Abstract
Arbuscular mycorrhizal (AM) fungi are hypothesized to assist growth of northern white-cedar in acid peatlands, yet there is little direct evidence that they can provide sufficient resources, especially nitrogen (N), from unfertilized peat soils. Our objective was to determine mycorrhizal efficacy to support cedar growth and nutrient supply as part of a low-impact approach for ecological restoration of cedar in peatlands. We tested the effectiveness of AM inoculation in a greenhouse experiment in factorial combination with fertilization and liming. We also determined AM colonization rate in the different treatment combinations. We found that AM inoculation in the absence of fertilization significantly increased all growth parameters, phosphorus (P) concentrations, and N, P, and copper (Cu) content of the seedlings, and decreased N:P ratios. Fertilizer alone had a similar impact on plant growth and nutrient acquisition when compared to un-fertilized AM inoculation treatments. Liming alone was ineffective at increasing cedar growth and nutrient uptake. There were many interactions of AM inoculation with liming and fertilization. Specifically, the positive effect of AM inoculation on many growth and nutrition metrics was strongly reduced in the presence of fertilization, whereas the P benefit of mycorrhizas appeared to increase under liming. We conclude that addition of AM inoculation alone improved cedar growth and P acquisition, reducing the need for fertilizer and lime in peatlands. However, seedling N limitation might be a problem in strongly N-deficient peat soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Northern white-cedar (Thuja occidentalis L; cedar hereafter) grows in a variety of habitats including mesic forests, limestone cliffs, sand dunes, riparian systems, abandoned farm fields, and swamps (Johnston 1990; Kost et al. 2007; Zenner and Almendinger 2012). Cedar is a dominant tree in rich swamps or forested rich fens with calcium rich groundwater and a pH above six in northeastern North America (Johnston 1990; Fraver et al. 2009; Ott and Chimner 2016). Although cedar is most dominant in high pH soils, cedar can also be found in more acidic soils (pH < 5.5), often as an associate species in mixed stands (Johnston 1990; Hofmeyer et al. 2009). Cedar swamps are a valuable ecosystem in the Great Lakes region. They are a large carbon sink in the Great Lakes region (Ott and Chimner 2016), are valuable wildlife habitat, particularly as thermal cover and browse during winters for deer (Rooney et al. 2002; Boulfroy et al. 2012), and are one of the most biodiverse ecosystems in the region (Kost et al. 2007). Cedar occupies more than 2 million hectares of commercial forest land in the northern Lake states and is an important forestry tree because the rot- and termite-resistant wood is used for products in contact with water and soil (e.g., houses, fence posts, decks, saunas, furniture and shingles) (Johnston 1990). However, despite the importance of cedar swamps, they are an endangered ecosystem because there has been a problem regenerating cedar for over 70 years (Heitzman et al. 1997). In addition, cedar in upland habitats has undergone drastic declines over the past century (Zenner and Almendinger 2012).
Efforts are underway to better manage and restore cedar in both swamp and upland ecosystems over a range of habitat conditions, including pH (Kangas et al. 2015; Palik et al. 2015; Danneyrolles et al., 2017). In addition, because cedar has few pests or diseases (Johnston 1990), it is also being considered as an alternative species for planting in forested wetlands that are undergoing high mortality due to pests. For instance, cedar was found to be one of the viable species for planting in black ash forests that underwent simulated emerald ash borer (Agrilus planepennis Fairmaire) infestation (Bolton et al. 2018). As Minnesota is in the midst of an eastern larch beetle (Dendroctonus simplex LeConte) outbreak that has infected more than 440,000 acres of tamarack (Ward and Aukema 2019, cedar was also test planted in place of tamarack (Larix laricina (Du Roi) K. Koch) and was also found to be a viable alternative species (Schwartz 2016). Tamarack grows over a broad pH range (4.3–6.9; Uchytil 1991), so understanding the factors affecting the ability of cedar to establish over this range is important. Little is known about establishing cedar in more acid sites, but if cedar is to be considered for restoration in a range of habitats, more information is needed on controls on cedar establishment over an ecologically relevant range of soil acidity.
Historically, both fertilization and liming have been commonly used to improve seedling growth in marginal soils during restoration (Moore et al. 2000; Walker 2002; Jonard et al. 2010; Pabian et al. 2012). However, these practices can be costly and lead to eutrophication and accumulation of heavy metals in soils, aquatic ecosystems, and plants (Simola 1983; Brække 1999; Savci 2012; van der Ent et al. 2013; Marchand et al. 2014). Another option is using arbuscular mycorrhizal (AM) fungi that colonize cedar (Brundrett et al. 1989; Matthes-Sears et al. 1992; Bainard et al. 2011) and may assist its colonization in nutrient-poor peat soils by enhancing nutrient, especially P, uptake. However, AM fungi are relatively uncommon in many acidic peatlands dominated by ectomycorrhizal, ericoid mycorrhizal, or non-mycorrhizal plants, so inoculation with AM fungi could be especially important if naturally inoculum sources are limited. It is largely unknown whether AM fungi can play an important nutritional role for cedar colonization in these more acidic peatlands. Mycorrhizal inoculation can effectively reduce fertilizer (N and P) use by enhancing host plant capacity to take up these nutrients from soil (Singh 1998; Tawaraya et al. 2007). However, AM fungi effectively overcome P limitation but not N limitation (Hoeksema et al. 2015). Given that acid peatlands are commonly N limited, it is important to understand whether AM inoculation can be effective in these peatlands.
Both fertilization and liming could also reduce the efficacy and abundance of mycorrhizal fungi (Treseder 2004). Fertilizers, through enhancing N and P, generally eliminate nutritional benefits of mycorrhizas (Nijjer et al. 2010; Johnson 1993). A mutualism commonly occurs in high N and low P, whereas little or no benefit to hosts is more likely under low N and high P (Johnson et al. 2014). Liming could also alter the benefit of AM fungi because increasing pH from acid pH to about pH 7, increases availability of inorganic P (Schlesinger 1997), and reduces amount of fungi (Ivarson 1977). However, at pH > 7, P availability declines again and so mycorrhizal colonization and benefits could increase (Anderson et al. 1996; Børja and Nilsen 2008).
Copper availability in organic soils is generally low and can limit plant growth (Rehm 2002). Cu uptake by AM fungi might be hampered under high P concentration, which likely reduces absorption of Cu by plants (Lambert et al. 1979). However, the AM role in Cu acquisition is still unclear (Leyval et al. 1997). Some studies found increased Cu absorption by AM plants (Killham and Firestone 1983; Weissenhorn et al. 1995) whereas others showed the reverse (Leyval et al. 1991).
Given the paucity of information on the effect of AM fungi on cedar seedling growth and macro- and micronutrient uptake on poor peat soils, our objective was to fill this gap. The aims of the greenhouse study were to test whether (1) AM inoculation increased growth of cedar seedlings in peat soil, and (2) fertilization and liming of the soil modified results of inoculation. We hypothesized that (1) AM inoculation would increase cedar growth and P acquisition, driving plants toward N limitation; and (2) the positive impact of AM inoculation of cedar would be reduced in fertilized and limed seedlings.
Materials and methods
Study site
A greenhouse study was conducted at the School of Forest Resources and Environmental Science, Michigan Technological University. Seeds of cedar were obtained from the USDA Forest Service (J.W. Toumey Nursery, Watersmeet, MI). The peat soil (pH 4.4) used for this study was obtained from a forested poor fen near Painesdale, Houghton County, MI (N 47.01349°, W 88.43082°). The peatland is dominated by non-mycorrhizal mosses (Sphagnum spp. and Polytrichum spp.), dwarf shrubs belonging to Ericaceae colonized by ericoid mycorrhizal fungi, and trees dominated by black spruce and tamarack colonized by ectomycorrhizal fungi. To avoid contamination with native inoculum from sparsely distributed AM hosts, the soils were collected under Ericaceae from an area with no cedar or other AM host species present within 100 m.
Experimental treatments
Prior to sowing, cedar seeds were soaked overnight in cold water. The seeds were germinated on flats filled with a pasteurized (70 °C) mixture of vermiculite (Sunshine Vermiculite, Sun Gro Horticulture Canada Ltd) and potting soil (Sunshine Mix 1, Sun Gro Horticulture Canada Ltd) with a 1:2 volumetric ratio. The seeded flats were placed within a mist chamber in the greenhouse for approximately 2 months until the seeds germinated and grew to an average height of 2 cm.
The experiment was a 2 × 2 × 2 full-factorial completely random experimental design consisting of three factors: mycorrhizal inoculation (M), fertilization (F), and liming (L), each with two levels (with and without the factor). Each of the eight treatment combinations was replicated ten times. We used Osmocote Plus 15–9–12 (N–P–K) slow release fertilizer that included some micronutrients such as magnesium, sulfur, boron, copper, iron, manganese, molybdenum, and zinc (Everris NA, Inc., Dublin, OH). For lime, we used garden and lawn lime (Mayville Limestone Inc, Mayville, WI) consisting of 22% calcium (Ca) and 12% of magnesium (Mg). Fertilizer and lime were mixed with the soils about a month prior to the initiation of the experiment, applied based on their manufacture’s recommendation, with dosage 1.65 g fertilizer/500 ml soil and 1.15 g lime/500 ml soil. As a result of liming, initial soil pH for the unlimed and limed treatments were 4.38 and 6.21 respectively. Arbuscular mycorrhizal inoculum consisted of Rhizophagus intraradices, Glomus mosseae, G.aggregatum, and Claroideoglomusetunicatum (Tri-C Enterprises, Chino, CA) that contained 120 propagules/cc. Control inoculum material for other effects of inoculum was pasteurized at 70 °C. Mycorrhizal inoculum was applied in the growing media when the seedlings were transplanted. The inoculum was placed around seedling roots at the rate of 3.4 g per seedling as recommended by the manufacturer. On uninoculated treatments, the seedlings were given the same amount of pasteurized AM inoculum.
On March 12, 2014, the germinated seedlings were transplanted into Deepots 7 cm in diameter by 25 cm tall (Stuewe and Sons, Inc. Tangent, OR) containing 500 ml unsterilized field collected peat soils treated as described above. The pots were randomly arranged in racks on greenhouse benches. Day length was set at 16 h with supplemental lighting via Halco metal halide lamps (Prolume MP 400/BU), and temperature maintained at 22–24 °C. Seedlings were watered daily using tap water.
Data collection
The seedlings were harvested on February 7, 2015, 11 months from their transplanting date. At harvest, leader height and basal diameter of seedlings were measured. In addition, shoots and roots of the seedling were separated. Roots were washed with tap water, 0.3 g (wet weight) healthy fine root subsamples were taken from around the root collar where new roots emerged for measurement of mycorrhizal colonization (see below), then residual roots and shoots were placed into paper bags and oven dried (65 °C) until their weights were constant. After drying, we measured root and shoot dry weight.
To measure the effectiveness of mycorrhizal inoculum, we first cleared and stained the roots following the protocol of Vierheilig et al. (2005). Briefly, this entailed clearing the roots by submerging them in 30 ml 10% KOH solutions and placing them in a water bath at 90 °C. When KOH solution became colored, the solution was repeatedly changed until it remained clear. Cleared roots were rinsed with DI water and stained overnight at room temperature with the staining solution containing Chlorazol E Black (CEB, Acros Organic (0.3 g), lactic acid (100 ml), glycerol (200 ml), and DI water (200 ml). Finally, the roots were rinsed with DI water and placed in destaining solution consisting of lactic acid (200 ml), glycerol (100 ml), and DI water (400 ml). The destaining solution was changed until solution remained clear. Roots were mounted on slides in PVLG gel, which is a mixed solution of DI water (100 ml), lactic acid (100 ml), glycerol (100 ml), and polyvinyl alcohol (16.6 g) (van Diepen 2008). Next, we scored the percentage of fungal colonization on the stained roots under the microscope, based on presence of AM and other fungal structures including aseptate AM hyphae, septate non-AM hyphae, arbuscules, coils, and vesicles (van Diepen 2008). We measured the percentage of colonized roots under 200× magnification, with a total of 100 root transects per slide. Photos of mycorrhizal structures on colonized roots were taken using a microscope-mounted 5.0-megapixel digital camera (Leica DFC480, Cambridge, UK).
We measured leaf nutrient concentration and content (N, P, C, and Cu) in dried cedar leaves. The leaves were ground use a mortar and pestle, and analyzed at the Forest Ecology Stable Isotope Laboratory at Michigan Technological University. For %C and %N we used a Costech 4010 elemental analyzer (Costech Analytical Technologies Inc., Valencia, CA, USA) calibrated with atropine. For %P and %Cu, we used inductively coupled plasma optical emission spectrometry on a Perkin Elmer Optima 7000DV ICP-OES (PerkinElmer Inc., Waltham, MA, USA) using the dry ash method (Miller 1998). Foliar nutrient content was derived from dry mass and concentration data. To determine the efficacy of the liming treatment, we measured soil pH of each treatment at the termination of the experiment on pooled, 2 mm sieved soils. We measured soil pH using a pH/conductivity meter (Denver Instrument Model 220, Denver Instrument, Arcada, CO, USA). Soil pH at the end of the experiment was 5.96–6.14 for unlimed treatments, and 7.40–7.50 for limed treatments, with the pH increase attributable to the use of tap water for watering.
Statistics
The effect of treatment factors on cedar growth metrics (height, diameter, biomass, and biomass allocation), nutrient status (concentration and content of N, P, and Cu; N:P), and percentage of mycorrhizal colonization were statistically tested in SAS (SAS Institute Inc., Cary, NC, USA) using generalized linear models (GLIMMIX procedure). Models were 2 × 2 × 2 full-factorial with all 2 and 3 way interactions of the predictors (liming, fertilization and inoculation) included. We accounted for lack of normality using transformations when needed, and lack of homogeneity of variance was accounted for using an appropriate “group” term that permitted analysis under heterogeneous variance. There was no transformation needed for the variables height, total biomass, P and Cu content, %Cu, and NP ratio. We transformed other variables as following: square root for diameter, root biomass, root shoot ratio, N content; and log10 for shoot biomass, %N, %P. Response distributions were set at Gaussian, link function was identity, and estimation technique was restricted maximum likelihood. We used a significance level (alpha) of 0.05.
Results
AM structures presence
AM fungal structures such as aseptate hyphae, vesicles, and arbuscules were more abundant in inoculated than uninoculated treatments (Table 1). The most common structures were aseptate AM hyphae, which appeared in all inoculated treatments and no uninoculated treatments. Few arbuscules were observed in this experiment, and those that were observed appeared to be degraded. Vesicles were also found in limited number. Septate hyphae, indicating non-mycorrhizal root endophytes, were highest in the fertilized + inoculated treatment combination (Table 1). Within inoculated seedlings, mycorrhizal abundance was suppressed by fertilization, as evident in the significant F*M interaction terms for aseptate hyphae and arbuscules (Table 2).
Seedling Growth
For virtually all growth metrics, there was a significant and roughly equivalent positive effect of both fertilization and inoculation (Table 3; Fig. 1a–f). However, these effects were mostly non-additive: the positive effect of both treatments was much stronger alone than when in combination, resulting in many significant treatment interactions (Table 3; Fig. 2a–f).
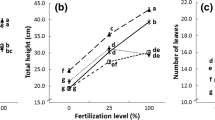
Effect of mycorrhizal inoculation, liming, and fertilization on growth of the cedar seedlings on: a height; b diameter; c shoot biomass; d root biomass; e total biomass; f root shoot ratio. g N concentration; h N content; i P concentration; j P content; k Cu concentration; l Cu content; m N:P ratio. Abbreviations as in Table 1. Error bars indicate standard error. Note that for the non-mycorrhizal treatments the sample size for analysis of P and Cu was only 5 because low needle biomass precluded analysis of the smaller replicates
Interaction plots for effects of fertilization (a–l) and liming (m–q) with mycorrhizal inoculation on the seedling growth. Fertilization: a height; b diameter; c root biomass; d shoot biomass; e total biomass; f root shoot ratio; g N concentration; h N content; i P content; j Cu concentration; k Cu content, and l N:P ratio. Liming: m root biomass; n total biomass; o root:shoot ratio; p N concentration, and q P concentration. (M0 = uninoculated, M1 = inoculated, F0 = unfertilized, F1 = fertilized, L0 = unlimed, L1 = limed). P values are for the interaction term, in the full model, for the variables included in each plot
In comparison with the other two treatments, the liming main effects were weaker, and its interactions with inoculation and fertilization differed. There were significant negative main effects of liming on shoot and total biomass (Fig. 1; Table 3). In the presence of fertilization, the negative effects of liming were reversed, leading to significant interactions for height, root biomass, shoot biomass, and total biomass (Table 3; Fig. 1; interactions plots not shown). In contrast, negative effects of liming were evident in the presence of mycorrhizal inoculation, leading to large reductions in root, shoot and total biomass in the mycorrhizal limed treatment relative to the mycorrhizal un-limed treatment, which manifested as significant liming × inoculation interactions for root biomass and total biomass (Table 3; Figs. 1, 2).
Nutrient acquisition
For P concentration and content of all nutrients, both fertilization and mycorrhizal inoculation showed the same positive effects, and both led to reductions in foliar N:P (Table 4, Fig. 1). When combined, fertilization and inoculation had small or no additive effects, leading to significant fertilization x inoculation interactions for all variables except %P (Table 4; Fig. 2).
Liming showed different effects from both fertilization and inoculation. There were significant negative main effects of liming on %P, P content, and N content, and significant positive main effects of liming on N:P ratio (Table 4, Fig. 1). Liming had a weak significant interaction with inoculation for both %N and %P (Table 4; Fig. 2). In both cases this resulted from a positive effect of inoculation in limed treatments vs. negative (%N) or non-significant (%P) effects of inoculation in unlimed treatments.
Discussion
Overall, AM inoculation positively affected all growth metrics of cedar seedlings and nutrient measurements except N concentration, supporting our hypothesis that on unfertilized acid peat soil AM fungal inoculation was able to improve cedar growth and nutrient supply, especially P, reducing the need for fertilizer and liming. Our study indicates that mycorrhizal growth response (MGR) for cedar was very strong and positive. Likewise, we found mycorrhizal inoculation effects on host growth primarily in the absence of fertilization. The fertilization treatment reduced efficacy of AM fungi (Figs. 1, 2), whereas liming marginally increased efficacy.
Many studies have found that benefits of AM fungi for plants are predominantly obtained in sites with limited nutrients, especially P (Liu et al. 2000; Tawaraya 2003; Smith and Read 2008; Smith and Smith 2011). This is consistent with their importance in enhancing plant P uptake, whereas their role in N uptake is less clear (Smith and Smith 2011). Mycorrhizal inoculation was able to reduce the foliar N:P ratio, which is a good indicator of increasing P availability to the plants. These results suggest that availability of the limiting resource (P) stimulated plant growth with an assumption that other resources were not limiting. Liebig’s law of the minimum states that plant growth is predominantly determined by the most limiting resource, although plant growth is often co-limited by multiple resources (Harpole et al. 2011; Johnson et al. 2014). Tissue N concentration, P concentration, and N:P ratios in our study reveal that mycorrhizal inoculation successfully reduced P deficiency, but may have driven plants towards N limitation (Fig. 1). In general, tissue N:P > 16 indicates P limitation (Koerselman and Meuleman 1996; Johnson et al. 2014), where AM fungi act as mutualistic symbionts, supplying surplus P to hosts. Meanwhile, tissue N:P < 14 indicates potential N limitation where AM fungi might act as commensal or parasitic symbiont (Johnson et al. 2014). For conifers the thresholds are similar: the N/P that indicates possible P deficiency is 12.5, and P deficiency is clearly indicated at 16 (Ballard 1985). By this criterion all of the mycorrhizal treatment combinations are N deficient. In a congener (Thuja plicata), the moderate deficiency threshold for nitrogen is 1.5% and for P is 0.13% (Walker et al. 1955; Ballard 1985). By this criterion, the unlimed non-mycorrhizal seedlings were just below these deficiency levels for N and the mycorrhizal treatment was below the deficiency threshold for %N suggesting high potential for N limitation. However, although the mycorrhizal plants tended to go up in P concentration, when unfertilized they were still below the critical threshold.
We found that the effect of inoculation on host N depended on the liming treatment, with a slight decline in N concentration with inoculation in the unlimed treatment vs. a slight increase with inoculation in the limed treatment (Fig. 2p). This increase might have been due to higher nitrification under the higher pH conditions (Ste-Marie and Paré 1999). Thuja plicata has been found to grow better with nitrate than ammonium as an N source (Krajina et al. 1973).
Although AM fungi are capable of N acquisition from either inorganic or organic forms (Smith and Smith 2011), many studies report that AM fungi do not increase N availability as much as P availability (Liu et al. 2000; Valentine et al. 2001; Jin et al. 2012). In acidic peatlands, where N is often limiting (Bayley et al. 2005) and ectomycorrhizal and ericoid mycorrhizal species have specific adaptations to increase organic N uptake (Smith and Read 2008) that could reduce the efficacy of AMF of cedar relative to competitors. Under limited soil N availability, AM fungi cannot supply sufficient N to their hosts (Hoeksema et al. 2015). There are several possible reasons for this. First, their N demand (per unit biomass) is higher than their hosts (Johnson et al. 2014; Hodge and Storer 2015), and it is assumed that AM fungi use N to fulfill their own nutritional needs before supplying it to host plants. AM extraradical hyphae had N concentration seven- to ten-fold higher than that of plant shoots and roots (Hodge and Fitter 2010). Second, in pot experiments AM external hyphae and plant root systems have to compete using the same soil volume so it is less likely AM external hyphae would explore different soil resources (Hodge 2000, 2001). However, this would also be true for P, yet we see a clear P benefit. Third, AM fungi lack the extracellular proteases and oxidative enzymes that can mobilize N from protein polyphenol complexes that can dominate N availability in peat soils (Read et al. 2004).
Regulation of Cu uptake is a critical challenge for plants, because both deficiency and excess can be detrimental to plant growth. Although all treatments were several-fold above the deficiency threshold of ~ 4 ppm for other conifers (Schmitt and White 1988), our findings indicate that AM fungi reduced rather than enhanced foliar Cu concentration, with uptake not keeping up with pool dilution associated with enhanced growth. Reduction of Cu concentration in the roots and shoots has been reported (Zhang et al. 2009; Meier et al. 2015). Timmer and Leyden (1980) reported a negative correlation between P availability and Cu uptake by plants, where increasing P supply led to diminished Cu acquisition by plants. In addition, increasing Cu uptake is also influenced by N, when soluble organic N associates with Cu and then is translocated throughout the plant (Singh and Swarup 1982). Hence, if AM mediated proportional reduction of N uptake occurred, it might also negatively affect Cu uptake. Additionally, glomalin produced by AM fungi could decrease Cu uptake by sequestering Cu and other heavy metals (Gil-Gardeza et al. 2014) and preventing Cu transfer from the roots to shoots (Joner and Leyval 1997; Joner et al. 2000; Toler et al. 2005; Zhang et al. 2009). Whatever the mechanisms, we found no evidence that AM inoculation will help to overcome Cu limitations that can occur in peatlands.
Presence of AM fungal structures such as arbuscules and vesicles can vary both with fungal species and hosts. Our study showed limited arbuscules and vesicles in AM-colonized cedar roots. Duke et al. (1994) suggested that lack of arbuscules might indicate plant roots are in nutrient-rich conditions and the plant is less responsive to P supply by AM fungi. Abbott et al. (1984) and Braunberger et al. (1991) stated that proportion of arbuscules to vesicles might be used to understand relative benefit of mycorrhizal fungi to the plant. However, Brundrett and Kendrick (1988) suggested that arbuscules are ephemeral structures that sometimes are not present if roots are sampled at the wrong phenological stage. Consistent with this, most of the arbuscules we observed were in a degraded state, perhaps reflecting the seasonal dynamics of these ephemeral structures.
Fertilization and liming have generally been applied to increase plant growth, improve soil fertility, and reduce soil acidity in peatland restoration (Huotari et al. 2007; Bjork et al. 2010; Caporn et al. 2007), even though there are economic and ecological costs of these practices. Our study showed benefits of inoculation are large in the absence of fertilization at both moderately acid and mildly alkaline pH (Fig. 1), indicating that inoculation is an important alternative to fertilization under these conditions.
In conclusion, AM inoculation successfully improved nutrient status and growth of cedar seedlings in acidic and mildly alkaline peat soils, with benefits approaching those of fertilization. This indicates that AM fungi might be a preferable alternative to enhance success of cedar restoration projects in areas with environmental sensitivity to liming and fertilization. However, two factors might limit our ability to infer success in acid peatland soil. First, our study was a greenhouse experiment, hence eliminating plant competition. Presence of other plants might restrict cedar growth because other species have adaptations, including mycorrhizal partners better adapted to N uptake, which might favor them in competition for limited N (Johnston 1990; Read et al. 2004; Weber et al. 2005), hence enhancing N limitation of cedar. Second, AM efficacy is affected by environmental factors (e.g., more acidic field pH in many Sphagnum peatlands) and given the pH range of our tests, our inference regarding the most acid systems is limited. Therefore, the next step is to perform field trials of the efficacy of mycorrhizal inoculation of cedar in peatlands over a range of pH conditions.
References
Abbott LK, Robson AD, Boer GD (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol 97:437–446
Andersson S, Jensen P, Soderstrom B (1996) Effects of mycorrhizal colonization by Paxillus involutus on uptake of Ca and P by Picea abies and Betula pendula grown in unlimed and limed peat. New Phytol 122:695–704
Bainard LD, Klironomos JN, Gordon AM (2011) The mycorrhizal status and colonization of 26 tree species growing in urban and rural environments. Mycorrhiza 21:91–96
Ballard TM (1985) Evaluating forest stand nutrient status. Land Management Report #20, Ministry of Forests, Province of British Columbia, Victoria, Canada
Bayley SE, Thormann MN, Szumigalski AR (2005) Nitrogen mineralization and decomposition in western boreal bog and fen peat. Ecoscience 12:455–465
Bjork RG, Ernfors M, Sikstrom U, Nilsson MB, Andersson MX, Rutting T, Klemedtsson L (2010) Contrasting effects of wood ash application on microbial community structure, biomass and processes in drained forested peatlands. FEMS Microbiol Ecol 73:550–562
Bolton N, Shannon J, Davis J, Grinsven M, Noh N, Schooler S, Kolka R, Pypker T, Wagenbrenner J (2018) Methods to improve survival and growth of planted alternative species seedlings in black ash ecosystems threatened by emerald ash borer. Forests 9:146
Børja I, Nilsen P (2008) Long term effect of liming and fertilization on ectomycorrhizal colonization and tree growth in old Scots pine (Pinus sylvestris L.) stands. Plant Soil 314:109–119
Boulfroy E, Forget E, Hofmeyer PV, Kenefic LS, Larouche C, Lessard G, Lussier J-M, Pinto F, Ruel J-C, Weiskittel A (2012) Silvicultural guide for northern white-cedar (eastern white cedar). USDA Forest Service, Newtown Square
Brække FH (1999) Drainage, liming and fertilization of organic soils. I. Long-term effects on acid/base relations. Scand J For Res 14:51–63
Braunberger PG, Miller MH, Peterson RL (1991) Effect of phosphorous nutrition on morphological characteristics of vesicular-arbuscular mycorrhizal colonization of maize. New Phytol 119:107–113
Brundrett MC, Kendrick WB (1988) The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can J Bot 66:1153–1173
Brundrett M, Murasea G, Kendrick B (1989) Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can J Bot 68:551–578
Caporn S, Sen R, Field C, Jones E, Carroll J, Dise N (2007) Consequences of lime and fertiliser application for moorland restoration and carbon balance. Research report to Moors for the Future (May 2007) Department of Environmental and Geographical Sciences. Faculty of Science and Engineering. Manchester Metropolitan University, Chester Street, Manchester, United Kingdom
Danneyrolles V, Dupuis S, Arseneault D, Terrail R, Leroyer M, de Römer A, Fortin G, Boucher Y, Ruel J-C (2017) Eastern white cedar long-term dynamics in eastern Canada: implications for restoration in the context of ecosystem-based management. For Ecol Manage 400:502–510. https://doi.org/10.1016/j.foreco.2017.06.024
Duke SE, Jackson RB, Caldwell MM (1994) Local reduction of mycorrhizal arbuscule frequency in enriched soil microsites. Can J Bot 72:998–1001
Fraver S, White AS, Seymour RS (2009) Natural disturbance in an old-growth landscape of northern Maine, USA. J Ecol 97:289–298
Gil-Cardeza ML, Ferri A, Cornejo P, Gomez E (2014) Distribution of chromium in a Cr-polluted soil: presence of Cr (III) in glomalin related protein fraction. Sci Total Environ 493:828–833
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken MES, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14:852–862
Heitzman E, Pregitzer KS, Miller RO (1997) Origin and early development of northern white-cedar stands in northern Michigan. Can J For Res 27:1953–1961
Hodge A (2000) Microbial ecology of the arbuscular mycorrhiza. FEMS Microbiol Ecol 32:91–96
Hodge A (2001) Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol 151:725–734
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA 107:13754–13759
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 286:1–19
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2015) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Hofmeyer PV, Kenefic LS, Seymour RS (2009) Northern white-cedar ecology and silviculture in the northeastern United States and southeastern Canada: a synthesis of knowledge. North J Appl For 26:21–27
Huotari N, Sutela ET, Kauppi A, Kubin E (2007) Fertilization ensures rapid formation of ground vegetation on cut-away peatlands. Can J For Res 37:874–883
Ivarson KC (1977) Changes in decomposition rate, microbial population and carbohydrate content of an acid peat bog after liming and reclamation. Can J Soil Sci 57:129–137
Jin H, Liu J, Liu J, Huang XW (2012) Forms of nitrogen uptake, translocations, and transfer via arbuscular mycorrhizal fungi: A review. Sci China Life Sci 55:474–482
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2014) Mycorrhiza: phenotypes and the Law of the Minimum. New Phytol 205:1–12
Johnston WF (1990) Thuja occidentalis L. northern-white cedar. In: Burns, RM; Honkala, BH (eds) Silvics of North America. Vol. 1. US Department of Agriculture, Forest Service, Washington, pp 580−589
Jonard M, Andre F, Giot P, Weissen F, Van der Perre R, Ponette Q (2010) Thirteen-year monitoring of liming and PK fertilization effects on tree vitality in Norway spruce and European beech stands. Eur J For Res 129:1203–1211
Joner EJ, Leyval C (1997) Uptake of 109Cd by roots and hyphae of a Glomus mosseae/Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol 135:353–360
Joner EJ, Briones R, Leyval C (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 226:227–234
Kangas LC, Schwartz R, Rennington M, Webster CR, Chimner RA (2015) Artificial microtopography and herbivory protection facilitates wetland tree (Thuja occidentalis L.) survival and growth in created wetlands. New For 47:1–14
Killham K, Firestone MK (1983) Vesicular arbuscular mycorrhizal mediation of grass response to acidic and heavy metal depositions. Plant Soil 72:39–48
Koerselman W, Meuleman AFM (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Kost MD, Albert D, Cohen J, Slaughter B, Schillo R, Weber C, Chapman K (2007) Natural communities of Michigan: classification and description, pp. 1–10. Lansing, MI
Lambert DH, Baker DE, Cole H (1979) The role of mycorrhizae in the interactions of phosphorus with zinc, copper, and other elements. Soil Sci Soc Am J 43:976–980
Leyval C, Berthelin J, Schontz D, Weissenhorn I, Morel JL (1991) Influence of endomycorrhizas on maize uptake of Pb, Cu and Cd applied as mineral salts or sewage sludge. In: Farmer JG (ed) Heavy Metals in the Environment. CEP Consultants, Edinburgh, pp 204–207
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological, and applied aspects. Mycorrhiza 7:139–153
Liu A, Hamel C, Hamilton RI, Ma BL, Smith DL (2000) Acquisition of Cu, Mn, and Fe by mycorrhizal maize (Zea mays L.) grow in soil at different P and micronutrient levels. Mycorrhiza 9:331–336
Marchand L, Nsanganwimana F, Lamy JB, Quintela Sabaris C, Gonnelli C, Colzi I, Mench M (2014) Root biomass production in populations of six rooted macrophytes in response to Cu exposure: intra-specific variability versus constitutive-like tolerance. Environ Poll 193:205–215
Matthes-Sears U, Neeser C, Larson DW (1992) Mycorrhizal colonization and macronutrient status of cliff-edge Thuja occidentalis and Acer saccharum. Ecography 15:262–266
Meier S, Cornejo P, Cartes P, Borie F, Medina J, Azcon R (2015) Interactive effect between Cu-adapted arbuscular mycorrhizal fungi and biotreated agrowaste residue to improve the nutritional status of Oenothera picensis growing in Cu-polluted soils. J Plant Nutr Soil Sci 178:126–135
Miller RO (1998) High temperature oxidation: dry ashing. In: Kalra YP (ed) Handbook of reference methods for plant analysis. Taylor & Francis, Boca Raton, pp 53–56
Moore DJ, Camiré C, Ouimet R (2000) Effects of liming on the nutrition, vigor, and growth of sugar maple at the Lake Clair Watershed, Québec, Canada. Can J For Res 30:725–732
Nijjer S, Rogers WE, Siemann E (2010) The Impacts of fertilization and mycorrhizal production and investment in Western Gulf Coast grasslands. Am Midl Nat 163:124–133
Ott CA, Chimner RA (2016) Long term peat accumulation in temperate forested peatlands (Thuja occidentalis Swamps) in the Great Lakes region. Mires Peat. https://doi.org/10.19189/MaP.2015.OMB.182
Pabian SE, Rummel SM, Sharpe WE, Brittingham MC (2012) Terrestrial liming as a restoration technique for acidic forest ecosystem. IJFR. Article ID 976809, 1–11. https://doi.org/10.1155/2012/976809
Palik BJ, Haworth BK, David AJ, Kolka RK (2015) Survival and growth of northern white-cedar and balsam fir seedlings in riparian management zones in northern Minnesota, USA. For Ecol Manag 337:20–27
Read DJ, Leake JR, Perez-Moreno J (2004) Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can J Bot 82:1243–1263
Rehm G (2002) Copper for crop production. University of Minnesota Extension, St Paul
Rooney TP, Solheim SL, Waller DM (2002) Factors affecting the regeneration of northern white cedar in lowland forests of the Upper Great Lakes region, USA. For Ecol Manag 163:119–130
Savci S (2012) An agricultural pollutant: Chemical fertilizer. Int J Environ Sci Te 3:77–80
Schlesinger WH (1997) Biogeochemistry: an analysis of global change, 2nd edn. Academic Press, San Diego, p 588p
Schmitt MDC, White EH (1988) Conifer growth on residual organic soils: a greenhouse study. Plant Soil 108:253–261
Schwartz, R (2016). Carbon cycling and restoration in temperate forested peatlands. ProQuest Dissertations Publishing, Web. Michigan Technological University.
Simola H (1983) Limnological effects of peatland drainage and fertilization as reflected in the varved sediment of a deep lake. Hydrobiologia 106:43–57
Singh S (1998) Role of mycorrhiza in the tree nurseries. Part 1. Evaluation of mycorrhizal efficiency with and without application of fertilizers. Mycorrhiza News 10:2–11
Sing DV, Swarup C (1982) Copper nutrition of wheat in relation to nitrogen and phosphorus fertilization. Plant Soil 65:433–436
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. 3rd Edition. Academic Press, Cambridge
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Ann Rev Plant Biol 62:227–250
Ste-Marie C, Paré D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Bioch 31:1579–1589
Tawaraya K (2003) Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci Plant Nut 49:655–668
Tawaraya K, Turjaman M, Ekamawanti HA (2007) Effect of arbuscular mycorrhizal colonization on nitrogen and phosphorus uptake and growth of Aloe vera L. Hortscience 42:1737–1739
Timmer LW, Leyden RF (1980) The relationship of mycorrhizal infection to phosphorus-induced copper deficiency in sour orange seedlings. New Phytol 85:15–23
Toler HD, Morton JB, Cumming JR (2005) Growth and metal accumulation and arbuscular mycorrhizal colonization of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ Pollut 133:233–242
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorous, and atmospheric CO2 in field studies. New Phytol 164:347–355
Uchytil RJ (1991) Larix laricina. In: Fire effects information system, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). https://www.fs.fed.us/database/feis/plants/tree/larlar/all.html Access 12 06 2019
Valentine AJ, Osborne BA, Mitchell DT (2001) Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization growth and photosynthesis of cucumber. Sci Hortic 88:177–189
van der Ent A, Baker AJ, Reeves RD, Pollard AJ, Schat H (2013) Hyper-accumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 362:319–334
van Diepen L (2008) The role and diversity of arbuscular mycorrhizal fungi in Acer saccharum dominated forest ecosystems under natural and N-amended conditions. PhD dissertation, Michigan Technological University, Houghton, USA
Vierheilig H, Schweiger P, Brundrett M (2005) An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol Plant 125:393–404
Walker RF (2002) Response of Jeffrey pine on a surface mine site to fertilizer and lime. Restor Ecol 10:204–212
Walker RB, Gessel SP, Haddock PG (1955) Greenhouse studies in mineral requirements of conifers: western red cedar. For Sci 1:51–60
Ward SF, Aukema BH (2019) Anomalous outbreaks of an invasive defoliator and native bark beetle facilitated by warm temperatures, changes in precipitation and interspecific interactions. Ecography 42:1068–1078. https://doi.org/10.1111/ecog.04239
Weber A, Karst J, Gilbert B (2005) Thuja plicata exclusion in ectomycorrhizal-dominated forests: testing the role of inoculum potential of arbuscular mycorrhizal fungi. Oecologia 143:148–156
Weissenhorn I, Leyval C, Berthelin J (1995) Bioavailability of heavy metals and abundance of arbuscular mycorrhiza in a soil polluted by atmospheric deposition from a smelter. Biol Fertil Soils 19:22–28
Zenner EK, Almendinger JC (2012) Identifying restoration opportunities for northern white cedar by contrasting historical and modern inventories in an ecological classification system context. Ecol Restor 30:169–179
Zhang XH, Lin AJ, Gai YL, Reid RJ, Wong MH, Zhu YG, (2009) Arbuscular mycorrhizal colonization increases copper binding capacity of root cell walls of Oryza sativa L. and reduces copper uptake. Soil Biol Biochem 41:930–935
Acknowledgements
The authors wish to thank the Directorate General of Resource, Science, Technology, and Higher Education, Ministry of Research, Technology, and Higher Education of the Republic of Indonesia for funding support “the Dikti-Funded Fulbright” scholarship to GA, the Fulbright Program in Jakarta (Indonesia) and Chicago/Midwest area (USA); Michigan Technological University and the USDA Forest Service Northern Research Station for support for GA; the Michigan Technological University Ecosystem Science Center for grant support to GA; University of Bengkulu; John Stanovick for statistical advice; Lynette Potvin, Joe Plowe, Karena Schmidt, Tia Scarpelli, Sara Kelso, Sarah Hartung, Brandon Stimac, and others for assistance in the field and the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anwar, G., Lilleskov, E.A. & Chimner, R.A. Arbuscular mycorrhizal inoculation has similar benefits to fertilization for Thuja occidentalis L. seedling nutrition and growth on peat soil over a range of pH: implications for restoration. New Forests 51, 297–311 (2020). https://doi.org/10.1007/s11056-019-09732-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-019-09732-x