and root N (RNC and RC:N). We observed a shift along this axis from communities dominated by species with high RDMC and RNC (i.e., negative values on the PCAroot), to communities dominated by species with high SRL and high RC:N (i.e., positive values on the PCAroot). In the case of fine roots, the site with lowest soil P and K and highest N:P ratios (PH) was dominated by communities with high RDMC and RNC, whereas S1, a site with high P and K and low N:P ratios, was dominated by communities with high SRL and high C:N (Fig. 2a). The other two sites (QR and S2) had slightly positive and negative score values on the first PCAroot axis respectively, but these did not differ from each other (F3,36 = 44.653, P < 0.001; Fig. 2b). The second PCAroot axis, which accounted for 24.1% of the total variability, was positively associated to communities with species with high β and RNC and negatively with RDMC. Additionally, the distribution of the four sites along the second axis of variation revealed significant differences among sites (F3,36 = 4.796, P = 0.006; Fig. 2c).
a) Principal components analysis (PCA) for root traits at the community level (community-weighted mean values), b) mean (± SE) scores of the first PCA axis for roots of the four different sites and c) mean (± SE) scores of the second PCA axis for roots of the four different sites. Vectors represent root traits and symbols denote individual plant communities sampled at each site. Different lowercase letters above bars indicate significant differences among sites. Trait abbreviations are as follows: SRL = Specific root length, RDMC = Root dry matter content, β = Beta index, RC:N = Root carbon to nitrogen ratio and RNC = Root nitrogen concentration
Across sites, we found a positive relationship between the LEScom (i.e., communities dominated by species with acquisitive traits) with SRL, RC:N and β (Fig. 3a–e), and a negative relationship with RNC (Fig. 3b); RDMC did not exhibit a correlation with the LEScom (Fig. 3c). The slopes of all these correlations deviated from the 1:1 relationship (P < 0.05) revealing that fine root traits were not perfectly coordinated with the LEScom. In addition, the relationship between analogous leaf and root traits only exhibited a positive relationship between SLA and SLR, whereas the LNC – RNC and LC:N – RC:N relationships were negative, which is opposite to what is expected under a PES (Table S6, Fig. S5).
Standardized major axis (SMA) regressions between the first PCA axis of leaf traits (LEScom) and a) specific root length (SRL), (b) root N concentration (RNC), (c) root dry matter content (RDMC), (d) root C to N ratio (RC:N) and (e) β index at the community level. When significant, black lines represent SMA regressions. Colored lines represent significant SMA regressions within sites. Shown are the R2 and significance (P < 0.001) of the regressions
Overall, the observed changes in the acquisitive strategies of leaves at the community level along the resource gradient were driven mainly by species turnover (53.7%), rather than by changes in ITV (6.9%) (Fig. 4). Moreover, a proportion of the total variability in leaf trait values was related to a positive covariation between species turnover and ITV, indicating similar responses to environmental or soil factors across the four sites (14.6%). This pattern was consistent when considering individual traits with between 38.9–55.2% of the variability explained by turnover depending on the trait and between 4.4–33.7% by ITV (Fig. S6). In the case of roots, variability of trait values along the gradient was almost equally explained by species turnover (34.1%) or by ITV (29.0%), the latter being four times higher than for leaves (i.e., 29.0% vs. 6.9%) (Fig. 4). When considering individual traits, SRL, RNC and RC:N followed this pattern with a larger percentage of the variability explained by species turnover (45.8, 59.3 and 31.7%, respectively compared to 24.4, 5.1 and 6.4% explained by ITV), whereas the variability in RDMC and β-index were mainly driven by ITV (36.9 and 41.6%, respectively compared to 14.9 and 16.4% explained by species turnover, Fig. S7).
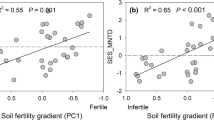
Across sites, the dominant plant strategies were strongly associated to soil properties and linked mainly to variation in P and K availability (i.e., soil N:P ratio and total K). The scores of the PCAleaf (LEScom) were negatively associated to soil N:P ratios (R2 = 0.92, P < 0.0001) and positively associated to soil K (R2 = 0.72, P < 0.001, Fig. 5). Thus, plant communities with negative scores in the LEScom (i.e., conservative strategies) were associated with higher soil N:P ratio (i.e., with P limited soils) and higher soil K, whereas communities with positive scores in the LEScom (i.e., acquisitive strategies) were associated with lower N:P ratios (e.g., higher P availability) and high total soil K.
Discussion
Our results revealed that at the landscape scale, differences in soil nutrient availability mediated mainly by changes in K and P availability, promote the existence of a LEScom in shrub assemblages across sites in the southern limit of the Atacama Desert, which shifted from more acquisitive to more conservative resource acquisition strategies as the environment became more nutrient-limited. In contrast, the response of root traits did not show a clear pattern of covariation according to the resource economics spectrum; we found two axes of variation, one associated to root morphology and root N, and another to rooting depth (β index). We also found that except for RDMC, most of the individual root traits covaried with the LEScom. However, only SRL exhibited a coordinated response with the LEScom according to what is expected by the PES theory; that is, lower and higher SRL in more conservative and more acquisitive communities, respectively. Finally, our results show that the shift in covaration of leaf traits across sites was determined mainly by species turnover, while the shift in root traits was determined by both species turnover and intraspecific variability.
Our first hypothesis that shrub communities should shift from more conservative to more acquisitive with increasing soil nutrient availability was partially supported. Resource acquisition strategies of shrub communities at the leaf level differed among soils of different ages; in the oldest site (PH), which had the lowest P and K availability, plant communities had the most conservative strategy (high LDMC and C:N). In contrast, in the youngest sites where P and K were less limiting, plant communities had more acquisitive strategies (high SLA and nutrient concentration). These results suggest that leaf resource acquisition strategies are responding mainly to limitations in soil P and K, with N colimiting the availability of P (N:P ratios). This may be because P and K are rock-derived nutrients, which gradually disappear through leaching and erosion as the soil ages (Lambers et al. 2010); in contrast, N is gradually incorporated into the ecosystem via atmospheric deposition and biological N-fixation and is usually higher in older soils (Walker and Syers 1976; Vitousek and Sanford 1986; Vitousek and Howarth 1991; Baribault et al. 2012; Coomes et al. 2013). Therefore, older sites have typically lower P and K availability than younger sites. A similar pattern was reported across a chronosequence where temperate forest species growing in younger soils had traits associated to an acquisitive strategy, whereas in older, P-limited sites, plant traits were associated to conservative strategies (Holdaway et al. 2011). Furthermore, in an experiment examining nutrient deficiency in perennial grasses, Minden and Venterink (2019) found that when K was not limiting, plant species exhibited more acquisitive leaf traits. From a functional perspective, plants with high SLA and leaf nutrients also have high CO2 assimilation rates and high transpiration rates, which allows them to acquire and use resources more rapidly (Wright et al. 2004; Reich 2014; Prieto et al. 2018). Additionally, because P and K control gas exchange through stomatal regulation (Jones 1992), having high LPC and LKC has a strong functional significance in arid ecosystems since it can enhance photosynthetic processes and the C uptake capacity of plants (Reich et al. 2009; Thomas et al. 2006), as well as increase water use efficiency and drought resistance both at the plant and community-level (Tomlinson et al. 2012; Sardans and Peñuelas 2015; Prieto et al. 2018). It was surprising that LNC was lower in sites with high soil N, departing from what is expected by the resource economics theory (Chapin et al. 1993; Aerts and Chapin 2000a; Westoby et al. 2002; Comas and Eissenstat 2004). This decoupling between soil and leaf N may be associated with the strong P limitation also limiting N uptake in plants (Richardson et al. 2004). In our study leaf N:P ratios were higher than 15 in most sites and greater than 20 in PH, an indication of P limitation for plant growth (sensu Güsewell 2004, Fig. S3). Since both N and P uptake by plants are influenced by soil P, rather than N availability (Drenovsky and Richards 2004; He et al. 2014), our results suggest that soil P availability is an important constraint in this desert ecosystem.
We did not find evidence supporting the existence of only one axis of variation in line with the RES; instead, we found that root trait covariation was multidimensional (Chen et al. 2013; Weemstra et al. 2016; Kramer-Walter et al. 2016; Wang et al. 2017). Our results showed a first axis of variation where SRL was negatively related to RDMC, as expected by RES predictions (Roumet et al. 2016; Prieto et al. 2015), but this axis was also positively related with RC:N and negatively related with RNC, revealing an opposite pattern to what would be expected in light of the RES (see Weemstra et al. 2016). Furthermore, we found a second axis of variation linked to plant rooting depth (Fig. 2a). Low SRL and high RDMC values have been linked to communities with a conservative acquisition strategy, characterized by low root turnover rates and long lifespans, which in turn allows roots to conserve nutrients and even to act as a “nutrient sinks” for other organs (Withington et al. 2006; McCormack et al. 2012; He et al. 2015; Weemstra et al. 2016). In addition low SRL can result from larger root diameter or higher tissue density (e.g., RDMC), which could have different implications for a root to be acquisitive or conservative (Chen et al. 2013). Specifically, roots with low SRL and high RNC and root diameter have a greater capacity of colonization by mycorrhizal fungi (Kong et al. 2014). The association with mycorrhizal fungi may enhance the ability of roots with an a priori low foraging capacity (e.g., low SRL), to acquire more resources (Kong et al. 2019; McCormack and Iversen 2019). Considering that several species of the Atacama Desert have shown affinity for arbuscular mycorrhizal fungi (Dhillion et al. 1995; Aguilera et al. 2016), this constitutes a plausible alternative strategy for plants to take up nutrients in our study system. Nutrient root traits, however, also changed along with soil N availability, because RNC was positively linked to N soil availability (Table S7) and negatively correlated to RC:N (Table S6). A possible explanation for this result is that RNC in shrub communities depends strongly on the concentration of soil N (Zangaro et al. 2008; Zhang et al. 2018; Chapin et al. 1993). Similar to what has been reported elsewhere (Poorter et al. 2012, Freschet et al. 2015), our findings reveal that when several soil resources are co-limiting, the response of root traits is not entirely clear. Therefore, to classify resource acquisition strategies of roots as acquisitive or conservative in these environmental conditions, it is key to consider different types of traits (e.g., morphological and nutrient traits, mycorrhizal colonization and/or the presence of cluster roots or exudates).
In our study, the community located in the poorest soil also had the lowest β index (see Fig. S4), indicating that a greater proportion of roots is allocated to soil layers near the surface (Jackson et al. 1996). This preferential root allocation to surface soils may compensate the lower exploration capacity of roots with low SRL and enhance nutrient acquisition in poor soils given that P and K availability is highest in the top soil (Jobbágy and Jackson 2001; White et al. 2013; Laliberté et al. 2015). In addition, in deserts this strategy may enable plants to acquire water from small episodic rainfall events (Fort et al. 2013; Prieto et al. 2014; Carvajal et al. 2019). In contrast, in the most acquisitive site (S2), shrubs had a greater proportion of roots allocated to deeper soil layers, allowing access to more reliable and stable water sources and potentially enabling plants to sustain greater transpiration rates (Moreno-Gutiérrez et al. 2012) through hydraulic lift (Prieto et al. 2010, 2012). Thus, adjustments in root biomass allocation could be an efficient strategy to cope with the multi resource limitation imposed by desert soils to plant communities.
It has been suggested that harsh environmental conditions, such as those characterizing the Atacama Desert, can generate strong selection pressures and promote the convergence of functional traits in distinct organs (de la Riva et al. 2016), leading to a PEScom (Reich 2014). In our study, however, we found a negative relationship between LEScom and RNC, which does not have the functional significance that is proposed by the PES hypothesis (Reich 2014). We associate this result to an allocation of N (at the plant level) to leaves in order to compensate for the limited N available in the soil and enable plants to maintain a high photosynthetic capacity in the most acquisitive communities (He et al. 2016). Similarly, the positive relationship between LEScom and the β index may also bring leaf nutrient ratios closer to values that are optimal for growth (Chapin et al. 2011). We also found a positive relationship between SRL and LEScom, revealing that a fast resource use by leaves was associated with a fast resource uptake by roots. However, along an aridity gradient in the Atacama Desert, Carvajal and collaborators (2019) found the opposite pattern between LEScom and SRL. This contrasting result may arise due to the different spatial scales in which both our studies were conducted since different assembly processes can operate at different scales to determine trait variation (Cornwell and Ackerly 2009; de Bello et al. 2013). For example, at a regional scale, the interaction between aridity and biotic processes can result in different above- and below-ground strategies (Carvajal et al. 2019); in contrast, at a landscape level as revealed here, it is the limitation of multiple soil resources that may drive the different observed responses between root and leaf traits.
The shift in the functional structure of leaves and roots was mainly driven by changes in species turnover, but the relative importance of ITV was almost four times higher for root than for leaf traits. These results suggest that changes in nutrient availability act as an environmental filter on both leaf and root traits, but are mediated by different processes. Previous studies have found that along edaphic or nutrient gradients, the shift in leaf traits is mainly determined by changes in species turnover (e.g., Pérez-Ramos et al. 2012; Siefert et al. 2015). Conversely, the shift in root traits along the nutrient gradient was driven almost equally by species turnover and ITV, although it depended on the trait considered. This suggests high within-species phenotypic variability at the root level, either as a result of phenotypic plasticity or ecotypic differentiation (Albert et al. 2011; Carvajal et al. 2017). High root ITV could be explained because root traits are subject to more simultaneous constraints than leaves (Weemstra et al. 2016); thus, high ITV may allow species to achieve an optimal acquisition strategy in response to the co-limitation of multiple resources, which is particularly important in highly heterogeneous environments (Freschet et al. 2015; Read et al. 2017; de la Riva et al. 2018). In summary, our findings suggest that whereas species turnover is responsible for most of the variation in leaf traits across sites (Perez-Ramos et al. 2012, de la Riva et al. 2016a), ITV is an important driver of the shift of root traits in plant communities in response to differences in soil nutrient availability in the southern edge of the Atacama Desert and thus, likely plays a particularly important role in structuring plant communities.
In conclusion, we provide evidence that a nutrient availability gradient did not lead to community-level coordination between leaf and root traits as predicted by the PES framework. This lack of an integrated plant economics spectrum appears to be related to the co-limitation of multiple soil resources, which promote different responses in different types of root traits. Contrary to leaves, roots also exhibited high intraspecific variation, which may allow them to optimize resource acquisition. In summary, our results highlight the importance of including both leaf and root traits to achieve a more thorough understanding how of the limitation of multiple resources can determine plant ecological strategies.
Abbreviations
- LES:
-
Leaf economic spectrum
- RES:
-
Root economic spectrum
- PES:
-
Plant economic spectrum
- SLA:
-
Specific leaf area
- LDMC:
-
Leaf dry matter content
- SRL:
-
Specific root length
- RDMC:
-
Root dry matter content
- β index:
-
Root depth distribution
- LNC:
-
Leaf nitrogen concentration
- LPC:
-
Leaf phosphorus concentration
- LKC:
-
Leaf potassium concentration
- LC:N:
-
Leaf C:N Ratio
- RNC:
-
Root nitrogen concentration
- RC:N:
-
Root C:N ratio
- N:
-
Nitrogen
- P:
-
Phosphorus
- K:
-
Potassium
- N:P:
-
Soil N:P ratio
- PCA:
-
Principal component analysis
- ITV:
-
Intraspecific trait variation
References
Aerts R, Chapin FS (2000a) The mineral nutrition of wild plants revisited: a re-evaluating of processes and patterns. Adv Ecol Res 30:1–67. https://doi.org/10.1016/S0065-2504(08)60016-1
Aerts R, Chapin FS (2000b) The mineral nutrition of wild plants revisited: a re-evaluatin of processes and patterns. Adv Ecol Res 30:1–67. https://doi.org/10.1016/S0065-2504(08)60016-1
Aguilera LE, Armas C, Cea AP et al (2016) Rainfall, microhabitat, and small mammals influence the abundance and distribution of soil microorganisms in a Chilean semi-arid shrubland. J Arid Environ 126:37–46. https://doi.org/10.1016/j.jaridenv.2015.11.013
Albert H, Thuiller W, De Bello F, Lavorel S (2011) Quantifying the relevance of intraspecific trait variability for functional diversity. Methods Ecol Evol 2:163–174. https://doi.org/10.1111/j.2041-210X.2010.00071.x
Augusto L, Achat DL, Jonard M, Vidal D, Ringeval B (2017) Soil parent material—a major driver of plant nutrient limitations in terrestrial ecosystems. Glob Chang Biol 23:3808–3824. https://doi.org/10.1111/gcb.13691
Baribault T, Kobe R, Finley A (2012) Data from: tropical tree growth is correlated with soil phosphorus, potassium, and calcium, though not for legumes. Dryad digital repository. Ecol Monogr 82:189–203
Carvajal DE, Loayza AP, Rios RS et al (2019) A hyper-arid environment shapes an inverse pattern of the fast–slow plant economics spectrum for above-, but not below-ground resource acquisition strategies. J Ecol 107:1079–1092. https://doi.org/10.1111/1365-2745.13092
Carvajal DE, Loayza AP, Rios RS et al (2017) Population variation in drought-resistance strategies in a desert shrub along an aridity gradient : interplay between phenotypic plasticity and ecotypic di ff erentiation. Perspect Plant Ecol Evol Syst 29:12–19. https://doi.org/10.1016/j.ppees.2017.10.001
Chapin FS, Kellar A, Pugnaire P (1993) Evolution of suites of traits in response to environmental stress. Am Nat 142:78–92
Chapin SF, Matson PA, Vitousek PM (2011) Principles of Terrestrial Ecosystem Ecology, 2st edn. Springer-Verlag, New York.
Chen W, Zeng H, Eissenstat DM, Guo D (2013) Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Glob Ecol Biogeogr 22:846–856. https://doi.org/10.1111/geb.12048
Comas LH, Eissenstat DM (2004) Linking fine root traits to maximum tree species rate among 11 mature temperate. Funct Ecol 18:388–397
Coomes DA, Bentley WA, Tanentzap AJ, Burrows LE (2013) Soil drainage and phosphorus depletion contribute to retrogressive succession along a New Zealand chronosequence. Plant Soil 367:77–91. https://doi.org/10.1007/s11104-013-1649-5
Cornwell WK, Ackerly DD (2009) Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California published by : ecological Society of America Community across an environmental in plant trait distributions assembly and shifts gradient and. Ecol Monogr 79:109–126. https://doi.org/10.1890/07-1134.1
de Bello F, Vandewalle M, Reitalu T et al (2013) Evidence for scale- and disturbance-dependent trait assembly patterns in dry semi-natural grasslands. J Ecol 101:1237–1244. https://doi.org/10.1111/1365-2745.12139
de la Riva EG, Tosto A, Pérez-Ramos IM et al (2016) A plant economics spectrum in Mediterranean forests along environmental gradients: is there coordination among leaf, stem and root traits? J Veg Sci 27:187–199. https://doi.org/10.1111/jvs.12341
de la Riva EG, Pérez-Ramos IM, Tosto A et al (2016a) Disentangling the relative importance of species occurrence, abundance and intraspecific variability in community assembly: a trait-based approach at the whole-plant level in Mediterranean forests. Oikos 125:354–363. https://doi.org/10.1111/oik.01875
de la Riva EG, Marañón T, Pérez-Ramos IM, Navarro-Fernández CM, Olmo M, Villar R (2018) Root traits across environmental gradients in Mediterranean woody communities : are they aligned along the root economics spectrum ? Plant Soil 424:35–48. https://doi.org/10.1007/s11104-017-3433-4
De Martonne E (1926). Une nouvelle fonction climatologique: L’indice d’aridité. La Meteorologie, 2, 449–458.
Dhillion SS, Vidiella PE, Aquilera LE, Friese CF, Leon E, Armesto JJ, Zak JC (1995) Mycorrhizal plants and fungi in the fog-free Pacific coastal desert of Chile. Mycorrhiza 5:381–386. https://doi.org/10.1007/BF00207410
Drenovsky RE, Richards JH (2004) Critical N : P values: Predicting nutrient deficiencies in desert shrublands. Plant Soil 259:59-69
El-keblawy A, Abdelfattah MA, Khedr AA (2015) Relationships between landforms , soil characteristics and dominant xerophytes in the hyper-arid northern United Arab Emirates. J Arid Environ 117:28–36. https://doi.org/10.1016/j.jaridenv.2015.02.008
Emparán C, Pineda G (2000) Hoja La Serena-La Higuera, región de Coquimbo. Servicio Nacional de Geología y Minería, Mapas Geológicos 18, 1 mapa escala 1:100.000. Santiago.
Fajardo A, Siefert A (2018) Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 99:1024–1030. https://doi.org/10.1002/ecy.2194
Fernandez-Going BM, Harrison SP, Anacker BL, Safford HD (2013) Climate interacts with soil to produce beta diversity in Californian plant communities. Ecology 94:2007–2018. https://doi.org/10.1890/12-2011.1
Fort F, Jouany C, Cruz P (2013) Root and leaf functional trait relations in Poaceae species: implications of differing resource-acquisition strategies. J Plant Ecol 6:211–219. https://doi.org/10.1093/jpe/rts034
Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R (2010) Evidence of the “plant economics spectrum” in a subarctic flora. J Ecol 98:362–373. https://doi.org/10.1111/j.1365-2745.2009.01615.x
Freschet GT, Swart EM, Cornelissen JHC (2015) Integrated plant phenotypic responses to contrasting above- and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytol 206:1247–1260. https://doi.org/10.1111/nph.13352
Fu BJ, Liu SL, Ma KM, Zhu YG (2004) Relationships between soil characteristics , topography and plant diversity in a heterogeneous deciduous broad-leaved forest near Beijing, China. Plant Soil 261:47–54
Gale MR, Grigal DF (1987). Vertical root distributions of northern tree species in relation to successionalstatus. Canadian Journal of Forest Research‐Revue Canadienne De Recherche Forestiere, 17, 829–834. doi:https://doi.org/10.1139/x87-131
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x
Hartley, A, Barger, N, Belnap, J, Okin, G (2007) Dryland ecosystems. Pp. 271-308 in P. Marschner and Z. Rengel, eds. Nutrient cycling in terrestrial ecosystems. Springer, Berlin & Heidelberg
He M, Dijkstra FA, Zhang K, et al (2014) Leaf nitrogen and phosphorus of temperate desert plants in response to climate and soil nutrientavailability. Sci Rep 4:1–7. doi:https://doi.org/10.1038/srep06932
He M, Song X, Tian F, Zhang K, Zhang Z, Chen N, Li X (2016) Divergent variations in concentrations of chemical elements among shrub organs in a temperate desert. Nat Publ Gr 6:1–10. https://doi.org/10.1038/srep20124
He M, Zhang K, Tan H, Hu R, Su J, Wang J, Huang L, Zhang Y, Li X (2015) Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongorica in relation to geographical, climatic, and soil conditions. Ecol Evol 5:1494–1503. https://doi.org/10.1002/ece3.1441
Holdaway RJ, Richardson SJ, Dickie IA et al (2011) Species- and community-level patterns in fine root traits along a 120000-year soil chronosequence in temperate rain forest. J Ecol 99:954–963. https://doi.org/10.1111/j.1365-2745.2011.01821.x
Hou SL, Lü XT, Yin JX, Yang JJ, Hu YY, Wei HW, Zhang ZW, Yang GJ, Liu ZY, Han XG (2019) The relative contributions of intra- and inter-specific variation in driving community stoichiometric responses to nitrogen deposition and mowing in a grassland. Sci Total Environ 666:887–893. https://doi.org/10.1016/j.scitotenv.2019.02.322
INIA, 2006. Métodos de análisis recomendados para suelos de Chile. Serie Actas INIA N 34. Santiago. Chile. 164 p.
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411. https://doi.org/10.1007/BF00333714
Jager MM, Richardson SJ, Bellingham PJ et al (2015) Soil fertility induces coordinated responses of multiple independent functional traits. J Ecol 103:374–385. https://doi.org/10.1111/1365-2745.12366
Jobbágy EG, Jackson RB (2001) The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53:51–77. https://doi.org/10.1023/A:1010760720215
Jones HG (1992) Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 428 pp., Cambridge Univ. Press, New York.
Kembel SW, Cahill JF (2011) Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS One 6:12–15. https://doi.org/10.1371/journal.pone.0019992
Kong D, Ma C, Zhang Q et al (2014) Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol 203:863–872. https://doi.org/10.1111/nph.12842
Kong D, Wang J, Wu H, Valverde-Barrantes OJ, Wang R, Zeng H, Kardol P, Zhang H, Feng Y (2019) Nonlinearity of root trait relationships and the root economics spectrum. Nat Commun 10:1–14. https://doi.org/10.1038/s41467-019-10245-6
Kramer-Walter KR, Bellingham PJ, Millar TR et al (2016) Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. J Ecol 104:1299–1310. https://doi.org/10.1111/1365-2745.12562
Kramer-walter KR, Laughlin DC (2017) Root nutrient concentration and biomass allocation are more plastic than morphological traits in response tonutrient limitation. Plant Soil 416:539–550. doi:https://doi.org/10.1007/s11104-017-3234-9
Laliberté E, Lambers H, Burgess TI, Wright SJ (2015) Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol 206:507–521. https://doi.org/10.1111/nph.13203
Lambers H, Brundrett MC, Raven JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 348:7–27. https://doi.org/10.1007/s11104-011-0977-6
Lepš J, de Bello F, Šmilauer P, Doležal J (2011) Community trait response to environment: disentangling species turnover vs intraspecific trait variability effects. Ecography (Cop) 34:856–863. https://doi.org/10.1111/j.1600-0587.2010.06904.x
Liu G, Freschet GT, Pan X, Cornelissen JH, Li Y, Dong M (2010) Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol 188:543–553. https://doi.org/10.1111/j.1469-8137.2010.03388.x
Maestre FT, Reynolds JF (2006) Small-scale spatial heterogeneity in the vertical distribution of soil nutrients has limited effects on the growth and development of Prosopis glandulosa seedlings. Plant Ecol 183:65–75. https://doi.org/10.1007/s11258-005-9007-1
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195:823–831. https://doi.org/10.1111/j.1469-8137.2012.04198.x
McCormack ML, Iversen CM (2019) Physical and functional constraints on viable belowground acquisition strategies. Front Plant Sci 10:1–12. https://doi.org/10.3389/fpls.2019.01215
Minden V, Venterink HO (2019) Plant traits and species interactions along gradients of N , P and K availabilities. Funct Ecol 00:1–16. https://doi.org/10.1111/1365-2435.13387
Morales J, Squeo FA, Tracol Y, et al (2014) Resource economics and coordination mong above- and below-ground functional traits of threedominant shrubs from the Chilean coastal desert. J Plant Ecol 8:70–78. doi:https://doi.org/10.1093/jpe/rtu010
Noy-Meir I (1973) Desert ecosystems: Environment and producers. Annu Rev Ecol635 Syst 4: 25–51. https://doi.org/10.1146/annurev.es.04.110173.000325
Ordoñez JC, Van Bodegom PM, Witte JPM et al (2009) A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob Ecol Biogeogr 18:137–149. https://doi.org/10.1111/j.1466-8238.2008.00441.x
Pakeman R, Quested H (2007) Sampling plant functional traits : what proportion of the species need to be measured ? Appl Soil Ecol 10:91–96
Pérez-Harguindeguy N, Díaz S, Garnier E et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 62:167–234
Pérez-Ramos IM, Roumet C, Cruz P et al (2012) Evidence for a “plant community economics spectrum” driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J Ecol 100:1315–1327. https://doi.org/10.1111/1365-2745.12000
Poorter H, Niklas KJ, Reich PB, et al (2012) Biomass allocation to leaves, stems and roots : meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. doi:https://doi.org/10.1111/j.1469-8137.2011.03952.x
Prieto I, Pugnaire FI, Ryel RJ (2014) Water uptake and redistribution during drought in a semiarid shrub species. Funct Plant Biol 41:812–819. https://doi.org/10.1071/FP13300
Prieto I, Querejeta JI, Segrestin J et al (2018) Leaf carbon and oxygen isotopes are coordinated with the leaf economics spectrum in Mediterranean rangeland species. Funct Ecol 32:612–625. https://doi.org/10.1111/1365-2435.13025
Prieto I, Roumet C, Cardinael R et al (2015) Root functional parameters along a land-use gradient: evidence of a community-level economics spectrum. J Ecol 103:361–373. https://doi.org/10.1111/1365-2745.12351
Qiang Zhang, Gaoming Xiong, Jiaxiang Li, Zhijun Lu, Yuelin Li, Wenting Xu, Yang Wang, Changming Zhao, Zhiyao Tang, Zongqiang Xie, (2018) Nitrogen and phosphorus concentrations and allocation strategies among shrub organs: the effects of plant growth forms and nitrogen-fixation types. Plant and Soil 427 (1-2):305-319
R Development Core Team (2014) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Read QD, Henning JA, Sanders NJ (2017) Intraspecific variation in traits reduces ability of trait-based models to predict community structure. J Veg Sci 28:1070–1081. https://doi.org/10.1111/jvs.12555
Reich PB, Oleksyn J, Wright IJ (2009) Leaf phosphorus influences the photosynthesis-nitrogen relation: A cross-biome analysis of 314 species. Oecologia 160:207-212. doi: 10.1007/s00442-009-1291-3
Reich PB (2014) The world-wide “fast-slow” plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Richardson SJ, Peltzer DA, Allen RB, McGlone M, Parfitt RL (2004) Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139:267–276. https://doi.org/10.1007/s00442-004-1501-y
Roumet C, Birouste M, Picon-Cochard C, Ghestem M, Osman N, Vrignon-Brenas S, Cao KF, Stokes A (2016) Root structure-function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytol 210:815–826. https://doi.org/10.1111/nph.13828
Ruili Wang, Qiufeng Wang, Ning Zhao, Zhiwei Xu, Xianjin Zhu, Cuicui Jiao, Guirui Yu, Nianpeng He, Shuli Niu, (2017) Different phylogenetic and environmental controls of first‐order root morphological and nutrient traits: Evidence of multidimensional root traits. Functional Ecology 32 (1):29-39
Sardans J, Peñuelas J (2015) Potassium : a neglected nutrient in global change. Glob Ecol Biogeogr 24:261–275. https://doi.org/10.1111/geb.12259
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. doi:https://doi.org/10.1038/nmeth.2089
Schlesinger WH, Raikks JA, Hartley AE, Cross AF (1996) On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77:364–374. https://doi.org/10.2307/2265595
Siefert A, Violle C, Chalmandrier L, Albert CH, Taudiere A, Fajardo A, Aarssen LW, Baraloto C, Carlucci MB, Cianciaruso MV, de L Dantas V, de Bello F, Duarte LD, Fonseca CR, Freschet GT, Gaucherand S, Gross N, Hikosaka K, Jackson B, Jung V, Kamiyama C, Katabuchi M, Kembel SW, Kichenin E, Kraft NJ, Lagerström A, Bagousse-Pinguet YL, Li Y, Mason N, Messier J, Nakashizuka T, Overton JM, Peltzer DA, Pérez-Ramos IM, Pillar VD, Prentice HC, Richardson S, Sasaki T, Schamp BS, Schöb C, Shipley B, Sundqvist M, Sykes MT, Vandewalle M, Wardle DA (2015) A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol Lett 18:1406–1419. https://doi.org/10.1111/ele.12508
Squeo, FA, Aravena, R, Aguirre, E, et al. (2006). Groundwater dynamics in a coastal aquifer in north‐central Chile: Implications for groundwaterrecharge in an arid ecosystem. J Arid Environ, 67, 240–254. doi: https://doi.org/10.1016/j.jaridenv.2006.02.012
Stevens MHH, Carson WP (2002) Resource quantity , not resource heterogeneity , maintains plant diversity. Ecol Lett 5:420–426
Thomas DS, Montagu KD, Conroy JP (2006) Leaf inorganic phosphorus as a potential indicator of phosphorus status, photosynthesis and growth of Eucalyptus grandis seedlings. For Ecol Manage 223:267-274. doi: 10.1016/j.foreco.2005.11.006
Tomlinson KW, Sterck FJ, Bongers F et al (2012) Biomass partitioning and root morphology of savanna trees across a water gradient. J Ecol 100:1113–1121. https://doi.org/10.1111/j.1365-2745.2012.01975.x
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea:how can it occur? Biogeochemistry 13:87–115
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) Smatr 3- an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259. https://doi.org/10.1111/j.2041-210X.2011.00153.x
Weemstra M, Mommer L, Visser EJW, van Ruijven J, Kuyper TW, Mohren GM, Sterck FJ (2016) Towards a multidimensional root trait framework: a tree root review. New Phytol 211:1159–1169. https://doi.org/10.1111/nph.14003
Westoby M, Falster DS, Moles AT et al (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159. https://doi.org/10.1146/annurev.ecolsys.33.010802.150452
White PJ, George TS, Dupuy LX et al (2013) Root traits for infertile soils. Front Plant Sci 4:1–7. https://doi.org/10.3389/fpls.2013.00193
Withington JM, Reich PB, Oleksyn J, Eissenstat DM (2006) Comparison of structure and life span in roots and leaves among temperate trees. Ecol Monogr 76:381–397. https://doi.org/10.1890/0012-9615(2006)076[0381:COSALS]2.0.CO;2
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Yavitt JB (2000) Nutrient dynamics of soil derived from different parent material on Barro Colorado Island, Panama. Biotropica 32:198–207
Zangaro W, de Assis RL, Rostirola LV, de Souza PB, Gonçalves MC, Andrade G, Nogueira MA (2008) Changes in arbuscular mycorrhizal associations and fine root traits in sites under different plant successional phases in southern Brazil. Mycorrhiza 19:37–45. https://doi.org/10.1007/s00572-008-0202-5
Acknowledgments
We thank all those who assisted with data collection in the field. This research was supported by grants from FONDECYT Regular 1151020 and CONICYT PIA APOYO CCTE AFB170008. C.A.D. and D.E.C. were supported by CONICYT doctoral fellowships (21150334 and 21140050, respectively). D.E.C. was also supported by ARBOLES Project (NERC-NEWTON-CONICYT NE S011811/1). A.P.L. was supported by FONDECYT initiation grant (11140400). I.P was supported by “Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia” through an “Ayudas a Proyectos para la Generación de Nuevo Liderazgo Científico “Jóvenes Líderes en Investigación” (20654/JLI/18).
None of the authors declare conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Luke McCormack.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 951 kb)
Rights and permissions
About this article
Cite this article
Delpiano, C.A., Prieto, I., Loayza, A.P. et al. Different responses of leaf and root traits to changes in soil nutrient availability do not converge into a community-level plant economics spectrum. Plant Soil 450, 463–478 (2020). https://doi.org/10.1007/s11104-020-04515-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04515-2