Abstract
Background and aims
Concentrations of chemical analogs arsenic (As) and phosphorus (P) were measured in As-hyperaccumulator (Pteris vittata; PV) and three non As-hyperaccumulators (Thelypteris kunthii, Nephrolepsis brownii, and N. falcata) to draw inferences regarding their uptake from soils to roots and translocation to fronds and spores.
Methods
Frond and root samples of 150 ferns at peak spore maturation were collected with associated soils between July 2012 and July 2014.
Results
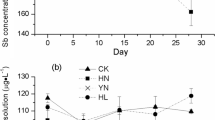
Arsenic in PV spores (45.4–336 mg kg−1) exceeded soil As (0.60–111 mg kg−1) in all sites and at clean sites (0.60–1.17 mg kg−1) for non-hyperaccumulator spores (1.83–8.60 mg kg−1). In PV, As in fronds and spores correlated positively with soil As (r = 0.71–0.74) with bioconcentration factors (tissue As:soil As) of 14.3–654 and 3.26–53.6 compared to 0.08–0.44 and 0.03–8.37 for three non-hyperaccumulators. However, P in PV spores (1977–4832 mg kg−1) correlated negatively with frond (1028–2439 mg kg−1; r = −0.43) and soil (76.2–170 mg kg−1; r = −0.34) P.
Conclusions
PV hyperaccumulates As into fronds and spores from soils with trace As. Since PV spores constituted ~9 % of frond biomass, the elevated spore As may deserve further investigation in their role as a potential health hazard and metal cycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) has no known biological function and is toxic to all biota. Even at low concentrations, As negatively impacts physiological functions in humans. Thus, its presence in the environment constitutes an imperative health threat (Ajmone-Marsan and Biasioli 2010). In addition to being a carcinogen, As exposure can inactivate enzymes, inhibit functional groups, displace essential elements and produce reactive oxygen species (Nagajyoti et al. 2010). Identifying potential As exposure pathways, especially those that occur naturally in the environment are important to protect public health (Hughes et al. 2011).
Several fern species have been identified as arsenic hyperaccumulators, including Pteris vittata (PV) (Ma et al. 2001), Pteris cretica, Pteris longifolia, and Pteris umbrosa (Zhao et al. 2002), and Pityrogramma calomelanos (Visoottiviseth et al. 2002). PV is one of the most efficient As-hyperaccumulators, with As bioconcentration factor (BF) exceeding 26 (ratio of frond As to soil As) (Lessl and Ma 2013). PV tissues including callus, gametophyte, and sporophyte all effectively accumulate As (Gumaelius et al. 2004; Yang et al. 2007).
However, despite extensive research on As-hyperaccumulators, especially PV, little is known about As concentrations in fern spores. Lombi et al. (2002) reported 376 mg kg−1 As in PV spores growing in a soil spiked with 300 mg kg−1 As. Given its As hyperaccumulation nature and the known dispersal range of fern spores (Wolf et al. 2001), it is important to examine the As contents in PV spores grown under natural conditions. Though there is little research on fern spores, there are reports of zinc, copper, and lead accumulation in pollen grains of common ragweed (Ambrosia artemisiifolia), averaging 94, 18, and 3.1 mg kg−1 (Cloutier-Hurteau et al. 2014). Furthermore, pollen from annual blue grass (Poa annua) grown from a cadmium-contaminated soil contain 10 % more allergen compounds, exacerbating airway diseases (Aina et al. 2010). While there is limited information on metals in pollens, there is even less on fern spores.
As a phosphorus (P) analog, arsenic is taken up via P transporters in plants. P is an essential nutrient for plants and is taken up from soils as inorganic phosphate, which has low availability. Background concentrations of As in plant tissues are 0.1–1.0 mg kg−1 compared to ~3000 mg kg−1 P (Marschner 2012). Plant P uptake is not only highly regulated, but also inextricably tied to As uptake. Under low P conditions, As uptake by plants induces P deficiency (Finnegan and Chen 2012). In PV fronds, however, As concentrations can exceed P without hindering its growth (Lessl and Ma 2013), exemplifying the unique relationship of As and P in PV. Plants normally respond to As by suppressing P uptake, but this is not the case in PV, which has increased P uptake, especially in the roots (Luongo and Ma 2005). Evaluation of As and P content in different fern tissues will facilitate a better understanding of As resiliency in PV.
We investigated As-hyperaccumulator (PV) and three non-hyperaccumulators (T. kunthii, N. brownii, and N. falcata). Cultivated and non-cultivated ferns were collected from urban soils including contaminated and clean sites in North Florida, USA. The specific objectives of this study were to measure As and P in soils, and fern roots, fronds and spores to infer their uptake, translocation and accumulation characteristics in plants. The present study also reports on the implication elevated As concentrations in PV spores on As cycling in the environment in addition to the potential health risk of As exposure to humans.
Materials and methods
Fern collection and spore measurement
All samples were collected from Gainesville, Florida where P. vittata, T. kunthii, N. brownii, and N. falcata commonly grow. Sixteen sites were identified with fern plants containing mature fronds of 1–2.5 m. Frond and root samples of 114 PV, 24 T. kunthii, 6 N. brownii and 6 N. falcata were collected along with the associated soils between July 2012 and July 2014 when ferns exhibited peak spore maturation. Cultivated PV plants of 3–4 year old (sites 5–10) growing in As-contaminated soils were sampled on three separate occasions (18 samples per site). Samples were taken from each remaining site, which contained naturally growing ferns of ~6 year old. Soil samples were taken from the base of each fern, and roots were removed for separate analysis. After rinsing with deionized (DI) water to remove attached soil, roots were dried at 60 °C for 72 h. In order to obtain a sufficient quantity of spore, 3–5 large fronds (~1.5 m length) from each fern were clipped from the base of the rhizome, weighed, and placed over clean papers with sori side down. Spores were collected after the tissues were air-dried for 1 week at room temperature. After collection, frond tissues were placed at 60 °C for 72 h, weighed, and ground through a 2-mm sieve for analysis.
Spores from fern plants were sifted through a 50-μm sieve to remove extraneous fern tissues (Fig. 1). The sori on dry fronds was gently brushed off and sieved through a 250-μm sieve to collect sporangia. For quantification, 10 mg of PV spores from each site were suspended in 1.0 mL DI water and vortexed. Once the samples were homogenous, three 10.0 μL aliquots were placed onto glass slides for enumeration via light microscopy. Spore production was calculated as both mass and number of spores per gram of dry frond tissue.
Chemical analyses and As speciation
Soils were air-dried, sieved through a 2-mm screen and analyzed for pH (1:2 soil to water) and Mehlich extractable P (1:10 soil to solution ratio shaken at 100 rpm for 5 min). Total As and P concentrations were assessed by subjecting 1.00 g of soil or 0.10 g of plant tissue to HNO3/H2O2 digestion (USEPA Method 3051) on a hot block (Environmental Express, Ventura, CA). Briefly, samples were suspended in 15 mL 1:1 nitric acid (trace metal grade) and heated at 105 °C for 5 h. Samples were then cooled, adding 1 mL 20 % H2O2 and digested for an additional 30 min before bringing samples to a 50 mL volume with distilled deionized water.
The solubility of As and P from spores were assessed by shaking 0.100 g samples in 10 mL DI water for 15 min at 200 rpm. An aliquot of the solution was filtered (0.2 μm) while the remaining sample was digested as described previously. Analysis of As and P were performed by inductively coupled plasma mass spectrometry (ICP–MS; Perkin Elmer Corp., Norwalk, CT). Detection limits were calculated from multiple analyses of blank solutions. All standards, samples, and blanks were made up in acid-washed (2.5 % HNO3) glassware and plastic materials.
Filtered solutions of DI water after washing spores (described previously) in addition to spores ground in a mortar were used for As speciation via high performance liquid chromatography (HPLC, Waters e2695, Ireland) coupled with ICP−MS with a Hamilton PRP–X100 anion exchange column (4.1 × 250 mm, 10 μm) (Hamilton, UK). The operating conditions and elution program was slightly modified from Xu et al. (2014). The mobile phase consisted of 10 mM NH4NO3 and 10 mM (NH4)2HPO4 adjusted to pH 6.2 with a flow rate of 1.0 mL min−1. The As standards were purchased from the National Research Center for Certified Reference Materials of China and prepared daily.
Statistical analyses
Data are presented as mean of all replicates with standard error. Two-way Analysis of Variance (ANOVA) were used to determine significant differences between treatment means and compared by Tukey’s multiple range tests at p < 0.05. Pearson correlations were performed to test for possible relationships between As concentrations in soils, and fern roots, fronds and spores. Regression statistical analysis was performed using JMP®10 PRO (SAS Institute Inc., Cary, NC, 1989–2010).
Results and discussion
Arsenic is a non-essential element and its uptake by plants is probably an inadvertent consequence of co-transport with the essential element P. Certain plants such as As-hyperaccumulator PV have adapted traits to hyperaccumulate As from soils (Xie et al. 2009). Since its discovery, extensive research has focused on As uptake and translocation in PV sporophytes and gametophytes (Gumaelius et al. 2004; Liao et al. 2004; Li et al. 2005a; Pickering et al. 2006; Yang et al. 2007). However, PV reproductive tissues have received scant attention. For that matter, there is a lack of information regarding the elemental distribution in fern spores. The goal of this study was to examine the transfer of As (toxic element) and P (essential nutrient) from soils to As-hyperaccumulator PV and non-hyperaccumulators including T. kunthii, N. brownii and N. falcata with emphasis on their reproductive tissues.
Characteristics of sampling sites
Arsenic is ubiquitous in the environment, averaging 5 mg kg−1 in clean soils worldwide (Reimann and Garrett 2005). Florida soils typically contain low As due to their sandy nature, with a background concentration of ~0.4 mg kg−1 (Chen et al. 2001). PV is a naturalized fern plant originally from China while the other three ferns are native to subtropical regions and often grow together (Nelson 2000). Sites were grouped into three categories: natural ferns in clean soils (0.6–1.2 mg kg−1 As; sites 1, 2, 11, 12, 15, and 16), natural ferns in As-contaminated soils (4.0–59 mg kg−1 As; sites 3, 4, 13, and 14), and cultivated PV in As-contaminated soils (17–111 mg kg−1 As; sites 5–10) (Table 1). Soils were considered clean if the As concentration was lower than Florida cleanup level for residential soils (2.1 mg kg−1 As) (FDEP 2005). Natural PV and T. kunthii in As-contaminated soils were growing underneath structures made from As-treated wood in residential apartments (Gress et al. 2014). Sites 5–10 contained cultivated PV plants from As-contaminated soils, which were maintained in raised beds and harvested in 6 month intervals from December 2009 through July 2014 (Lessl and Ma 2013).
Arsenic uptake in roots and translocation to fronds
Grown in the presence of As, PV rapidly takes it up and transports it to the fronds where it is stored in the vacuoles of epidermal cells (Shen et al. 2014). The As content in PV tissues were consistent with this observation at 14–698 mg kg−1 in the roots and 454–4973 mg kg−1 in the fronds (Table 1). Comparatively, As in the three non-hyperaccumulators were 0.2–43 mg kg−1 in the roots and 0.3–6.9 mg kg−1 in the fronds (Table 1). Taking the lowest As concentration for example, the As concentration in PV was 70–151 times greater than those in the three non-hyperaccumulators. In PV, root and frond As concentrations correlated positively with soil As (Table 2).
However, As bioconcentration factor (BF; ratio of tissue As to soil As) differed among the three sites (Table 3). Arsenic in the roots and fronds of cultivated PV (sites 5–10) were 52–158 and 455–2065 mg kg−1, with a BF of 3.5 for roots and 26 for fronds and an arsenic translocation factor (TF; ratio of frond As to root As) of 7.8 (Table 3). Comparatively, BFs in natural PV from As-contaminated soils (sites 3 and 4) were significantly larger at 25 for roots and 232 for fronds with a TF of 17 (Table 3). The disparity in As accumulation between natural and cultivated PV could be attributable to differences in canopy size, plant age and climatic factors. Mobile elements like P can be redistributed from older to younger tissues to support active growth (Sánchez-Calderón et al. 2010). It is possible that in PV, As and P behave similarly. Since cultivated PV fronds were removed biannually, there was less opportunity for continued As translocation from the roots to fronds. Natural PV were unattended for years, so higher translocation was expected.
Compared to contaminated soils, higher As BF was observed in natural PV growing in clean soils (0.8–1.1 mg kg−1). Despite limited As (sites 1 and 2), the roots (~19 mg kg−1 As) and fronds (~509 mg kg−1) in natural PV had the highest BF at 643 for fronds (Table 1). It is interesting to note that even at low soil As concentrations (0.8–1.1 mg kg−1), PV continued to accumulate As from soils. These observations suggest that PV does not simply tolerate As but probably actively takes it up typified by BF of 643 for fronds in natural PV growing in clean soils (Table 3). At low soil concentrations, As is preferentially allocated to young PV fronds (Tu and Ma 2005). So uptake and translocation of As is more characteristic of a nutrient than a toxic metal in PV. Even though As has no known biological function, in the presence of As, PV has been observed to exhibit increased growth (Lessl et al. 2013; Xu et al. 2014).
To put results of As-hyperaccumulator PV in context, naturally growing non-hyperaccumulating ferns were sampled from As-contaminated (14–59 mg kg−1) and clean sites (0.6–1.2 mg kg−1) (Table 1). Regardless of soil As concentrations, their BFs were ~1 for roots and <0.6 for fronds (Tables 3 and 4). This is typical of As-sensitive plants, which limit As uptake (Meharg and Hartley-Whitaker 2002). There was a slight increase in frond As of T. kunthii from < 0.3 in clean soils to 5.7 mg kg−1 in contaminated soils (Table 1). Arsenic BF for fronds in T. kunthii, N. brownii, and N. falcata was 0.3–0.4 compared to 26–654 in PV (Table 3).
Arsenic transfer from fronds to spores
Arsenic in PV spores was 45–487 mg kg−1 (Table 1), which was positively correlated with As concentrations in soils, fronds and roots (r = 0.71–0.78) (Table 2). Similar to As BF for fronds, the BF for spores was the highest in natural PV from clean sites and lowest in cultivated PV (sites 5–10) (Table 3). Compared to fronds, PV spores contained ~17 % of the As with no difference between sites (Table 3). The source of As in spores is unclear, but since As is sequestered as arsenite (AsIII) (≥94 %) in fronds (Chen et al. 2004), we compared As speciation in PV spores and sporangia. Similar to PV fronds (Singh and Ma 2006), no methylated As species were detected in the reproductive tissues (data not shown). However, ~64 % of the As in spores and sporangia was AsIII (Table 5), a striking difference from the fronds (Chen et al. 2004). The increase in oxidized arsenate (AsV) in reproductive tissues compared to fronds suggests that it was probably oxidized in the spore tissue.
In homosporous ferns like PV, following its single-cell origin, the sporangium divides into 16 sporogenous cells, which undergo three rounds of meiosis to form 64 spores surrounded by a single-layered tapetum and a microsporangial wall (Fig. 1) (Raghavan 1989). The tapetum forms a loose meshwork of tissue that surrounds spores and coats the inside walls of the sporangia. We attempted to differentiate internal and external As by shaking spores in DI water for 15 min and determining As speciation in solution. Washing spores with DI water solubilized ~31 % of the total As, with 81 % being AsV (Table 5). This led us to suspect AsV in spores may be associated with tapetal tissues, which was exposed to air and subjected to oxidation following spore release. Due to its toxicity, the soluble As associated with PV spores could benefit gametophyte establishment by excluding As sensitive plants in the environment. Furthermore, since As has been shown to improve growth of PV gametophytes (Lessl et al. 2013), it is possible the As in spores is advantageous for PV establishment and subsequent growth.
Spores from three non-hyperaccumulators all contained higher As concentrations than normally found in plant tissues. This is uncharacteristic for plant reproductive tissues, which accumulate limited non-essential elements (Kranner and Colville 2011). Interestingly, As in T. kunthii spores from clean soils (0.6 mg kg−1 in sites 11 and 12) contained ~2.5 times more As than spores from contaminated soils (14–59 mg kg−1 in sites 13 and 14), i.e., 4.7 vs. 1.9 mg kg−1 (Table 1). This was despite the fact that T. kunthii from contaminated soils contained 40 times more As in the roots and 21 times more As in the fronds (Table 1). A similar trend was observed in N. brownii and N. falcata, which had ~27 times higher As concentration in the spores (4.4–8.6 mg kg−1) than fronds and roots (0.23–0.85 mg kg−1) (Table 4).
Enrichment of non-essential elements in reproductive tissues is unusual, especially for plants growing in clean soils (Kranner and Colville 2011). The paucity of information pertaining to elemental distribution in fern spores makes it difficult to hypothesize the reason behind this observation. Non-essential metals are often accumulated in plant roots where they are sequestered in the vacuoles, limiting their translocation to reproductive tissues (Li et al. 2005b; Kranner and Colville 2011). This is an adaptive trait for plants, as metals are known to have detrimental impacts on plant germination and growth (Zhang et al. 2010; Nagajyoti et al. 2010). This is especially true for As, which is toxic to plants (Robinson et al. 2009).
The low As in T. kunthii spores in contaminated soils may be due to As-triggered defense mechanisms, which inhibited As translocation explaining the high root As and low spore As (Table 1). When exposed to toxic As concentrations, defense systems in non-hyperaccumulating plant are postulated to reduce rate of As uptake (Catarecha et al. 2007). Comparatively, the relative high As in T. kunthii, N. brownii, and N. falcata spores in clean soils could be a byproduct of nutrient co-transport with P. Fern spores amass sufficient nutrients to allow germination of the free-living gametophyte stage (Raghavan 1989). Elevated cobalt, lead, chromium, and silver in Onoclea sensibilis spores are hypothesized to be co-transported with essential trace metals, which act as a reservoir for the developing gametophyte (Wayne and Hepler 1985).
The presence of As in spores had no apparent impact on their viability. Spores from all ferns had the same relative germination rate after 10 days in sterile DI water (data not shown). Although if there were a discrepancy, it would be difficult to definitively confirm if As were the sole cause. Further experiments are required to better our understanding of this relationship.
Phosphorus in fern tissues
Total P concentrations in fern roots and fronds averaged 1800 and 1700 mg kg−1 (Table 6). Half of the cultivated PV ferns (sites 5–10) received soluble P applications (site 7, 8, and 10) (Lessl and Ma 2013), having ~39 % higher root and frond P concentrations than non-cultivated PV (Table 6). However, regardless of P status, growth of PV and T. kunthii appeared equally healthy and vigorous. Since the range of P for optimal growth is species specific, little can be gleaned from P concentration alone. For example, PV frond P concentrations were substantially lower than those for PV growing under hydroponic conditions, which averaged 5100 mg kg−1 in the roots and 3400 mg kg−1 in the fronds (Wang et al. 2002; Singh and Ma 2006; Baldwin and Butcher 2007). Cultivated fern fronds from 24 species averaged 3900 mg kg−1 P (Mills and Jones 1996). The low P concentrations from this study were not surprising because most of the ferns were grown under natural conditions, which lacked the P inputs of hydroponic and cultivated ferns.
Spore P concentrations were highly variable, ranging from 847 to 6427 mg kg−1 but were generally higher than other fern tissues (Table 6). Spore P concentration correlated negatively with frond P in PV (r = −0.43) but positively with those in three non-hyperaccumulators (r = 0.56; Table 2). Little is known about the elemental distribution in fern spores, although Onoclea sensibilis spores contain 8200 mg kg−1 P (Wayne and Hepler 1985). Fern spores contain all essential elements to allow germination and establishment of the gametophyte (Raghavan 1989). In PV, P concentrations in the spores increased with decreasing soil and frond P concentrations. The data suggested that under P limiting conditions, PV allocated additional P to the spores to improve successful establishment of gametophyte offspring. However, the opposite trend was observed for three non-hyperaccumulators. Hence, additional fern species are needed to assess if this observation is unique to PV or As hyperaccumulators.
Arsenate and phosphate are chemical analogs and share the same transporters in plants. This poses a problem for plants, as P acquisition under P limiting condition may promote plant As uptake. Arsenic is generally less mobile than P with respect to translocation from plant roots to shoots (Meharg et al. 1994). The exception is As-hyperaccumulators like PV. In almost every regard, the relationship between spore As and P had the opposite correlation when comparing PV to the three non-hyperaccumulators (Table 2). Spore As correlated positively with frond (r = 0.49) and root (r = 0.37) P in PV and negatively in three non-hyperaccumulators. There was no relationship between spore As and spore P in PV but were negatively correlated in three non-hyperaccumulators. This suggested that As translocation from fronds to spores was not competing with P in PV, but competed with P in three non-hyperaccumulators. We speculated that PV has evolved a high capacity of As transport from the fronds to the spores as an adaptive trait.
Are PV spores a potential source of As to humans?
There is substantial concern pertaining to As exposure because of its link to human carcinogenesis and the development of health disorders. Ingestion of contaminated soils, especially by children, is an important exposure pathway for risks associated with As exposure (Smith et al. 2009). Similarly, there has been growing interest in investigating the role of pollen-induced health risks (Makra et al. 2010), particularly due to evidence that pollutants associated with pollen contributing to respiratory allergy symptoms (Motta et al. 2006).
Like-wise, fern spores are wind-dispersed propagules that are produced in large numbers and capable of traveling thousands of kilometers (Wolf et al. 2001). PV spores had a diameter of ~40 μm (Fig. 2), comprising ~9.7 % of total dry frond biomass and accounting for ~3.7 × 109 spores kg−1 frond (Table 7). Total PV frond biomass was 40–200 g plant−1 (dry weight), averaging 70 g plant−1 (data not shown). Under Florida conditions, young PV fiddleheads can mature to spore-bearing fronds in 5–6 months and grow year round (Lessl and Ma 2013). Thus, in a year, a single PV fern could produce ~7.76–38.8 g spore accounting for 0.9–7.8 mg As in clean soils (site 1 and 2) and 2.3–12 mg As in contaminated soils (site 3 and 4). World Health Organization (WHO) determined the lower limit on the target dose for an increased incidence of cancer to be 2–7 μg/kg body weight per day based on the range of estimated total dietary exposure (WHO 2010). Additional risk of As exposure from PV spores would depend on a person’s proximity to and density of PV plants. While spores can be distributed aerially over long distances, the spatial pattern of PV spore dispersal has yet to be established.
The As in PV spores was ~31 % soluble and mostly as AsV, which would designate a base level of bioavailable As since additional As could become available upon ingestion or inhalation. While not a significant source of As, it could be a contributing factor in a consortium of other particulates known to negatively impact human health. Pollen has been demonstrated to enter and deposit in the airways while small pollen and broken fragments (≤20 μm) have been shown to deposit in the lung, especially in the bronchial region (Horváth et al. 2011). The influx of As-enriched PV spores could exacerbate the impact that other airborne particulates have on allergy symptoms and respiratory diseases.
Conclusions
This is the first study to investigate the transfer of toxic As and nutrient P from soils to the roots, fronds, and spores in As-hyperaccumulator and non-hyperaccumulators. Arsenic in PV spores exceeded soil As concentrations at all sites. The As BF in PV was the highest in tissues from clean sites, indicating a threshold of As for optimal growth may exist for PV. The remarkable accumulation of As in PV tissues despite negligible soil concentrations necessitates further investigation of its role in its growth and function.
Unexpectedly, in clean soils, As content of typical ferns T. kunthii, N. brownii, and N. falcata were the highest in the spores. The significant bioaccumulation of As from soils to the spores deviates from the typical avoidance mechanisms preventing metal transfer to reproductive tissues. The results from this study pointed to the need for new data to better understand the mechanisms involved in the transfer of metals from soils to spores, especially for the purpose of evaluating their impact on human health.
References
Aina R, Asero R, Ghiani A et al (2010) Exposure to cadmium-contaminated soils increases allergenicity of Poa annua L. pollen. Allergy 65:1313–1321. doi:10.1111/j.1398-9995.2010.02364.x
Ajmone-Marsan F, Biasioli M (2010) Trace elements in soils of urban areas. Water Air Soil Pollut 213:121–143. doi:10.1007/s11270-010-0372-6
Baldwin PR, Butcher DJ (2007) Phytoremediation of arsenic by two hyperaccumulators in a hydroponic environment. Microchem J 85:297–300. doi:10.1016/j.microc.2006.07.005
Catarecha P, Segura MD, Franco-Zorrilla JM et al (2007) A Mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell Online 19:1123–1133. doi:10.1105/tpc.106.041871
Chen M, Ma LQ, Hoogeweg CG, Harris WG (2001) Arsenic background concentrations in Florida, U.S.A. surface soils: determination and interpretation. Environ Forensic 2:117–126. doi:10.1006/enfo.2001.0050
Chen R, Smith BW, Winefordner JD et al (2004) Arsenic speciation in Chinese brake fern by ion-pair high-performance liquid chromatography-inductively coupled plasma mass spectroscopy. Anal Chim Acta 504:199–207. doi:10.1016/j.aca.2003.10.042
Cloutier-Hurteau B, Gauthier S, Turmel M-C et al (2014) Trace elements in the pollen of Ambrosia artemisiifolia: what is the effect of soil concentrations? Chemosphere 95:541–549. doi:10.1016/j.chemosphere.2013.09.113
FDEP (2005) Technical Report: Development of Cleanup Target Levels (CTLs) for Chapter 62–777, F.A.C.
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol. doi:10.3389/fphys.2012.00182
Gress JK, Lessl JT, Dong X, Ma LQ (2014) Assessment of children’s exposure to arsenic from CCA-wood staircases at apartment complexes in Florida. Sci Total Environ 476–477:440–446. doi:10.1016/j.scitotenv.2014.01.018
Gumaelius L, Lahner B, Salt DE, Banks JA (2004) Arsenic hyperaccumulation in gametophytes of Pteris vittata. A new model system for analysis of arsenic hyperaccumulation. Plant Physiol 136:3198–3208. doi:10.1104/pp. 104.044073
Horváth A, Balásházy I, Farkas Á et al (2011) Quantification of airway deposition of intact and fragmented pollens. Int J Environ Health Res 21:427–440. doi:10.1080/09603123.2011.574269
Hughes MF, Beck BD, Chen Y et al (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332. doi:10.1093/toxsci/kfr184
Kranner I, Colville L (2011) Metals and seeds: biochemical and molecular implications and their significance for seed germination. Met Toler Plants Lichens 72:93–105. doi:10.1016/j.envexpbot.2010.05.005
Lessl JT, Ma LQ (2013) Sparingly-soluble phosphate rock induced significant plant growth and arsenic uptake by Pteris vittata from three contaminated soils. Environ Sci Technol 47:5311–5318. doi:10.1021/es400892a
Lessl JT, Ma LQ, Rathinasabapathi B, Guy C (2013) Novel phytase from Pteris vittata resistant to arsenate, high temperature, and soil deactivation. Environ Sci Technol 47:2204–2211. doi:10.1021/es3022073
Li W, Chen T, Chen Y, Lei M (2005a) Role of trichome of Pteris vittata L. in arsenic hyperaccumulation. Sci China C Life Sci 48:148–154. doi:10.1007/BF02879667
Li W, Khan MA, Yamaguchi S, Kamiya Y (2005b) Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul 46:45–50. doi:10.1007/s10725-005-6324-2
Liao X-Y, Chen T-B, Lei M et al (2004) Root distributions and elemental accumulations of Chinese brake (Pteris vittata L.) from As-contaminated soils. Plant Soil 261:109–116. doi:10.1023/B:PLSO.0000035578.24164.fa
Lombi E, Zhao F-J, Fuhrmann M et al (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156:195–203. doi:10.1046/j.1469-8137.2002.00512.x
Luongo T, Ma LQ (2005) Characteristics of arsenic accumulation by Pteris and non-Pteris ferns. Plant Soil 277:117–126. doi:10.1007/s11104-005-6335-9
Ma LQ, Komar KM, Tu C et al (2001) A fern that hyperaccumulates arsenic. Nature 409:579–579. doi:10.1038/35054664
Makra L, Sánta T, Matyasovszky I et al (2010) Airborne pollen in three European cities: detection of atmospheric circulation pathways by applying three-dimensional clustering of backward trajectories. J Geophys Res Atmos 115, D24220. doi:10.1029/2010JD014743
Marschner H (2012) Marschner’s mineral nutrition of higher plants. Academic, London
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43. doi:10.1046/j.1469-8137.2002.00363
Meharg AA, Naylor J, Macnair MR (1994) Phosphorus nutrition of arsenate-tolerant and nontolerant phenotypes of velvetgrass. J Environ Qual 23:234–238. doi:10.2134/jeq1994.00472425002300020003x
Mills HA, Jones JB (1996) Plant analysis handbook II: a practical sampling, preparation, analysis, and interpretation guide. Micro–macro, Athens
Motta AC, Marliere M, Peltre G et al (2006) Traffic-related air pollutants induce the release of allergen-containing cytoplasmic granules from grass pollen. Int Arch Allergy Immunol 139:294–298. doi:10.1159/000091600
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. doi:10.1007/s10311-010-0297-8
Nelson G (2000) The ferns of Florida: a reference and field guide. Pineapple Press, Sarasota
Pickering IJ, Gumaelius L, Harris HH et al (2006) Localizing the biochemical transformations of arsenate in a hyperaccumulating fern. Environ Sci Technol 40:5010–5014. doi:10.1021/es052559a
Raghavan V (1989) Developmental biology of fern gametophytes. Cambridge University Press, New York
Reimann C, Garrett RG (2005) Geochemical background—concept and reality. Sci Total Environ 350:12–27. doi:10.1016/j.scitotenv.2005.01.047
Robinson BH, Bañuelos G, Conesa HM et al (2009) The phytomanagement of trace elements in soil. Crit Rev Plant Sci 28:240–266. doi:10.1080/07352680903035424
Sánchez-Calderón L, Chacon-López A, Pérez-Torres C-A, Herrera-Estrella L (2010) Phosphorus: plant strategies to cope with its scarcity. In: Hell R, Mendel R-R (eds) Cell biology of metals and nutrients. Springer, Berlin, pp 173–198
Shen H, He Z, Yan H et al (2014) The fronds tonoplast quantitative proteomic analysis in arsenic hyperaccumulator Pteris vittata L. J Proteomics 46–57. doi: 10.1016/j.jprot.2014.01.029
Singh N, Ma LQ (2006) Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ Pollut 141:238–246. doi:10.1016/j.envpol.2005.08.050
Smith E, Weber J, Juhasz AL (2009) Arsenic distribution and bioaccessibility across particle fractions in historically contaminated soils. Environ Geochem Health 31:85–92. doi:10.1007/s10653-009-9249-2
Tu C, Ma LQ (2005) Effects of arsenic on concentration and distribution of nutrients in the fronds of the arsenic hyperaccumulator Pteris vittata L. Environ Pollut 135:333–340. doi:10.1016/j.envpol.2004.03.026
Visoottiviseth P, Francesconi K, Sridokchan W (2002) The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ Pollut 118:453–461. doi:10.1016/S0269-7491(01)00293-7
Wang J, Zhao F-J, Meharg AA et al (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561. doi:10.1104/pp. 008185
Wayne R, Hepler PK (1985) The atomic composition of Onoclea sensibilis spores. Am Fern J 75:12–18. doi:10.2307/1546574
WHO (2010) Exposure to arsenic: a major public health concern
Wolf PG, Schneider H, Ranker TA (2001) Geographic distributions of homosporous ferns: does dispersal obscure evidence of vicariance? J Biogeogr 28:263–270
Xie Q-E, Yan X-L, Liao X-Y, Li X (2009) The arsenic hyperaccumulator fern Pteris vittata L. Environ Sci Technol 43:8488–8495. doi:10.1021/es9014647
Xu J, Li H, Liang S et al (2014) Arsenic enhanced plant growth and altered rhizosphere characteristics of hyperaccumulator Pteris vittata. Environ Pollut 194:105–111. doi:10.1016/j.envpol.2014.07.017
Yang X, Chen H, Xu W et al (2007) Hyperaccumulation of arsenic by callus, sporophytes and gametophytes of Pteris vittata cultured in vitro. Plant Cell Rep 26:1889–1897. doi:10.1007/s00299-007-0388-6
Zhang H, Tan Z-Q, Hu L-Y et al (2010) Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol 52:556–567. doi:10.1111/j.1744-7909.2010.00946.x
Zhao FJ, Dunham SJ, McGrath SP (2002) Arsenic hyperaccumulation by different fern species. New Phytol 156:27–31. doi:10.1046/j.1469-8137.2002.00493.x
Acknowledgments
This research was supported in part by the National Natural Science Foundation of China (No. 21277070), Jiangsu Provincial Innovation Fund, and Opportunity Seed Fund from UF/IFAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Henk Schat.
Capsule Pteris vittata hyperaccumulates As into fronds and spores from soils with little As, more characteristic of a nutrient than a toxic metal
Rights and permissions
About this article
Cite this article
Lessl, J.T., Guan, D.X., Sessa, E. et al. Transfer of arsenic and phosphorus from soils to the fronds and spores of arsenic hyperaccumulator Pteris vittata and three non-hyperaccumulators. Plant Soil 390, 49–60 (2015). https://doi.org/10.1007/s11104-014-2376-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2376-2