Abstract
This study aimed to investigate the Sb and As co-accumulating processes of Pteris vittata under soil culture condition, including the transformation of Sb and As, and the difference in co-accumulating ability among different plant species/populations. Two populations of P. vittata and one population of As-tolerant species Holcus lanatus L. were grown on soil co-contaminated by Sb and As. Sb and As speciation in plants was assessed by X-ray absorption near-edge structure (XANES) spectroscopy. P. vittata displayed strong As- but limited Sb-accumulating ability, with the highest shoot concentrations of As and Sb reaching 455 and 26 mg kg−1, respectively. After 28 days culture, the concentrations of Sb and As in the soil solution were reduced by up to 22% and 36% in the P. vittata treatments, respectively. Holcus lanatus showed limited uptake for both metalloids. In P. vittata, the reduction of arsenate to arsenite occurred (with As in shoots all existing as arsenite), but limited reduction of antimonate to antimonite (with more than 90% of Sb in shoots existing as antimonate) was observed. In terms of the differences in metalloid uptake between the two P. vittata populations, the population from the habitat with higher soil As concentration showed 35% higher As uptake than the population from the habitat with lower As concentration. This populational difference may partly result from varying As transformation efficiencies. However, no significant difference was observed in Sb accumulation between the two populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimony (Sb) and arsenic (As) are two harmful elements belonging to group 15 of the periodic table. Sb and As are often found together at high concentrations in mining and smelting sites, especially in mining sites of sulfide ore deposits (Sun et al. 2017). China is the world’s largest Sb producer, with an average annual production of 80% of the global produce (US Geological Survey 2013). Serious Sb contamination was detected in Hunan province (Li et al. 2014; Wei et al. 2015). Excess Sb and As in soil can cause health risks to surrounding residents and consumers of agricultural products (Abad-Valle et al. 2018; Arslan et al. 2018; Filella et al. 2002a, b; Pierart et al. 2015). The risks posed by soils co-contaminated by As and Sb must be urgently evaluated and controlled (Doherty et al. 2017; Mirza et al. 2017; Tandy et al. 2017; Xiao et al. 2017).
Phytoremediation recently received increasing attention due to its adaptability to large-scale farmland cleanup (Escapa et al. 2017; Schneider et al. 2016). Phytoremediation of As-contaminated soil has been successfully applied to several cases in China and other countries (Jankong et al. 2007; Niazi et al. 2012). Despite the growing interest in phytoremediation and the success of pilot studies, fundamental studies are still needed to disclose the complex interactions among contaminants, soil, and plants in the rhizosphere, especially under multi-metal(loid)s co-contamination (Chirakkara et al. 2016; Feng et al. 2017).
Given the advantages of phytoextraction, such as its harmlessness to soil quality and lower cost than most techniques, studies have been conducted to investigate the possibility of utilizing phytoextraction to soil contaminated with multi-metals. Several Brassicaceae and Sedum species can co-accumulate Zn and Cd (Fellet et al. 2013; Xing et al. 2012). Hydroponic experiments indicated that the shoot Sb and As concentrations in Vetiveria zizanioides reached 217 and ~ 500 mg kg−1 after 21-day culture, respectively (Mirza et al. 2017). One of our previous hydroponic experiments indicated that Pteris vittata can co-accumulate As and Sb in the pinnae, with the aboveground Sb and As concentrations measuring higher than 1000 mg kg−1 (Wan et al. 2016). However, thus far, no soil culture experiment has confirmed the co-accumulation of Sb and As by P. vittata.
The capacity of P. vittata to accumulate As varies among P. vittata populations (Wan et al. 2013a; Wu et al. 2009). The population difference in As accumulation may be strongly related to the conversion of As species (Wu et al. 2009). P. vittata populations with stronger ability to reduce As(V) to As(III) showed higher As-accumulating ability because As(III) was transported upward more easily than As(V) (Su et al. 2008). This intraspecies difference has become an important aspect in phytoextraction practice because it can produce large variation in extraction efficiency. However, no study determined whether these differences also exist in Sb accumulation among P. vittata populations.
Similar to As, earlier hydroponic experiments indicated that the trivalent form of Sb can be taken up by P. vittata more easily than the pentavalent one. When provided with Sb(III), Sb concentrations in the pinnae of P. vittata reached values 765% higher than those of plants exposed to Sb(V) (Wan et al. 2016). On the other hand, the Sb(III) percentage of the total Sb in the pinnae is lower than that of As (Wan et al. 2016). The transformation of Sb has been rarely reported in soil culture systems.
Our earlier study (Wan et al. 2016) on P. vittata and Mirza et al. (2017) on V. zizanioides have both revealed a mutual beneficial effect between As and Sb; thus, addition of As can improve Sb uptake by plants, and the addition of Sb can improve As uptake. These aforementioned studies involved hydroponic experiments. Thus, studying metalloid uptake in co-contaminated soils may bear importance.
The current experiment included a population of P. vittata that can co-accumulate Sb and As from solution spiked with both metalloids, a population of P. vittata with As-accumulating ability only, and an As-tolerant plant Holcus lanatus (Fernandez et al. 2017). Holcus lanatus was included to compare the differences in metalloid uptake between accumulators and non-accumulators.
Comparisons were performed on Sb and As uptake by these plants. The targeted processes included Sb and As species transformation in the soil–plant system by X-ray absorption near-edge structure (XANES) spectroscopy and transformation from solid to liquid phases via soil solution extraction. The present study aimed to (1) confirm the Sb and As co-accumulating ability of P. vittata under soil culture condition; (2) compare the transformation of Sb and As during the uptake process; and (3) investigate the difference in co-accumulating ability among different plant species/populations.
Materials and methods
Spores and seeds
Two populations of P. vittata were used in the current experiments. Spores of one population were collected from a soil in an Sb smelting area located in Xikuangshan, Hunan, China (abbreviated as HN population). This area features high concentrations of both As and Sb. Hunan province is the largest Sb ore area in the world. Xikuangshan Sb ore contains high amounts of Sb and As, and it has been mined for more than 110 years. The total concentrations of Sb and As in this soil reach approximately 453 and 147 mg kg−1, respectively.
Spores of the other P. vittata population were collected from a Pb–Zn mining area with high As concentrations in Yunnan province, China (abbreviated as YN population). Arsenic is a common accompanying element of Pb/Zn mines. The area where we sampled P. vittata spores features a Pb/Zn mining history of more than 200 years. The total Sb and As concentrations in this soil were approximately 10.9 and 572 mg kg−1, respectively. Table 1 provides background information of these sampling sites. Holcus lanatus seeds were provided by Emorsgate Seeds (Norfolk, England).
Preculture of P. vittata and Holcus lanatus
Spores of P. vittata were mixed together with water and then dispensed in soil (sieved through 2 mm mesh) at a density of 0.1 g m−2 to produce progenies of P. vittata. To get a uniform growth of P. vittata sporelings, the re-growth scenario was adopted. When the sporelings of P. vittata reached the height around 40~50 cm, the shoots were cut for the re-growth of them. The re-growing sporelings at the height of 10 cm were used in the experiment. Holcus lanatus seeds were directly placed on soil at a density of 10 g m−2 after soaking in 150 mg L−1 gibberellic acid (Merck) for 48 h.
The soils used for germination of spores or seeds were collected from a farmland in Beijing. These soils contained 18.5 g kg−1 organic matter, 0.88 g kg−1 total P, 1.04 g kg−1 total N, 14.2 cmol kg−1 cation exchange capacity, 26% clay (< 0.002 mm), and 8 mg kg−1 total As concentration.
Pot experiment
Four treatments were studied as follows: (1) HN population of P. vittata (HN treatment), (2) YN population of P. vittata (YN treatment), (3) Holcus lanatus (HL treatment), and (4) no plant (CK treatment). Each treatment consisted of four replicates.
Sporelings and seedlings with a height of ~ 10 cm were transferred to a pot filled with 1 kg culture soil. The culture soil used for pot experiments was collected from a mining area in Hunan province contaminated by both As and Sb due to the nearby Sb smelting activities. Top soil (0–20 cm) was collected, and plant material was removed. Then, the soil was homogenized on site and air-dried and then sieved with a 2 mm mesh in laboratory. The soil had a pH of 6.6 and contained 20.0 g kg−1 organic matter, 0.78 g kg−1 total P, 1.10 g kg−1 total N, 11.2 cmol kg−1 cation exchange capacity, and 28% clay (< 0.002 mm). The total Sb and As concentrations of the culture soil were 64.8 and 65.8 mg kg−1, respectively.
A soil solution sampler (Macro Rhizon Flex, 10 cm porous material, pore size of 0.2 mm, and 4.5 mm outer diameter, Rhizosphere Research, the Netherlands) was inserted at an angle of 45° over the entire pot width. Soil solution was extracted once every week and analyzed for total As and Sb concentrations. To collect adequate samples, water content was increased 24 h before soil solution sampling. At the time of soil solution sampling, the water content was ~ 25%.
After 28 days of growth, plants were harvested, separated into shoots and roots, and rinsed for 1 h with tap water. To remove apoplastic As and Sb, we immersed the roots in an ice-cold solution (4 °C) containing 1 mM K2HPO4, 5 mM Morpholino ethane sulfonic acid buffer, and 0.5 mM Ca(NO3)2 for 20 min before the plant materials were rinsed thrice with deionized water (Ren et al. 2014). Half of the plant samples were immediately frozen using liquid nitrogen and freeze-dried under vacuum at − 50 °C until a constant weight was obtained. Then, the plant samples were stored in a − 30 °C freezer prior to measurements for As and Sb speciation in plants using XANES. The remaining plant samples were oven-dried at 60 °C until a constant weight was attained for chemical analysis. When plant roots were removed from the pots, the soil adhering to roots was collected and defined as the rhizosphere soil. The remaining soil was defined as the bulk soil.
Experiments were conducted in a greenhouse at a day/night temperature of 23 to 25 °C/20 to 22 °C (16 h/8 h) and a relative humidity of 60%. Soil water content was kept at ~ 27% (w/w) by weighing every 2 days.
Chemical analyses
Soil pH was determined in a 1:2.5 soil/water mixture. Total P and N in the soil were determined using titrimetric and gravimetric methods with ascorbic acid (John 1970) and Kjeldahl method (Anantakrishnan and Srinivasa Pai 1952), respectively. Available P was measured by Olsen method (Olsen et al. 1954). Total organic matter was calculated using the Walkley–Black method (Nelson and Sommers 1982). Cation exchange capacity was measured using the ammonium acetate method (Ciesielski and Sterckeman 1997).
To measure metalloid concentrations, we digested soils with HNO3–H2O2 following method 3050B of the U.S. EPA (1996). Plant samples were dried, ground, and digested with a mixture of HNO3–HClO4. Sb concentrations in soil solution, soil, and plant samples were determined with inductively coupled plasma–mass spectrometry (ICP–MS; ELAN DRCe, PerkinElmer, USA). Arsenic concentrations in soil solution, soil, and plant samples were determined by atomic fluorescence spectrometer (Haiguang AFS-2202, Haiguang Instrumental Co., China).
XANES of As and Sb in plants
Immediately prior to XANES measurements, freeze-dried samples were ground into powder and packed in a 3 cm × 0.7 cm sample holder. The X-ray absorption spectra of As and Sb were collected at the X-ray absorption fine structure (XAFS) station on the 14 W1 beam line of the Shanghai Synchrotron Radiation Facility (Shanghai, China). To reduce beam damage during measurements, we analyzed all samples in a liquid-helium cryostat chamber. Pre-edge background was removed and normalized. Measurement and data analysis procedures for XANES of As and Sb followed the methods reported by Huang et al. (2008) and Ji et al. (2017), respectively. Linear combination fitting was utilized to calculate the percentages of different compounds in the samples.
Quality control
Certified standard reference materials for soils (GSS-6) and plants (GSV-2) from the China National Standard Materials Center were digested along with samples for quality control. Mean ± standard errors of our measurements for GSS-6 reached 225 ± 16 mg kg−1 for As and 62 ± 6 mg kg−1 for Sb. These values agreed well with certified values (220 ± 14 mg kg−1 for As and 60 ± 7 mg kg−1 for Sb) of the reference material. Mean ± standard errors of our measurements for GSV-2 reached 1.28 ± 0.12 mg kg−1 for As and 0.010 ± 0.016 mg kg−1 for Sb, in agreement with the certified values (1.25 ± 0.15 mg kg−1 for As and 0.095 ± 0.014 mg kg−1 for Sb) of the reference material.
Data processing
Data were analyzed using PASW Statistics 18.0 and are provided in the form of average ± standard deviation. One-way ANOVA with Tukey’s HSD test as post hoc test was performed to determine the significance of treatment effects. A general linear model with repeated measures was used to compare As and Sb concentrations in soil solution over time. Significance level was set at an error probability of 0.05. Bioaccumulation factor (BAF) and translocation factor (TF) were calculated for both As and Sb, as follows:
Results
Sb and As concentrations in soil solution
Initial Sb concentration in soil solution ranged from 207 to 222 μg L−1 under different treatments (Fig. 1a). The Sb concentration in soil solution showed no significant change over time in CK and HL treatments. By contrast, in the two P. vittata treatments (HN and YN), Sb concentration in soil solution significantly decreased with time. On day 28, Sb concentration in soil solution reached 162 μg L−1 in HN and 183 μg L−1 in YN treatment.
The As concentration in soil solution showed changes similar to that of Sb over time (Fig. 1b). In CK and HL treatments, As concentration in soil solution exhibited no significant change with time; while in the two treatments with P. vittata, the As concentration in the soil solution remarkably decreased with time. On day 28, As concentration in soil solution reached 86 μg L−1 in HN and 66 μg L−1 in YN treatment.
Plants’ growth
P. vittata and Holcus lanatus grew well in soil and exhibited no toxicity symptoms. The two populations of P. vittata showed no significant difference in terms of plant height, biomass, and root/shoot biomass ratio (Table 2). HN population showed significantly lower pinna density than the YN population.
Sb and As concentrations and speciation in plants
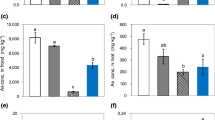
Shoot concentrations of Sb were lower than 30 mg kg−1 in all the analyzed plants (Fig. 2a). Sb concentrations in the roots were higher than those in the shoots of YN populations of P. vittata and Holcus lanatus. However, an opposite trend was observed in the HN population of P. vittata. Holcus lanatus exhibited significantly lower Sb concentration in the aboveground parts than P. vittata.
Sb (a) and As (b) concentrations in three plant species/populations. HN: treatment with HN population of P. vittata growing; YN: treatment with YN population of P. vittata growing; HL: treatment with Holcus lanatus growing. Different lowercase letters indicate significant difference in shoot Sb or As concentrations among three plant species/populations (P < 0.05, n = 4). Different capital letters indicate significant difference in root Sb or As concentrations among three plant species/populations (P < 0.05, n = 4). Asterisk indicates significant difference in Sb or As concentrations between the shoots and roots of the same species (P < 0.05, n = 4)
The concentration of As in plants was higher than that of Sb (Fig. 2b). The As concentration was lower in roots than in the aboveground parts of P. vittata, whereas the opposite trend was observed in Holcus lanatus. Holcus lanatus showed significantly lower As concentration in both roots and shoots than P. vittata. Comparing the two populations of P. vittata, YN population presented significantly higher As concentrations in shoots than the HN population.
These results indicated the higher accumulating capacity for As and Sb of P. vittata as compared to Holcus lanatus. In terms of the difference between two P. vittata populations, YN population showed a higher As-accumulating ability than HN population (P < 0.05). There was no significant difference in the shoot concentration of Sb between two P. vittata populations.
Because of the low Sb and As concentrations in Holcus lanatus, speciation analysis was only conducted for P. vittata. Speciation analysis of Sb in plants indicated that Sb existed mainly as Sb(V) in the pinnae and roots of both populations of P. vittata (Figs. 3a and 4)a, and no significant Sb transformation was observed. In contrast to Sb, As manifested notable transformation in P. vittata (Figs. 3b and 4)b. In the roots, As(V) was the predominant As species, whereas As(III) was the main As species in the pinnae.
Between the two P. vittata populations, YN population showed a lower As(V) percentage in the pinnae than the HN population. This finding indicated that the YN population transformed As(V) into As(III) more efficiently than HN population. YN population originated from a habitat with high As concentration and was confirmed as a high As-accumulating population with well-developed aboveground parts. HN population originated from an area with less As concentration but with high Sb concentration (Table 1).
The culture soil for the pot experiment was also tested for the speciation of As and Sb, and both were present predominantly as pentavalent forms (Fig. S1).
Sb and As concentrations in rhizosphere and bulk soils
No significant difference was observed in Sb concentrations between rhizosphere and bulk soils for the three plant species/populations (Fig. 5a). The As concentration in rhizosphere soil was significantly lower than that in bulk soil in the YN population of P. vittata (Fig. 5b). Rhizosphere and bulk soils showed no significant difference for the HN population of P. vittata and Holcus lanatus.
Sb (a) and As (b) concentrations in the rhizosphere and bulk soils under different treatments. HN: treatment with HN population of P. vittata growing; YN: treatment with YN population of P. vittata growing; HL: treatment with Holcus lanatus growing. Different lowercase letters indicate significant difference in Sb or As concentrations in rhizosphere soil among different treatments (P < 0.05, n = 4). Same capital letter indicates that there was no significant difference in Sb or As concentrations in bulk soils among different treatments. Asterisk indicates significant difference in As concentrations between rhizosphere and bulk soils for YN population (P < 0.05, n = 4)
BAF and TF of Sb and As
P. vittata showed significantly higher BAF and TF for both Sb and As than Holcus lanatus (Table 3). Between the two P. vittata populations, YN population showed significantly higher BAF and TF for As than HN population. Although Sb concentration in shoots and BAF showed no significant difference between the two P. vittata populations, HN population showed a significantly higher TF for Sb than the YN population of P. vittata.
Discussion
Sb and As uptake from co-contaminated soil by P. vittata and their speciation
Similarly to literature data (Lombi et al. 2002), P. vittata displayed a strong As-accumulating capacity. Considering the YN population of P. vittata as an example, the As concentration in sporelings approximated 95 mg kg−1 before transplanting. After growing on the soil containing 65.8 mg As kg−1 for 4 weeks, As concentrations in shoots reached 450 mg kg−1 (Fig. 2b). Therefore, P. vittata extracted ~ 3.9 μg of As from the soil within the indicated period. This result was in accordance with the decrease in As content in soil (Fig. 5). The concentration of As in the rhizosphere soil decreased by ~ 10 mg kg−1, and the rhizosphere amount accounted for approximately 70% of the total soil. Therefore, ~ 3.5 μg of As was removed from the soil. The minor difference between the two calculation methods may result from sampling and measurement errors.
To date, plant uptake experiments for Sb have been mostly carried out in hydroponic systems, quartz sand cultures, or field surveys (Feng et al. 2015; Hajiani et al. 2017; Mirza et al. 2017). Soil culture experiments are still limited. Similar to a previous study (Muller et al. 2009), the current soil culture experiment revealed that P. vittata exhibits a certain Sb uptake ability, which is higher than that of Holcus lanatus, and of several crop plants such as Raphanus sativus (Ngo et al. 2016) and Triticum aestivum (Shtangeeva et al. 2014), wild species like V. zizanioides (Mirza et al. 2017), and a well-known metal hyperaccumulator species as Brassica juncea (Wang et al. 2018).
Compared to As extraction, P. vittata had lower Sb extraction efficiency. A previous study showed an Sb concentration as high as 767 mg kg−1 in P. vittata gametophytes under Sb(III) exposure in a hydroponic experiment (Tisarum et al. 2015). One of our previous hydroponic experiments also revealed an Sb concentration as high as 1000 mg kg−1 in P. vittata shoots when supplied with Sb(III) (Wan et al. 2016). In both studies, a high shoot Sb concentration was observed in the Sb(III) treatment. Shoot Sb concentrations in the gametophytes of P. vittata after 4-week exposure to 4.8 mg Sb(V) L−1 and in the sporophytes of P. vittata after 2-week exposure to 1.0 mg Sb(V) L−1 reached 39.6 (Tisarum et al. 2015) and 604 mg kg−1 (Wan et al. 2016), respectively. Similarly, a quartz substrate culture experiment yielded a shoot Sb concentration of 8.1 mg kg−1 in P. vittata after 7-week exposure to 16 mg Sb(V) L−1 (Mullner et al. 2013). The shoot concentration of Sb at ~ 26 mg kg−1 under exposure to 0.2 mg Sb(V) L−1 in the soil solution found by the current study was generally in the same range as those of the aforementioned studies. Therefore, compared to the high concentration of Sb in the shoots of P. vittata found in an earlier hydroponic experiment (Wan et al. 2016), the lower shoot Sb concentration found by the current study resulted from the following: (1) lower availability of Sb in the soil and (2) most Sb in soil are in the form of Sb(V), which can hardly enter P. vittata roots compared to Sb(III).
The preference for Sb(III) over Sb(V) uptake was also observed in Lolium perenne L., Festuca pratensis H., T. aestivum L., Hordeum vulgare L., Secale cereale L., and Helianthus annuus L. but not in Holcus lanatus L. and Oryza sativa L. (Ji et al. 2017, 2018; Wan et al. 2013b). Sb(III) in weakly acidic to neutral solutions mainly exists as neutral Sb(OH)3, whereas Sb(V) exists as oxyanion Sb(OH)6−. It has been suggested that Sb(OH)3 enters plant roots through aquaglyceroporin (Bhattacharjee et al. 2008; Porquet and Filella 2007) and Sb(OH)6− via unselective monovalent anion channels or through leaks in the Casparian strip (Bienert et al. 2008; Hajiani et al. 2017; Tschan et al. 2009). Further studies are required toward the different transport and translocation ratios of two Sb species in plants.
Similarly to the results of previous studies (Lei et al. 2012), reduction of As(V) to As(III) occurred in P. vittata since As(III) was detected mainly in the pinnae, while As(V) mainly in the roots (Figs. 3b and 4)b. The reduction rate of Sb(V) to Sb(III) was much lower than the former. Sb(V) was the main Sb species in P. vittata, with only a limited amount of Sb(III) (Figs. 3a and 4)a. Since the reduction of As(V) to As(III) and Sb(V) to Sb(III) are in close pH–Eh ranges (Cornelis et al. 2008), such difference may be biochemical instead of pure chemical. It has been found that arsenate reductase have contributed to the efficient reduction of As(V) to As(III) in P. vittata (Cesaro et al. 2015). Perhaps the lack of enzyme facilitating the reduction of Sb(V) to Sb(III) is the reason behind the limited Sb uptake. We did not find any organic Sb species using XANES, in accordance with the findings obtained by high-performance liquid chromatography–ICP–MS (HPLC–ICP–MS) (Mullner et al. 2013), but in contrast with another field investigation which has found some unknown Sb species in wild plants (Wei et al. 2015). The biochemical reactions of Sb showed difference among plant species. A study on L. perenne indicated that under Sb(V) exposure, Sb in the roots of this plant existed exclusively as Sb(V), whereas half of Sb in the shoots existed as Sb(V) and half as Sb(III) (Ji et al. 2017). One of our previous hydroponic studies showed that ~ 35% of Sb in P. vittata shoots existed as Sb(III) (Wan et al. 2016). In the current study, less than 10% of Sb in P. vittata shoots existed as Sb(III). Considering the limited reduction ability for Sb and the strong preference of P. vittata for Sb(III), the limited uptake of Sb by P. vittata in the current study may have partly resulted from the low Sb(III) percentage of the total Sb in soil. Further studies on plant’s reduction mechanisms, especially in vivo dynamics, intra- and interspecies differences, and their relationship with Sb uptake by plants are required.
Until now, studies on the interaction between As and Sb in the soil–plant system are limited. Hydroponic and sand culture studies have both found a positive interaction between As(V) and Sb(V), i.e., the co-existence of As and Sb improved the uptake of both elements, compared to As or Sb alone (Mullner et al. 2013; Wan et al. 2017). In the current study, only one type of soil (contaminated by both As and Sb) was used; therefore, it is hard to make a conclusion about the interaction between As and Sb. Further studies on different types of soils with different contamination levels may help to better elucidate the interaction between the two elements.
Comparison of Sb and As uptake by the two P. vittata populations
The two populations of P. vittata showed differences in As transformation ability (Figs. 3b and 4b). The population that more efficiently reduced As(V) to As(III) was also able to accumulate more As in the pinnae, thereby confirming the results of a previous study and suggesting the importance of As transformation in As hyperaccumulation (Ghosh et al. 2015). Another reason for the variance in As uptake between two P. vittata populations may be the different P concentrations in their habitats (Table 1). The soil where YN population was collected had apparently lower concentrations of both total and available P than the soil of HN population. Under such P-starving circumstance, it is likely that YN population has evolved a transporter with higher affinity to P, which was suggested to be also the transporter of As(V) in P. vittata (Singh and Ma 2006).
The difference in Sb uptake between the two P. vittata populations was not as apparent as for As uptake. There was no significant difference in Sb shoot concentration between the two populations. The TF of Sb in HN population was significantly higher than in YN population. A previous hydroponic study on Sb accumulation by Sb-tolerant species Achillea wilhelmsii showed that metallicolous A. wilhelmsii population can accumulate more Sb in shoots than the nonmetallicolous population, but the difference was only significant at the exposure level of 3 μM of either Sb(III) or Sb(V) (Hajiani et al. 2017). These intraspecific differences still require further investigation.
Implication to risk control measures for soil co-contaminated by Sb and As
The utilization of P. vittata has been confirmed as an effective measure to remove As from contaminated soil (Kertulis-Tartar et al. 2006). Despite the potential of co-accumulating Sb and As by P. vittata in hydroponic studies, the current pot experiment indicated that P. vittata features a limited capacity to uptake Sb (but still higher than all the other reported plants under soil culture conditions). Recently, thiosulfate has been found to increase Sb and As uptake by B. juncea (Wang et al. 2018). Further studies on the mobilization of metalloids by chemical compounds may increase plants’ ability to extract Sb and As from soil.
P. vittata can be used to control the risks of soil co-contamination by Sb and As. Both Sb and As concentrations in soil were higher than the background values in Hunan (2.98 mg kg−1 for Sb and 14 mg kg−1 for As) (Pan and Yang 1988). The As concentration in soil was significantly higher than that recommended by China’s Environmental Quality Standard for Soils (GB15618–1995; grade II for soil, 6.5 ≤ pH < 7.5, As ≤ 30 mg kg−1). Average Sb and As concentrations in soil were also much higher than the maximum permissible pollutant concentrations recommended by the World Health Organization for receiving soils (i.e., 36 and 8 mg kg−1, respectively (Chang et al. 2002). As and Sb concentrations in the soil solution exceeded the Environmental Quality Standard for Surface Water (GB3838–2002, class V, and 100 μg L−1 (As); GB3838–2002, Limit for Centralized Drinking Water Sources, and 5 μg L−1 (Sb)). Therefore, despite the low fractions of metalloids in soil solutions (0.33% for Sb and 0.17% for As), the potential pollution diffusion through surface run-off or leaching into groundwater should be a concern in this area (Macgregor et al. 2015).
Although the total concentrations of As and Sb in the culture soil were basically the same (being 65.8 and 64.8 mg kg−1, respectively), the concentration of Sb in the soil solution was apparently higher than that of As. Despite the earlier opinion that Sb is less mobile than As, Sb may be able to disperse into water system more easily than As, especially under iron-rich conditions. Wu et al. (2018) suggested that in iron-rich environments, As(V) can be easily adsorbed to solid phase due to its strong affinity to iron minerals, and Sb(V) facilitates this adsorption process. By contrast, Sb(V) may stay in solution due to its weak affinity to iron minerals and the competition with As(V) (Wu et al. 2018).
After growing HN or YN populations of P. vittata for less than 30 days in soil, As concentration in the soil solution (Fig. 1b) decreased to below the standard set by the Environmental Quality Standard for Surface Water. Also, As was depleted in the rhizosphere, thereby favoring risk control. For Sb, planting the HN population from a habitat with high concentrations of both As and Sb decreased the mobilization of Sb from solid to liquid phase in soil to a certain extent but not as significantly as for As.
Compared with the efficiency of iron-based immobilizing agents (zero valent iron and ferrihydrite), which can decrease As and Sb in pore water by at least 79% after 7 days (Doherty et al. 2017), the immobilization ability of P. vittata is lower. However, the total metalloid concentrations will continue to decrease over time while plants are growing and over many cultivation cycles. Thus, planting accumulators may be an appropriate strategy if the final aim is to decrease the total concentrations of metalloids without urgent time constraints. In addition, considering the high extraction ability of P. vittata for the trivalent forms of As and Sb, this fern may be a suitable plant to decrease pollution risks under reducing and waterlogged conditions, with Sb(III) and As(III) being the dominant metalloid species in soil.
Conclusion
Planting the hyperaccumulator P. vittata significantly decreased soil solution concentrations of As, thus reducing the risks of As diffusion to ground or surface water. However, in the current study, planting P. vittata showed a limited effect in Sb stabilization for the soil used. The two P. vittata populations employed showed differences in Sb and As uptake, with the latter being related to changes in As speciation within the plant. The control of excess Sb and As in soil is an important and serious issue. However, information on Sb uptake remains limited and further investigations should focus on this issue.
References
Abad-Valle P, Alvarez-Ayuso E, Murciego A, Munoz-Centeno LM, Alonso-Rojo P, Villar-Alonso P (2018) Arsenic distribution in a pasture area impacted by past mining activities. Ecotoxicol Environ Saf 147:228–237
Anantakrishnan SV, Srinivasa Pai KV (1952) The Kjeldahl method of nitrogen determination. Proc Indian Acad Sci (Math Sci) 36:299
Arslan H, Erdemir US, Guleryuz G, Kiazolu H, Gucer S (2018) Assessment of trace elements in Plantago holosteum Scop. (Plantaginaceae) from abandoned tungsten mine works using inductively coupled plasma-mass spectrometry. Anal Lett 51:279–291
Bhattacharjee H, Mukhopadhyay R, Thiyagarajan S, Rosen BP (2008) Aquaglyceroporins: ancient channels for metalloids. J Biol 7:33
Bienert GP, Thorsen M, Schussler MD, Nilsson HR, Wagner A, Tamas MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)(3) and Sb(OH)(3) across membranes. BMC Biol 6:15
Cesaro P, Cattaneo C, Bona E, Berta G, Cavaletto M (2015) The arsenic hyperaccumulating Pteris vittata expresses two arsenate reductases. Sci Rep 5:10
Chang AC, Pan GX, Page AL, Asano T (2002) Developing human health-related chemical guidelines for reclaimed water and sewage sludge applications in agriculture. Division of Environmental Health, World Health Organization, Geneva, p 94
Chirakkara RA, Cameselle C, Reddy KR (2016) Assessing the applicability of phytoremediation of soils with mixed organic and heavy metal contaminants. Rev Environ Sci Biotechnol 15:299–326
Ciesielski H, Sterckeman T (1997) A comparison between three methods for the determination of cation exchange capacity and exchangeable cations in soils. Agronomie 17:9–16
Cornelis G, Johnson CA, Van Gerven T, Vandecasteele C (2008) Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: a review. Appl Geochem 23:955–976
Doherty SJ, Tighe MK, Wilson SC (2017) Evaluation of amendments to reduce arsenic and antimony leaching from co-contaminated soils. Chemosphere 174:208–217
Escapa C, Coimbra RN, Paniagua S, Garcia AI, Otero M (2017) Comparison of the culture and harvesting of Chlorella vulgaris and Tetradesmus obliquus for the removal of pharmaceuticals from water. J Appl Phycol 29:1179–1193
Fellet G, Marchiol L, Zerbi G (2013) Potential for metal phytoextraction of Brassica oilseed species. In: Anjum NA et al (eds) Phytotechnologies: remediation of environmental contaminants. CRC Press, Boca Raton, pp 179–203
Feng R, Wang X, Wei C, Tu S (2015) The accumulation and subcellular distribution of arsenic and antimony in four fern plants. Int J Phytoremediat 17:348–354
Feng RW, Wei CY, Tu SX, Tang SR, Wu FC (2017) Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions. Microchem J 97:38–43
Fernandez S, Poschenrieder C, Marceno C, Gallego JR, Jimenez-Gamez D, Bueno A, Afif E (2017) Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J Geochem Explor 174:10–20
Filella M, Belzile N, Chen Y-W (2002a) Antimony in the environment: a review focused on natural waters I. Occurrence. Earth Sci Rev:125–176
Filella M, Belzile N, Chen YW (2002b) Antimony in the environment: a review focused on natural waters II. Relevant solution chemistry. Earth Sci Rev 59:265–285
Ghosh P, Rathinasabapathi B, Teplitski M, Ma LQ (2015) Bacterial ability in AsIII oxidation and AsV reduction: relation to arsenic tolerance, P uptake, and siderophore production. Chemosphere 138:995–1000
Hajiani NJ, Ghaderian SM, Karimi N, Schat H (2017) A comparison of antimony accumulation and tolerance among Achillea wilhelmsii, Silene vulgaris and Thlaspi arvense. Plant Soil 412:267–281
Huang ZC, Chen TB, Lei M, Liu YR, Hu TD (2008) Difference of toxicity and accumulation of methylated and inorganic arsenic in arsenic-hyperaccumulating and -hypertolerant plants. Environ Sci Technol 42:5106–5111
Jankong P, Visoottiviseth P, Khokiattiwong S (2007) Enhanced phytoremediation of arsenic contaminated land. Chemosphere 68:1906–1912
Ji Y, Sarret G, Schulin R, Tandy S (2017) Fate and chemical speciation of antimony (Sb) during uptake, translocation and storage by rye grass using XANES spectroscopy. Environ Pollut 231:1322–1329
Ji Y, Vollenweider P, Lenz M, Schulin R, Tandy S (2018) Can iron plaque affect Sb(III) and Sb(V) uptake by plants under hydroponic conditions. Environ Exp Bot 148:168–175
John MK (1970) Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci 109:214–220
Kertulis-Tartar GM, Ma LQ, Tu C, Chirenje T (2006) Phytoremediation of an arsenic-contaminated site using Pteris vitrata L.: a two-year study. Int J Phytoremediat 8:311–322
Lei M, Wan XM, Huang ZC, Chen TB, Li XW, Liu YR (2012) First evidence on different transportation modes of arsenic and phosphorus in arsenic hyperaccumulator Pteris vittata. Environ Pollut 161:1–7
Li JN, Wei Y, Zhao L, Zhang J, Shangguan YX, Li FS, Hou H (2014) Bioaccessibility of antimony and arsenic in highly polluted soils of the mine area and health risk assessment associated with oral ingestion exposure. Ecotoxicol Environ Saf 110:308–315
Lombi E, Zhao FJ, Fuhrmann M, Ma LQ, McGrath SP (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156:195–203
Macgregor K, MacKinnon G, Farmer JG, Graham MC (2015) Mobility of antimony, arsenic and lead at a former antimony mine, Glendinning, Scotland. Sci Total Environ 529:213–222
Mirza N, Mubarak H, Chai LY, Yong W, Khan MJ, Khan QU, Hashmi MZ, Farooq U, Sarwar R, Yang ZH (2017) The potential use of Vetiveria zizanioides for the phytoremediation of antimony, arsenic and their co-contamination. Bull Environ Contam Toxicol 99:511–517
Muller K, Daus B, Mattusch J, Stark HJ, Wennrich R (2009) Simultaneous determination of inorganic and organic antimony species by using anion exchange phases for HPLC-ICP-MS and their application to plant extracts of Pteris vittata. Talanta 78:820–826
Mullner K, Daus B, Mattusch J, Vetterlein D, Merbach I, Wennrich R (2013) Impact of arsenic on uptake and bio-accumulation of antimony by arsenic hyperaccumulator Pteris vittata. Environ Pollut 174:128–133
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. Madison, American Society of Agronomy, pp 539–579
Ngo LK, Pinch BM, Bennett WW, Teasdale PR, Jolley DF (2016) Assessing the uptake of arsenic and antimony from contaminated soil by radish (Raphanus sativus) using DGT and selective extractions. Environ Pollut 216:104–114
Niazi NK, Singh B, Van Zwieten L, Kachenko AG (2012) Phytoremediation of an arsenic-contaminated site using Pteris vittata L. and Pityrogramma calomelanos var. austroamericana: a long-term study. Environ Sci Pollut Res 19:3506–3515
Olsen SR, Watanabe FS, Cosper HR, Larson WE, Nelson LB (1954) Residual phosphorus availability in long-time rotations on calcareous soils. Soil Sci 78:141–151
Pan YM, Yang GZ (1988) Background values of elemets in soils of Hunan Province and its research methods (in Chinese). Chines Environmental Science Press, Beijing
Pierart A, Shahid M, Sejalon-Delmas N, Dumat C (2015) Antimony bioavailability: knowledge and research perspectives for sustainable agricultures. J Hazard Mater 289:219–234
Porquet A, Filella M (2007) Structural evidence of the similarity of Sb(OH)3 and as(OH)3 with glycerol: implications for their uptake. Chem Res Toxicol 20:1269–1276
Ren JH, Ma LQ, Sun HJ, Cai F, Luo J (2014) Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci Total Environ 475:83–89
Schneider J, Bundschuh J, do Nascimento CWA (2016) Arbuscular mycorrhizal fungi-assisted phytoremediation of a lead-contaminated site. Sci Total Environ 572:86–97
Shtangeeva I, Niemela M, Peramaki P (2014) Effects of soil amendments on antimony uptake by wheat. J Soils Sediments 14:679–686
Singh N, Ma LQ (2006) Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ Pollut 141:238–246
Su YH, McGrath SP, Zhu YG, Zhao FJ (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 180:434–441
Sun WM, Xiao EZ, Xiao TF, Krumins V, Wang Q, Haggblom M, Dong YR, Tang S, Hu M, Li BQ, Xia BQ, Liu W (2017) Response of soil microbial communities to elevated antimony and arsenic contamination indicates the relationship between the innate microbiota and contaminant fractions. Environ Sci Technol 51:9165–9175
Tandy S, Meier N, Schulin R (2017) Use of soil amendments to immobilize antimony and lead in moderately contaminated shooting range soils. J Hazard Mater 324:617–625
Tisarum R, Chen YS, Dong XL, Lessl JT, Ma LQ (2015) Uptake of antimonite and antimonate by arsenic hyperaccumulator Pteris vittata: effects of chemical analogs and transporter inhibitor. Environ Pollut 206:49–55
Tschan M, Robinson BH, Schulin R (2009) Antimony in the soilplant system a review. Environ Chem 6:106–115
U.S. EPA (1996) Method 3050B: acid digestion of sediments, sludges, and soils. Revision 2. U.S. EPA, Washington, DC
US Geological Survey (2013) Mineral commodity summaries. US geological survey, Washington, DC
Wan XM, Lei M, Liu YR, Huang ZC, Chen TB, Gao D (2013a) A comparison of arsenic accumulation and tolerance among four populations of Pteris vittata from habitats with a gradient of arsenic concentration. Sci Total Environ 442:143–151
Wan XM, Tandy S, Hockmann K, Schulin R (2013b) Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake. Environ Pollut 172:53–60
Wan XM, Lei M, Chen TB (2016) Interaction of As and Sb in the hyperaccumulator Pteris vittata L.: changes in As and Sb speciation by XANES. Environ Sci Pollut Res 23(19):19173–19181
Wan XM, Lei M, Yang JX (2017) Two potential multi-metal hyperaccumulators found in four mining sites in Hunan Province, China. CATENA 148:67–73
Wang JX, Xing Y, Li P, Xia JC, Liu T, Feng XB (2018) Chemically-assisted phytoextraction from metal(loid)s-polluted soil at a typical carlin-type gold mining area in Southwest China. J Clean Prod 189:612–619
Wei CY, Ge ZF, Chu WS, Feng RW (2015) Speciation of antimony and arsenic in the soils and plants in an old antimony mine. Environ Exp Bot 109:31–39
Wu FY, Leung HM, Wu SC, Ye ZH, Wong MH (2009) Variation in arsenic, lead and zinc tolerance and accumulation in six populations of Pteris vittata L. from China. Environ Pollut 157:2394–2404
Wu DB, Sun SP, He MH, Wu ZX, Xiao J, Chen XD, Wu WD (2018) As(V) and Sb(V) co-adsorption onto ferrihydrite: synergistic effect of Sb(V) on As(V) under competitive conditions. Environ Sci Pollut Res 25:14585–14594
Xiao EZ, Krumins V, Xiao TF, Dong YR, Tang S, Ning ZP, Huang ZY, Sun WM (2017) Depth-resolved microbial community analyses in two contrasting soil cores contaminated by antimony and arsenic. Environ Pollut 221:244–255
Xing Y, Peng HY, Li X, Zhang MX, Gao LL, Yang XE (2012) Extraction and isolation of the salidroside-type metabolite from zinc (Zn) and cadmium (Cd) hyperaccumulator Sedum alfredii Hance. J Zhejiang Univ Sci B 13(10):839–845
Funding
Financial support was provided by the Program for “Bingwei” Excellent Talents in the Institute of Geographic Sciences and Natural Resources Research, CAS, and grants from the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roberto Terzano
Electronic supplementary material
Fig. S1
(DOCX 223 kb)
Rights and permissions
About this article
Cite this article
Wan, X., Yang, J. & Lei, M. Speciation and uptake of antimony and arsenic by two populations of Pteris vittata L. and Holcus lanatus L. from co-contaminated soil. Environ Sci Pollut Res 25, 32447–32457 (2018). https://doi.org/10.1007/s11356-018-3228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3228-z