Abstract
Pre-inoculation of transplants with arbuscular mycorrhizal fungi may increase the in-field P uptake through an increased exploitation of the soil volume and, thereby, reduce the need for P fertilizer application. The objective of this study was to investigate how pre-inoculation influences the post-transplanting rate of mycorrhizae development, nutrient uptake and growth of field-grown leek plants (Allium porrum L.) at various P levels. Field experiments were carried out in normal field soils supporting high crop production levels. This work demonstrated that pre-inoculation increased the post-transplanting rate of mycorrhizae development, the shoot and root concentration of P, Zn, Cu, and N, and the plant production. Therefore, module-raised pre-inoculated transplants should be adopted as a management strategy in leek production in order to ensure sufficient mycorrhization of young plants for uptake of P and, thereby, reduce the need for application of fertilizer P.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental concerns and requests for sustainable plant production lead to a demand for a reduction in the use of input factors. A reduced use of phosphorus (P) fertilizer is important as topsoil P may leach to drainage water (Grant et al. 1996) and then pollute the aquatic environment. Consequently, plant production should be based on an optimum use of biological resources, such as arbuscular mycorrhizae (AM) to reduce the need for high soil P levels.
Arbuscular mycorrhizae development frequently leads to increased plant uptake of P and several micro nutrients through an increased exploitation of the soil volume. An increased exploitation is especially important for the uptake of less mobile nutrients like P, Zn and Cu. In mycorrhizal plants, the uptake rate of P per unit root length is two to three times higher than in non-mycorrhizal plants (McGonigle and Fitter 1988). In, for example, leeks without mycorrhizae, optimum growth was obtained only if the soil P content was very high, i.e. above 80–100 mg kg−1 NaHCO3-soluble P (Jakobsen 1995). In contrast, optimum growth in leeks with well-established mycorrhizae was obtained in a soil with a P content of 15–25 mg kg−1. Consequently, the use of P fertilizers may be reduced or even omitted, due to the increased P bioavailability to plants with extensive growth of root-external AM hyphae.
Mycorrhizae are particularly important for a sufficient nutrient uptake in plant species that do not develop dense root systems or do not develop root hairs (Jakobsen et al. 2005; Schweiger et al. 1995). Leeks have shallow and less dense root systems devoid of root hairs (Burns 1980) and an early season P supply is important for such crops, especially at low spring temperatures (Grant et al. 2001). A well-developed AM may increase the sustainability in plant production by reducing the need for fertilizer P (Hooker and Black 1995; Kahiluoto et al. 2001).
Arbuscular mycorrhizal fungi occur naturally in most soils in communities composed of several species. Large inter- and intra-specific differences exist between AM fungi in their ability to supply the plant with P and other less mobile nutrients (Bürkert and Robson 1994; Jensen 1982; Munkvold et al. 2004). Furthermore, the content of mycorrhizal inoculum in the soil depends on the previous crop, cover crops, tillage, and other agronomic means as previously described (Sorensen et al. 2005).
Formation of AM is very much influenced by the content of soil P (Schubert and Hayman 1986)). In high-P soils, plants do not need mycorrhizae to take up sufficient P. In fact, it may even be a disadvantage due to the carbon cost of mycorrhizae formation (Graham and Eissenstat 1998; Ryan and Graham 2002).
A plant beneficial symbiosis may be obtained by inoculation of the plants with the desired AM species. Pre-inoculation with AM fungi is an obvious management practice in crops established as transplants and may lead to increased yield not only in low-P soils but also in soils with moderate and even high P availability (Cantrell and Linderman 2001; Charron et al. 2001; Douds and Reider 2003; Vosatka 1995). In leeks, pre-inoculation enhanced growth in a conventional cropping system, but not in an organic system (Kahiluoto and Vestberg 1998). This difference was explained by a more abundant and effective indigenous population of AM fungi in the organically cultivated soil.
Pre-transplant inoculation with AM fungi has been demonstrated to increase biomass production (Biermann and Linderman 1983b; Waterer and Coltman 1988). However, in transplant production, the importance of low soil-P availability is likewise essential. In onion transplants, Waterer and Coltman (1988) and Furlan and Bernier-Cardou (1989) showed that mycorrhizal infection may not succeed at high P supply.
The yield-increasing effect of pre-inoculation is significant after soil disinfection where the indigenous AM fungi have been eradicated. In a disinfected field soil with a content of 16–19 mg kg−1 NaHCO3-soluble P, Sasa et al. (1987) reported a significant increased nutrient-uptake rate and growth in pre-inoculated leeks. In untreated soil, pre-inoculation also increased nutrient uptake and growth, but less significant. Similar results have been reported for onions grown in a field soil with a content of 31 mg kg−1 NaHCO3-soluble P (Snellgrove and Stribley 1986).
Previously, inoculation with AM fungi has been extensively investigated in pot experiments with disinfected and low-P soil (Amijee et al. 1989; Bürkert and Robson 1994; Cantrell and Linderman 2001; Jensen 1982; Schubert and Hayman 1986). However, reports on field work without soil disinfection are less frequent but have increased (Douds and Reider 2003; Kahiluoto and Vestberg 1998; Khaliq and Sanders 2000) and recently, the management of AM pre-inoculation in plant production has been discussed (Kiers et al. 2002; Ryan and Graham 2002) and recommended to reduce the consumption of P fertilizer (Kahiluoto et al. 2001; Larsen et al. 2007).
The objective of this study was to investigate how pre-inoculated leek plants influence the post-transplanting rate of mycorrhizae development, nutrient uptake and growth in field soils supporting high crop production levels. A further objective was to investigate the interactive effects of pre-inoculation and soil P content. Our hypothesis is that AM pre-inoculation improves in-field mycorrhizae colonization, plant chemical composition, and biomass production especially at low soil P. To test this, we compare pre-inoculated and non-inoculated leek plants.
Materials and methods
This work with AM pre-inoculated leeks (Allium porrum L. cv. Carlton) comprised of two field experiments in 2000 and 2001, and one semi-field experiment in 2001. The climate at the experimental site is temperate coastal with monthly mean temperatures of 11–16°C from May to September.
Experimental design of field experiments
The two field experiments were conducted on a sandy loam soil containing 2.8% organic matter, 15% clay, 15% silt, and 67% sand. The pH (0.01 M CaCl2) was 6.1. The experiments were arranged in randomised complete block designs with four replicates.
Both experiments were carried out in an organically managed farming system. The previous crop was a full-year clover grass, i.e. a mixture of ryegrass (Lolium sp.) and various nitrogen fixing legume crops, mainly species belonging to Trifolium and Medicago. The clover grass was not harvested but incorporated in early spring for supplying plant nutrients to the experimental crop. This green manure was the only fertilizer source. The plant available pool of soil P at establishment of the experimental crop was 27 and 21 mg kg−1 NaHCO3-soluble P in 2000 and 2001, respectively. The corresponding contents of mineral N in the top 0.25 m soil were 142 and 61 kg ha−1, respectively.
Pre-inoculated transplants were produced in a greenhouse at 15–20°C. The AM fungi used for this inoculation were selected among seven isolates of AM inocula in a preliminary compatibility test with ten leek cultivars. In 2000, seeds were sown in trays containing peat mixed with 5% (vol) inoculum of Glomus intraradices 28A (BEG87), G. claroideum V12, G. mosseae V294 or with 10% field soil. The peat was added 127 N, 108 P, and 201 K mg L−1. Inocula were based on AM fungi colonised roots, spores and soil from dried pot cultures with Trifolium subterraneum L. Prior to use, AM fungi inocula had passed quality control standard procedures based on root colonization potential. The field soil used as inoculum was collected from the experimental field. During a plant raising period of two months, the plants were irrigated twice with 0.2% KNO3 and twice with 0.2% NH4NO3. The transplant AM colonization obtained by inoculation is given in Table 1. In 2001, seeds were sown in 90 mL peat pots (Jiffy Product no. 30611902) with a disinfected sand-soil mixture with 15% Leca® mixed with 5% inoculum of G. intraradices DAOM 197198 or mixed with 25% field soil. During the plant raising period, plants from all three treatments received N six times as NH4NO3 (in total 30 mg N per plant) and plants from the treatment without AM fungus inoculation and the treatment with field populations of AM fungi received KH2PO4 seven times (in total 8 mg P per plant). The plant size and AM colonization obtained by inoculation is given in Table 1.
Following rotavation to 20 cm soil depth, transplants were planted in non-disinfected soil at a row distance of 50 cm and an in-row density of 12 plants m−1. In May 2000, transplants were established as bare-root plants, whereas in June 2001, the intact peat pots with plants were planted in the field. The plants were grown without use of fungicides, weeds were removed mechanically and plants were irrigated when necessary. The leek crop received 339 mm and 304 mm of water from rainfall and irrigation in 2000 and 2001, respectively.
Shoots and roots were sampled 29, 43, 57, and 72 days after transplanting in 2000 and 23, 37, 51, and 65 days after transplanting in 2001 for biomass production and analysed for P, Zn, Cu, and N. Plants from 3 rows of 1 m each (corresponding to 36 plants) were used for each sampling. In 2000, roots of plants pre-inoculated with G. claroideum and G. mosseae were not chemically analysed, and in 2001, roots were not analysed for N. For root length determination and AM colonization, soil from the top 0.20 m was sampled with an auger in a distance of 0.05 m from the plants. Soil and roots were collected and roots analyzed for AM colonization. At the final harvest 147 and 121 days after transplanting in 2000 and 2001, respectively, the total biomass production was determined from 3 rows of 3 m each.
Experimental design of semi-field experiment
Pre-inoculated and non-inoculated leek transplants were planted in cylinders containing a non-disinfected sandy soil with 20, 32, or 44 mg kg−1 NaHCO3-soluble P. The cylinders were made of concrete with an internal diameter of 1.13 m and 1 m high, and inserted into the soil. The semi-field cultivation system was conventionally managed. The previous crop was ryegrass that was incorporated three month before planting. At planting, the pH (0.01 M CaCl2) was 6.1 and the content of mineral N in the top 0.25 m soil was 129 kg ha−1. The transplants were raised as described for the field experiment in 2001. The inoculum used was G. intraradices DAOM 197198. The experiment was arranged in randomised complete block design with three replicates.
Transplants were planted in the middle of June in three rows per concrete cylinder with a row distance of 0.25 m and an in-row density of 15 plants m−1. To avoid N deficiency, a supraoptimal amount of 206 kg ha−1 N was applied with mineral N fertilizer two weeks after planting. The plants were grown without use of fungicides, weeds were removed mechanically and plants were irrigated when necessary. The leek crop received 255 mm of water from rainfall and irrigation. Shoots and roots were sampled from the middle 1 m row 36 days after transplanting for biomass production, chemical analyses, and colonized root length. The root sampling procedure was identical to that described for the field experiments. In the middle of September, the total biomass production was determined from the remaining 2 m row, now 0.5 m apart.
Analytical details
Plant available pools of soil P and soil N were determined in spring, before leek establishment. Phosphorus was extracted with 0.5 M NaHCO3 (Olsen et al. 1954) and mineral N was determined by analysis of nitrate and ammonium in accordance with the methods of Best (1976) and Crooke and Simpson (1971), respectively.
At each sampling, shoots and roots were analysed for P, Zn, Cu, and N. Phosphorus was determined colorimetrically with ammonium molybdate after destruction with perchloric acid (Stuffins 1967). Zinc and copper were determined by atomic absorption spectrophotometry after microwave destruction in concentrated nitric acid (Milner and Whiteside 1981). Nitrogen was determined by thermal conductivity after combustion in pure oxygen (Hansen 1989). Root samples analysed for colonized root length had been washed from the soil, cleared in 10% KOH for twenty minutes at 90°C and stained in 0.05% trypan blue in lacto glycerol for three minutes at 90°C (modified after Phillips and Hayman (1970)). Dry matter production of the leek plants during growth and at the final harvest was determined after drying to constant weight at 80°C.
Analysis of variance was performed on each variable using the Statistical Analysis System (SAS Institute Inc. 1989). The effects were tested using the General Linear Models procedure.
Results
Pre-inoculation
In both experimental years, the AM colonization increased from around 40% to around 70% during the second month after transplanting into the field (Fig. 1). Pre-inoculation with G. intraradices produced the greatest colonization in both years whereas inoculation with field soil did not significantly increase the root length colonized. In 2000, pre-inoculation with G. claroideum or G. mosseae produced intermediate root length colonization that was not significantly different from non-inoculated plants. At the forth sampling, about 70 days after transplanting, colonization was not significantly influenced by any inocula used for pre-inoculation.
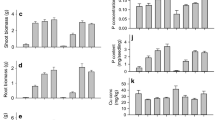
At the first field sampling, pre-inoculation with G. intraradices resulted in increased concentration of P in shoots (P < 0.01) and roots (P < 0.001) compared to non-inoculation (Fig. 2). Pre-inoculation with G. intraradices significantly increased the concentrations of Zn in shoots (P < 0.01) at this sampling date (Fig. 3). The concentration of Zn in roots was significantly increased by pre-inoculation with G. intraradices in 2000 but not in 2001. Still, at the first field sampling, the concentration of Cu in shoots was significantly increased by pre-inoculation with G. intraradices in 2001 but not in 2000 (Fig. 4). In roots, the concentration of Cu was significantly increased (P < 0.01) by pre-inoculation with G. intraradices. The concentration of N in shoots and roots was significantly increased (P < 0.05) by pre-inoculation with G. intraradices at the first field sampling (Fig. 5). Pre-inoculation with field soil produced intermediate results, although, the increase was significant (P < 0.05) only for the concentration of P. Inoculation with G. claroideum or G. mosseae in 2000 also produced intermediate results (data not shown).
At the second and later samplings, the concentrations of P, Zn, Cu, and N in shoots were, in general, not significantly influenced by pre-inoculation. One exception of this general finding was for Zn, as pre-inoculation with G. intraradices resulted in a significantly increased concentration in 2000 (Fig. 3). In roots, the concentration of P was significantly increased by G. intraradices in both years (Fig. 2), and the concentrations of Zn and Cu were significantly increased in one of two years (Figs. 3 and 4). The concentrations of Zn and Cu in roots were considerably higher than those in shoots. At the final harvest in October, pre-inoculation did not affect the concentrations of P, Zn, Cu, or N in shoots or roots (data not shown).
The concentration of plant nutrients in leek shoots and roots initially increased and then levelled off or decreased during the second month after field establishment (Figs. 2, 3, 4 and 5). The Cu concentration in roots at the first field sampling both years was nearly halved compared to the transplant root concentration (data not shown). Comparing these two dates of measurement, the Zn and Cu concentrations of shoots were of the same order, whereas the concentration of P decreased after transplanting and the concentration of N increased in 2000 (Table 1 and Figs. 2, 3, 4 and 5). Comparing the fourth field sampling and the final harvest (data not shown), the concentrations of P, Zn, Cu, and N in shoots slightly decreased.
In 2000, pre-inoculation did not increase plant biomass production either during growth (Fig. 6) or at final harvest. At the first field sampling, pre-inoculation even significantly depressed growth. At final harvest 147 days after transplanting, the biomass production was around 8.4 Mg ha−1 of dry matter on average of treatments. In 2001, however, inoculation with G. intraradices or field soil significantly increased the biomass production during growth (Fig. 6) and at final harvest. Non-inoculated leeks produced 4.6 Mg ha−1 whereas inoculated leeks produced 5.6 Mg ha−1 of dry matter 121 days after transplanting.
P supply and pre-inoculation interactions
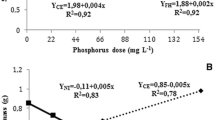
Colonized root length decreased with increasing soil P in both pre-inoculated and non-inoculated leeks (Fig. 7). At the highest soil P, colonized root length did not differ between pre-inoculated and non-inoculated leeks.
At increasing soil P, the concentration of P in shoots increased whereas that of Cu decreased in both pre-inoculated and non-inoculated leeks (Table 2). The concentrations of Zn and N were not influenced by the P supply. No interactive effects between P supply and pre-inoculation on the chemical composition was seen.
At increasing soil P content, the dry weight of young pre-inoculated plants was maximized at 32 mg kg−1 NaHCO3-soluble P and significantly higher than non-inoculated plants grown at 44 mg kg−1 (Fig. 7). At final harvest, the biomass production was significantly increased from 5.3 Mg ha−1 of non-inoculated plants to 7.1 Mg ha−1 of pre-inoculated plants. The biomass production was neither significantly influenced by the P supply or the interaction between P supply and pre-inoculation.
Discussion
Mycorrhizae formation
In accordance to our hypothesis, pre-inoculation with mycorrhizae improved the post-transplanting rate of mycorrhizae development. However, the present work demonstrated that there are at least three conditions that affect the mycorrhizae development of pre-inoculated transplants: 1) species of AM fungus used as inoculum, 2) handling of transplants and 3) P content of the field soil.
The root colonization during the second month after field establishment increased if the transplants were pre-inoculated with G. intraradices. However, pre-inoculation with G. claroideum, G. mosseae, or a field population did not significantly increase the root length colonized. This difference could be due to the observed differences in transplant root colonization where inoculation with G. intraradices was superior to the other inocula. Arbuscular mycorrhizal fungi have previously been described to differ in their ability to colonize plant roots. Clark et al. (1999), Jensen (1982), and Schubert and Hayman (1986) showed that root colonization varied extensively within isolates, and Vosatka (1995) reported that isolates were generally more effective than a community of indigenous fungi from non-sterilised soil. In the ability to increase the post-transplanting rate of mycorrhizae formation in the present experiment, pre-inoculation with G. intraradices was superior to G. claroideum, G. mosseae, or a field population.
The handling of transplants influenced the post-transplanting rate of mycorrhizae formation. The increased colonization during the second month after plant establishment of leeks pre-inoculated with G. intraradices was considerably greater in 2001 compared to 2000 and this difference between years could be ascribed to method of transplanting. When transplanting bare-root plants, as in 2000, many fine roots with extraradical mycelium were torn off and lost. This was not the case in 2001, using pot-raised plants where all roots were nearly undisturbed during the planting process. Soil disturbance has been reported to decrease the root length colonized due to changes in the extraradical mycelium (Evans and Miller 1988), and therefore, module-raised transplants are preferable compared to bare-root. The isolate of G. intraradices used as inoculum differed between the two experimental years and, therefore, the increased colonization obtained in 2001 also could be ascribed to this difference. Further, year differences in post-transplanting rate of mycorrhizae formation could be due the transplant AM colonization. The use of a sand-soil mixture in 2001 might explain the higher colonization of transplants obtained this year. In comparing different growth media Biermann and Linderman (1983a, b) showed that soil was superior to peat due to it’s higher P adsorption capacity.
In 2001, pre-inoculation with G. intraradices resulted in 79% root length colonization of the transplants. However, three weeks later the colonization was considerably lower. This apparent reduction could probably be explained by differences in method of sampling. For transplants, roots from the entire pot were used for the measurement, but for plants established in the field, roots were sampled with an auger in a distance of 0.05 m from the plant, i.e. outside the pot. At this early sampling, many roots were young and not yet colonized. The colonization was of the same order for all treatments, which indicate that the colonization measured could be ascribed to the indigenous mycorrhizae population in the field soil and not the pre-inoculated mycorrhizae. At the next sampling dates, five and seven weeks after transplanting, the root length colonized by G. intraradices was significantly higher than the other treatments and increased to around 80%.
Formation of AM is influenced by the content of soil P, and in high-P soils, plants are less dependent on mycorrhizae and less colonized (Amijee et al. 1989; Jakobsen 1995; Schubert and Hayman 1986). In the present experiment in non-sterilized sandy soil, pre-inoculation did not influence mycorrhizae formation at soil content of 44 mg kg−1 NaHCO3-soluble P, but increased the rate of mycorrhizae formation at 32 mg kg−1 or below. In our field experiments, the soil-P content was moderate in 2001 (21 mg kg−1) but a little higher in 2000 (27 mg kg−1). This difference could explain the slightly increased pre-inoculation effect on the post-transplanting rate of mycorrhizae formation in 2001. However, this effect could also be ascribed to the handling of transplants in respect to root disturbance, as described previously, and to the low tissue-P level of transplants inoculated with G. intraradices in 2001 compared to that of 2000.
In summary, the present experiments showed that pre-inoculation increased the post-transplanting rate of mycorrhizae development on condition that 1) the inoculum used was compatible with the plant cultivar, 2) the fine roots with extraradical mycelium were intact, and 3) the soil-P content was low.
Nutrient concentration
We hypothesised that pre-inoculation would improve nutrient uptake, but the present work demonstrated that increased nutrient concentration is dependent on the inoculum used. At the majority of sampling dates, pre-inoculation with G. intraradices increased the concentration of P, Zn, Cu, and N in roots. In shoots, however, this increase was less clear or even absent. These results are similar to those reported by Cantrell and Linderman (2001) and Sasa et al. (1987) in respect to the concentration of P, Zn and Cu, and by Vosatka (1995) who tested the effect of a range of AM species on the P concentration in roots and shoots of onions. However, Cantrell and Linderman (2001) and Smith et al. (1986) showed that AM infection decreased the N concentration in onion shoots and roots when plants were grown at low P supply, but increased the N concentration at high P supply.
In our P-supply experiment, pre-inoculation did not significantly increase the concentration of P, Zn, Cu, or N of leek shoots, although the root length colonized increased. However, pre-inoculation significantly increased plant growth. At increased plant growth, the concentration of plant nutrients has been shown to decrease due to the dilution effect (Jarrell and Beverly 1981). Such dilution of plant tissues might counteract an increased concentration and could explain why pre-inoculation did not increase the nutrient concentration in our experiment. Based on nutrient content, however, the uptake of P, Zn, Cu, and N was significantly increased by pre-inoculation.
At increasing soil P, we obtained an increased P concentration and a decreased Cu concentration in shoots of both pre-inoculated and non-inoculated leeks. These results are in agreement to Charron et al. (2001) and Lu and Miller (1989) who furthermore reported no P effect on the Zn plant tissue concentration, as in our experiment.
In our field experiments, pre-inoculation with G. intraradices significantly increased the root concentration of Zn and Cu in one of two years. At supraoptimal soil Zn and Cu content, AM infection has been shown to decrease the tissue concentration of Zn and Cu (Bürkert and Robson 1994; Gildon and Tinker 1983). However, it is not likely that the soils in the present experiments were neither supraoptimal nor deficient in Zn or Cu, and the significant effect of G. intraradices still need to be explained.
In the present experiments, the concentration of nutrients in shoots and roots increased at increasing plant age until 40–50 days after transplanting, and thereafter, the concentration levelled off. Increased nutrient concentration could be due to a high relative uptake rate as previously discussed (Sorensen et al. 2005). The nutrient concentration in roots levelled off at a later growth stage compared to that of shoots. An explanation to this difference could be that the nutrients, besides root tissue, also were stored in the AM fungi.
In summary, the present experiments showed that pre-inoculation increased the plant nutrient concentration on condition that inoculum and host was compatible.
Plant production
In accordance to our hypothesis, pre-inoculation with AM improved plant growth. However, the present work demonstrated that this result is dependent on transplant handling and the soil-P content.
Pre-inoculation with AM fungi increased plant production in one of two years. This increase coincided with a higher AM colonization this year. However, increased plant production could as well be due to use of pot-raised transplants with intact extraradical mycelium.
In 2001, the plant-available soil P was lower than in 2000 and this difference also could explain why pre-inoculation increased the plant production this year. At increasing P supply, it has previously been shown that the positive effect of pre-inoculation decreases or even turn to be negative (Schubert and Hayman 1986; Smith et al. 1986). In our P-supply experiment, pre-inoculation increased plant production even at 44 mg kg−1 NaHCO3-soluble P, which was the highest soil P content tested. However, the maximum plant production was obtained at a soil P content of 32 mg kg−1. In contrast to our organically cultivated field experiments, the P-supply experiment was conducted in a conventionally cropping system. However, the inoculum level from indigenous AM was not measured, but a more abundant and effective indigenous AM population in organically cultivated soils (Kahiluoto and Vestberg 1998) could explain why pre-inoculation did not increase plant production in 2000 in our field experiments on organically cultivated soil.
Besides differences in transplant handling and soil P content, the isolate of G. intraradices used as inoculum differed between the two experimental years. Therefore, the increased plant production obtained in 2001 also could be ascribed to the compatibility between the leek cultivar and the AM isolate. In addition, the increased plant growth and final plant production obtained by pre-inoculation could be due to non-uniform plant size at transplanting. However, the transplant biomass was not significantly influenced by pre-inoculation.
The effect of pre-inoculation on the final biomass production was reflected by the plant production during growth. In 2001, the final production increased probably due to low inoculum level from indigenous AM (P experiment on conventional soil) or to low soil-P content (organically cultivated soil). In contrast, pre-inoculation did not significantly increase plant production in 2000. This lack of response could be due to a slightly higher P supply this year or to the use of bare-root plants. Lack of crop response also might be due to the carbon cost of mycorrhizae formation as reported by Graham and Eissenstat (1998). Issues of soil-P level and indigenous AM inoculum level on cost/benefit of colonized AM fungi to plant growth has been discussed at length by Ryan and Graham (2002). They stated that AM fungi may not always increase plant growth even when soil-P is low and AM colonization is high. In our field experiment, the final production obtained in 2001 was considerably lower than that obtained the previous year. This difference was due to a late plant establishment and an early harvest in 2001.
In summary, the present experiments showed that pre-inoculation increased the plant production on condition that the inoculum was compatible and robust, that the growth medium for transplant production contained P fixing soil, that the extraradical mycelium of transplant roots was intact and that the P content of the field soil was low.
Overall conclusions
Our main hypothesis that pre-inoculation of transplants with AM fungi can improve post-transplant mycorrhizae development, nutrient uptake, and growth of leek plants was confirmed. Our results suggest that the performance of such pre-inoculated transplants depend on the characteristics of the AM fungus inocula, transplant growth medium, transplant tissue-P concentration, handling of transplants, and soil-P levels. As management strategies, module-raised pre-inoculated transplants should be used on low-P soils. For an increased sustainability, pre-inoculation should be applied to ensure sufficient mycorrhization of young plants for uptake of P and, thereby, reduce the need for fertilizer P application.
References
Amijee F, Tinker PB, Stribley DP (1989) The development of endomycorrhizal root systems. VII. A detailed study of effects of soil phosphorus on colonization. New Phytol 111:435–446
Best EK (1976) An automated method for determining nitrate-nitrogen in soil extracts. Queensl J Agric Anim Sci 33:161–166
Biermann BJ, Linderman RG (1983a) Effect of container plant growth medium and fertilizer phosphorus on establishment and host growth response to vesicular-arbuscular mycorrhizae. J Am Soc Hortic Sci 108:962–971
Biermann BJ, Linderman RG (1983b) Increased geranium growth using pretransplant inoculation with a mycorrhizal fungus. J Am Soc Hortic Sci 108:972–976
Bürkert B, Robson A (1994) 65Zn uptake in subterranean clover (Trifolium subterraneum L) by 3 vesicular-arbuscular mycorrhizal fungi in a root-free sandy soil. Soil Biol Biochem 26:1117–1124
Burns IG (1980) Influence of the spatial distribution of nitrate on the uptake of N by plants: a review and a model for rooting depth. J Soil Sci 31:155–173
Cantrell IC, Linderman RG (2001) Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 233:269–281
Charron G, Furlan V, Bernier-Cardou M, Doyon G (2001) Response of onion plants to arbuscular mycorrhizae 1. Effects of inoculation method and phosphorus fertilization on biomass and bulb firmness. Mycorrhiza 11:187–197
Clark RB, Zeto SK, Zobel RW (1999) Arbuscular mycorrhizal fungal isolate effectiveness on growth and root colonization of Panicum virgatum in acidic soil. Soil Biol Biochem 31:1757–1763
Crooke WM, Simpson WE (1971) Determination of ammonium in Kjeldahl digest of crops by an automated procedure. J Sci Food Agric 22:9–10
Douds DD, Reider C (2003) Inoculation with mycorrhizal fungi increases the yield of green peppers in a high P soil. Biol Agric Hortic 21:91–102
Evans DG, Miller MH (1988) Vesicular-arbuscular mycorrhizas and the soil-disturbance-induced reduction of nutrient absorption in maize. I. Causal relations. New Phytol 110:67–74
Furlan V, Bernier-Cardou M (1989) Effects of N, P, and K on formation of vesicular-arbuscular mycorrhizae, growth and mineral-content of onion. Plant Soil 113:167–174
Gildon A, Tinker PB (1983) Interactions of vesicular-arbuscular mycorrhizal infections and heavy metals in plants. II. The effects of infection on uptake of copper. New Phytol 95:263–268
Graham JH, Eissenstat DM (1998) Field evidence for the carbon cost of citrus mycorrhizas. New Phytol 140:103–110
Grant R, Laubel A, Kronvang B, Andersen HE, Svendsen LM, Fuglsang A (1996) Loss of dissolved and particulate phosphorus from arable catchments by subsurface drainage. Water Res 30:2633–2642
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224
Hansen B (1989) Determination of nitrogen as elementary N, an alternative to Kjeldahl. Acta Agric Scand 39:113–118
Hooker JE, Black KE (1995) Arbuscular mycorrhizal fungi as components of sustainable soil–plant systems. Crit Rev Biotechnol 15:201–212
Jakobsen I (1995) Transport of phosphorus and carbon in VA mycorrhizas. In: Varma A, Hock B (eds) Mycorrhiza. Springer-Verlag, Berlin, Heidelberg, pp 297–324
Jakobsen I, Chen B, Munkvold L, Lundsgaard T, Zhu YG (2005) Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using rootless barley mutant. Plant Cell Environ 28:928–938
Jarrell WM, Beverly RB (1981) The dilution effect in plant nutrition studies. Adv Agronomy 34:197–224
Jensen A (1982) Influence of four vesicular-arbuscular mycorrhizal fungi on nutrient-uptake and growth in barley (Hordeum vulgare). New Phytol 90:45–50
Kahiluoto H, Vestberg M (1998) The effect of arbuscular mycorrhiza on biomass production and phosphorus uptake from sparingly soluble sources by leek (Allium porrum L.) in Finnish field soils. Biol Agric Hortic 16:65–85
Kahiluoto H, Ketoja E, Vestberg M, Saarela I (2001) Promotion of AM utilization through reduced P fertilization 2. Field studies. Plant Soil 231:65–79
Khaliq A, Sanders FE (2000) Effects of vasicular-arbuscular mycorrhizal inoculation on the yield and phosphorus uptake of field-grown barley. Soil Biol Biochem 32:1691–1696
Kiers ET, West SA, Denison RF (2002) Mediating mutualisms: farm management practices and evolutionary changes in symbiont co-operation. J Appl Ecol 39:745–754
Larsen J, Ravnskov S, Sorensen JN (2007) Capturing the benefits of arbuscular mycorrhiza in horticulture. In: Hamel C, Plenchette C (eds) Mycorrhizae in crop production. The Haworth Press, Inc., New York, pp 123–149
Lu S, Miller MH (1989) The role of VA mycorrhizae in the absorption of P and Zn by maize in field and growth chamber experiments. Can J Soil Sci 69:97–109
McGonigle TP, Fitter AH (1988) Growth and phosphorus inflows of Trifolium repens L. with a range of indigenous vesicular-arbuscular mycorrhizal infection levels under field conditions. New Phytol 108:59–65
Milner BA, Whiteside PJ (1981) Introduction to Atomic Absorption Spectrophotometry. Pue Unicam Ldt., Cambridge, England
Munkvold L, Kjøller R, Vestberg M, Rosendahl S, Jakobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S.D.A. Circular 939
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Ryan MH, Graham JH (2002) Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil 244:263–271
SAS Institute Inc (1989) SAS/STAT User's Guide. Version 6, 4th edn., Volume 2. SAS Institute Inc., Cary, NC, USA
Sasa M, Zahka G, Jakobsen I (1987) The effect of pretransplant inoculation with VA mycorrhizal fungi on the subsequent growth of leeks in the field. Plant Soil 97:279–283
Schubert A, Hayman DS (1986) Plant growth responses to vesicular-arbuscular mycorrhiza – XVI: Effectiveness of different endophytes at different levels of soil phosphate. New Phytol 103:79–90
Schweiger PF, Robson AD, Barrow NJ (1995) Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytol 131:247–254
Smith SE, St.John BJ, Smith FA, Bromley JL (1986) Effects of mycorrhizal infection on plant growth, nitrogen and phosphorus nutrition in glasshouse-grown Allium cepa L. New Phytol 103:359–373
Snellgrove RC, Stribley DP (1986) Effects of preinoculation with a vesicular-arbuscular mycorrhizal fungus on growth of onions transplanted to the field as multi-seeded peat modules. Plant Soil 92:387–397
Sorensen JN, Larsen J, Jakobsen I (2005) Mycorrhiza formation and nutrient concentration in leeks (Allium porrum) in relation to previous crop and cover crop management on high P soils. Plant Soil 273:101–114
Stuffins CB (1967) Determination of phosphate and calcium in feeding stuffs. Analyst 92:107–111
Vosatka M (1995) Influence of inoculation with arbuscular mycorrhizal fungi on the growth and mycorrhizal infection of transplanted onion. Agric Ecosyst Environ 53:151–159
Waterer DR, Coltman RR (1988) Phosphorus concentration and application interval influence growth and mycorrhizal infection of tomato and onion transplants. J Am Soc Hortic Sci 113:704–708
Acknowledgement
This study was supported, in part, by the Danish Ministry of Food, Agriculture and Fisheries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter Christie.
Rights and permissions
About this article
Cite this article
Sorensen, J.N., Larsen, J. & Jakobsen, I. Pre-inoculation with arbuscular mycorrhizal fungi increases early nutrient concentration and growth of field-grown leeks under high productivity conditions. Plant Soil 307, 135–147 (2008). https://doi.org/10.1007/s11104-008-9591-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9591-7