Abstract
The vanilla cream1 (vac1) albino mutant is defective in a gene encoding a chloroplast-localized pentatricopeptide repeat protein of the DYW subgroup. However, the carboxyl-terminal DYW motif is truncated in VAC1. To identify vac1-specific phenotypes, we compared 34 chloroplast RNA editing sites and ~90 chloroplast gene expression patterns among wild type, vac1 and another albino mutant ispH, which is defective in the plastid isoprenoid biosynthesis pathway. We found that the editing of accD and ndhF transcripts is partially affected in vac1. In addition, steady-state levels of chloroplast rRNAs are significantly decreased in vac1. The expression of plastid-encoded RNA polymerase transcribed genes is down-regulated, whereas the expression of nucleus-encoded RNA polymerase transcribed genes is up-regulated in vac1. Although the development and function of mutant chloroplasts are severely impaired, steady-state mRNA levels of nucleus-encoded photosynthetic genes are not affected or are only slightly decreased in vac1. The ZAT10 gene encodes a transcription factor and its expression is down-regulated by norflurazon treatment in wild type. This norflurazon effect was not observed in vac1. These results suggest that the VAC1 protein may be involved in plastid-to-nucleus retrograde signaling in addition to its role in chloroplast RNA editing and gene expression. A defect in a key biosynthetic pathway can have many indirect effects on chloroplast gene expression as is seen in the ispH mutant. Similarly, the vac1 mutant has pleiotropic molecular phenotypes and most of which may be indirect effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloroplasts have their own genome and gene expression machinery. The chloroplast genome of higher plants contains ~120 genes, which are usually organized in polycistronic transcription units (Sugita and Sugiura 1996; Sato et al. 1999). In contrast, chloroplasts contain about 3,000 proteins. Thus more than 95% of chloroplast proteins are encoded by nuclear genes, synthesized in the cytosol and imported into chloroplasts post-translationally. Therefore, development and functions of chloroplasts are highly dependent on nuclear genes. The coordination of nuclear and chloroplast gene expression is also important for chloroplast biogenesis (Mullet 1988; Leon et al. 1998).
The expression and regulation of chloroplast genes are very different from that of nuclear genes. There are two types of RNA polymerase involved in transcribing chloroplast genes: a plastid-encoded multimeric RNA polymerase (PEP) and a nucleus-encoded RNA polymerase (NEP). The subunits of PEP are encoded by rpoA, rpoB, rpoC1 and rpoC2, which are similar to those of eubacterial RNA polymerase (Hu and Bogorad 1990; Igloi and Kössel 1992). In contrast, NEP is a phage-type monomeric RNA polymerase (Hedtke et al. 1997; Hess and Börner 1999). It has been suggested that these RNA polymerases are responsible for the transcription of distinct types of plastid genes. In general, PEP is involved in the transcription of photosynthesis genes whereas NEP preferentially transcribes housekeeping genes (Hajdukiewicz et al. 1997; Liere and Maliga 1999). The molecular mechanisms of selective usage of PEP and NEP in chloroplast gene expression are still unknown. Glutamyl-tRNA has been proposed to mediate the usage of PEP and NEP during chloroplast biogenesis (Hanaoka et al. 2005). Although the involvement of RNA polymerases in transcribing chloroplast genes has been identified, promoter sequences recognized by PEP and NEP have yet to be established. Recent studies on PEP and NEP promoters indicate that the usage of PEP and/or NEP promoters is very complicated and seems to be species-specific (Swiatecka-Hagenbruch et al. 2007, 2008).
The complexity of chloroplast gene expression system is not limited to the transcription level. The expression of chloroplast genes is also highly regulated at the post-transcriptional level (Sugita and Sugiura 1996). Many chloroplast genes are transcribed as polycistronic transcription units. These transcripts have to go through extensive modifications post-transcriptionally. For instance, endonucleolytic cleavage of di or polycistronic transcripts is required for efficient translation of mRNAs (Barkan et al. 1994; Sugiura et al. 1998). Other transcript maturation processes including RNA splicing and RNA editing are essential in higher plant chloroplasts (Maier et al. 1996; Sugita and Sugiura 1996). It appears that the gene expression system in plastids is more complicated than that of cyanobacteria and the other prokaryotes. It is not clear why plant plastids have evolved to have such complex transcriptional and post-transcriptional processes.
The complex chloroplast gene expression machinery requires the involvement of many proteins encoded by the nuclear genes. Recently, many chloroplast-localized pentatricopeptide repeat (PPR) proteins have emerged as primary nuclear factors that are involved in chloroplast gene expression and RNA metabolism in higher plants. PPR proteins are defined by tandem repeats of a degenerate 35 amino acid motif (Small and Peeters 2000). The PPR family is one of the largest protein families in plants, which contains ~450 and 477 PPR proteins in Arabidopsis and rice, respectively. A large majority (~80%) of the Arabidopsis and rice PPR genes are intronless (O’Toole et al. 2008). Moreover, most of the PPR proteins are predicted to localize to chloroplasts or mitochondria (Lurin et al. 2004). The PPR family is divided into P and PLS subfamilies based on the presence of classic PPR (P) motif and longer (L) or shorter (S) variant PPR motifs in the tandem arrays of PPR (Lurin et al. 2004). The PLS subfamily seems to be specific to land plants and is further divided into PLS, E and DYW subclasses based on the presence of E or DYW motifs in the C-terminal sequences (O’Toole et al. 2008; Schmitz-Linneweber and Small 2008). The huge number of different PPR proteins in plants indicates that most of the PPR proteins may have functions that are specific to plants.
PPR proteins have been shown to be involved in the transcription of chloroplast genes (Pfalz et al. 2006), RNA splicing (Schmitz-Linneweber et al. 2006; de Longevialle et al. 2007, 2008), RNA cleavage (Hashimoto et al. 2003; Meierhoff et al. 2003; Hattori et al. 2007; Okuda et al. 2009), RNA editing (Kotera et al. 2005; Okuda et al. 2007, 2008, 2009; Chateigner-Boutin et al. 2008; Cai et al. 2009; Hammani et al. 2009; Robbins et al. 2009; Yu et al. 2009; Zhou et al. 2009), translation (Fisk et al. 1999; Williams and Barkan 2003; Schmitz-Linneweber et al. 2005), and RNA stabilization (Yamazaki et al. 2004; Beick et al. 2008; Pfalz et al. 2009). These findings indicate that the PPR protein family has acquired essential roles in organelle gene expression in plants. The molecular bases underlying these PPR related functions are largely unknown. PPR proteins may directly bind to RNA or recruit effectors to the correct sites of target transcripts to perform splicing, editing, processing and translation (Lurin et al. 2004; Schmitz-Linneweber and Small 2008). Thus, PPR proteins have been proposed as sequence-specific RNA binding factors that are involved in post-transcriptional processes in organelles (Delannoy et al. 2007). In addition to organelle gene expression, PPR protein might also affect nuclear gene expression. For instance, GUN1 is a PPR protein that has been implicated in plastid-to-nucleus signaling (Koussevitzky et al. 2007). It is not yet known how the chloroplast-localized GUN1 PPR protein regulates nuclear gene expression.
To identify nuclear genes that are involved in chloroplast development and function, we have isolated a collection of pigmentation mutants in Arabidopsis. One of the albino mutants, vanilla cream1 (vac1), is caused by a T-DNA insertion in an intronless PPR gene. The vac1 mutant is allelic to ecb2, which has been shown to be totally impaired in editing of the 794th nucleotide from the AUG of accD mRNA (Yu et al. 2009). In order to identify the vac1-specific molecular phenotypes, we have included another albino mutant ispH as a reference line in gene expression and RNA editing analyses. The ispH is a null mutant of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase (HDR) from the plastid nonmevalonate pathway of isoprenoid biosynthesis (Hsieh and Goodman 2005). Our studies revealed that some molecular phenotypes are common but others are distinct between the vac1 and ispH albino mutants. One of the distinct features is that the editing efficiency of two sites in accD and one site in ndhF is partially reduced in vac1. We also analyzed the editing of accD and ndhF transcripts in ecb2 and found similar results. In addition to distinct features in chloroplast RNA editing, steady-state levels of rRNAs are specifically reduced in vac1. Moreover, the expression of NEP-transcribed genes is up-regulated, whereas steady-state mRNA levels of PEP-transcribed genes are decreased in vac1.
Materials and methods
Plant materials and growth conditions
The Arabidopsis vac1 albino mutant was originally isolated from ACR3 promoter-GUS transgenic lines (Hsieh and Goodman 2002). Progeny of the ACR3p-GUS 73 line segregated green and albino plants in a ratio of 3:1. This line was later named vanilla cream1 (vac1). Genetic analysis confirmed that the vac1 albino mutant co-segregated with the T-DNA insertion. TAIL-PCR analysis was used to identify the genomic flanking sequence of vac1 T-DNA mutant (Liu et al. 1995). Arabidopsis thaliana ecotype Columbia-0, vac1 and ispH mutants were grown on half-strength Murashige and Skoog (MS) plates [MS salts (Sigma), pH adjusted to 5.7 with 1 N KOH, 0.8% (w/v) agar] containing 2% sucrose, or in soil in the growth chamber at a light intensity of 80 μmolm−2 s−1 on a 16 h light/8 h dark cycle at 23°C. For norflurazon treatment, progeny of heterozygous vac1 and ispH mutants were grown on regular MS medium for 6 days to identify homozygous albino segregants. 6-day-old homozygous albino plants were then transferred to MS medium with or without 5 μM NF for 6 days. As a control, 6-day-old wild type Arabidopsis plants grown under a normal condition were transferred to MS medium with or without 5 μM NF for 6 days. Total RNA extracted from these samples was used for northern blot analysis.
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was measured using a pulse-amplitude-modulated (PAM) fluorometer (Walz, Germany). Minimal chlorophyll fluorescence (F 0 ) was measured under measuring light (650 nm) after 10 min of dark adaptation. Maximal chlorophyll fluorescence (F m ) was measured during a 1-s pulse of saturating white light (2,500 μmol m−2 s−1). The maximum quantum yield of PSII electron transport was calculated using the following equation: F v /F m = (F m − F 0 )/F m , where F v is variable chlorophyll fluorescence. For the extraction of chlorophylls and carotenoids, 2-week-old Arabidopsis seedlings were harvested and homogenized with 80% acetone (v/v). The determination of total chlorophylls and carotenoids in 3 independent samples of wild-type, vac1 and ispH seedlings grown in tissue culture was conducted as described (Lichtenthaler and Wellburn 1983).
Genomic Southern, RNA gel blot and RT-PCR analyses
Arabidopsis genomic DNA was extracted using a standard urea extraction buffer. For genomic Southern blot analysis, ten microgram of total DNA from 2-week-old wild-type, vac1 mutant and vac1 complemented line 9-2 was digested with Xho I. The following primers were used to make DIG-labeled single-stranded DNA probe for VAC1: 5′-TACTGGATATTCAGAGCGGG-3′, 5′-GTATCCATTCTAATCTCCAC-3′. Forty micrograms of total RNA extracted from 2-week-old Arabidopsis wild type and vac1 albino mutants grown in tissue culture were used for RNA gel blot analysis to detect the VAC1 transcripts. The same probe used for VAC1 genomic Southern blot analysis was used to detect the VAC1 transcripts in RNA gel blot analysis. The other primers used to make DIG-labeled probes for RNA gel blot analyses are listed in Supplemental Table S1. DIG probe labeling, pre-hybridization, hybridization, wash conditions and detection were performed according to Roche’s DIG Application Manual for Filter Hybridization. For RT-PCR analysis, total RNA was isolated from various organs of 6-week-old Arabidopsis grown in soil or 3-day-old etiolated seedlings exposed to light for 0, 1, 2 and 4 h using a phenol extraction protocol (Jackson and Larkins 1976). One microgram of total RNA treated with DNase I was used as a template for first-strand cDNA synthesis in a volume of 20 μl with 1 μl of Superscript III RT (Invitrogen). The PCR regime was 30 s at 94°C, 30 s at 55°C and 1 min at 72°C with 25 cycles for the UBQ10 and 30 cycles for the VAC1 genes. The following primers were used for RT-PCR analyses. VAC1, 5′-TAGCGATGACAAGTACCATC-3′, 5′-GTATCCATTCTAATCTCCAC-3′; UBQ10, 5′-CGATTACTCTTGAGGTGGAG-3′, 5′-AGACCAAGTGAAGTGTGGAC-3′. The primers used to make probes for 4.5S, 5S, 16S and 23S rRNAs were designed as described (Kishine et al. 2004). Chloroplast RNA editing sites were analyzed by RT-PCR and direct sequencing. Total RNA extracted from 2-week-old wild type, vac1 and ispH was used for RT-PCR with primers encompassing 34 known chloroplast RNA editing sites (Chateigner-Boutin and Small 2007). Excerpts of sequencing chromatograms for all 34 editing sites were shown in Fig. 9 and Supplemental Fig. S5.
Analysis of accD (S265L), accD (3′UTR) and ndhF (S97L) RNA editing efficiency
TOPO cloning kit (Invitrogen, Carlsbad, CA) was used to clone the amplified cDNAs derived from RT-PCR products harboring the accD (S265L), accD (3′UTR) and ndhF (S97L) editing sites from wild type, vac1 and ispH. Plasmids prepared from one hundred independent white colonies of each sample (total 900 clones) were sequenced to determine the editing efficiency of accD (S265L), accD (3′UTR) and ndhF (S97L). For the nucleotide at accD (S265L) editing site, 0 C and 100 T (100% edited) in wild type, 38 C and 62 T (62% edited) in vac1, and 19 C and 81 T in ispH (81%) were detected. For the nucleotide at accD (3′UTR) editing site, 45 C and 55 T (55% edited) in wild type, 92 C and 8 T (8% edited) in vac1 and 52 C and 48 T (48% edited) in ispH were detected. For the nucleotide at ndhF (S97L) editing site, 5 C and 95 T (95% edited) in wild type, 51 C and 49 T (49% edited) in vac1 and 13 C and 87 T (87% edited) in ispH were detected.
Transmission electron microscopy
The leaf samples from 2-week-old wild type or vac1 albino plants were fixed in 4% glutaraldehyde, 100 mM sodium cacodylate (pH7.2) for 16 h at 4°C, and postfixed with 1% osmium tetroxide in the same buffer for 6 h at 4°C. The fixed samples were dehydrated through a series of alcohol solutions and embedded in Spurr resin. Ultrathin sections were cut on a Reichert Ultracut-S (Leica Microsystems, Bannockburn, IL) and stained with uranyl acetate and lead citrate and viewed with a transmission electron microscope, JEOL 1200EX (JEOL USA, Peabody, MA).
Complementation of Arabidopsis vac1 mutant
A 3.3 kb VAC1 (At1g15510) genomic clone encompassing the entire predicted 5′ intergenic region (422 bp), the VAC1 open reading frame, and the 3′ intergenic region (281 bp) was amplified by PCR and cloned into the Kpn I site of the binary vector pCambia1301, which contains a hygromycin selectable marker. The resulting construct was transformed into kanamycin resistant heterozygous vac1 (+/−) plants by floral dip (Clough and Bent 1998). Successful transformants were selected from T1 plants grown on MS medium containing kanamycin and hygromycin. Genomic DNA extracted from green kanamycinR/hygromycinR primary transformants was used to determine the genotype of the VAC1 locus (+/− or −/−) by genomic Southern. Six of the 24 green hygromycinR primary transformants tested were vac1 (−/−) homozygous. In the T2 generation, about 100 seeds from each line were germinated on a hygromycin selective medium and all hygromycinR seedlings were green, an indication that the VAC1 genomic clone complements the albino phenotype in all lines. Genotypic and phenotypic characterizations of line 9-2, a representative T3 homozygous line for the VAC1 transgene and vac1 (−/−) locus are shown in Fig. 3c, d.
GFP fusion proteins
The computer program ChloroP (http://www.cbs.dtu.dk/services/ChloroP/) predicts that the length of VAC1 transit peptide is 52 amino acids. According to this prediction, the N-terminal cDNA sequence encoding the first 72 amino acids of VAC1 was amplified by PCR, digested with Nco I and Stu I, and cloned into the N-terminus of a GFP expression vector driven by a CaMV 35S promoter (Chiu et al. 1996). The primers used are: 5′-CCTTCCATGGCGTCTTCTGCTCAAAG-3′, 5′-CTCTAGGCCTATTCGCGCAGAGTCCATGTA-3′. The resulting construct, which encodes the first 72 N-terminal amino acids of VAC1 fused to GFP, was transformed into Arabidopsis protoplasts and observed under confocal laser scanning microscope 510 META Zeiss.
Results
Isolation and phenotypic analysis of Arabidopsis vanilla cream1 mutant
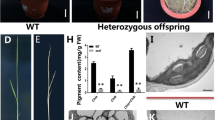
To isolate mutants impaired in chloroplast development and function, we generated a collection of Arabidopsis T-DNA insertion lines and screened for plants displaying albino, pale green or pale yellow phenotypes. One of the isolated mutants, vanilla cream1 (vac1), exhibits albino to pale yellow phenotype. Homozygous vac1 plants are albino lethal so the mutant is maintained as a heterozygous line. Progeny from a self-pollinated heterozygous plant segregate green and albino plants in a ratio of 3:1 on a half MS plus sucrose medium, i.e. the albino phenotype is inherited as a monogenic recessive mutation (Fig. 1a). The vac1 albino plants are lethal under either normal or low light condition. Some vac1 albino plants can grow to develop flower bud-like structures on a tissue culture medium (Fig. 1b). However, these flower buds never grow to mature before the plant dies. In 2-week-old vac1 mutants, total chlorophylls and carotenoids are 8.9 and 22.2%, respectively, of the amounts in wild-type plants (Fig. 1c). In contrast, the content of photosynthetic pigments was almost undetectable in the ispH albino plants, which are null mutants of the IspH gene encoding 1-hrdroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase (HDR) of the plastid nonmevalonate pathway for isoprenoid biosynthesis (Hsieh and Goodman 2005). In parallel with their photosynthetic pigment content, the PSII maximum quantum yield of vac1 mutants was about 8.7% of wild-type plants, whereas the photosynthetic activity was not detectable in the ispH albino plants (Fig. 1d).
Phenotypic analyses of Arabidopsis vac1 albino mutant. a Progeny of a self-pollinated heterozygous vac1 mutant segregate green and albino plants in a ~3:1 ratio on a non-selective medium. Plants shown are 2 weeks old. b A representative vac1 albino plant grown on a ½ MS plus 2% sucrose medium for 6 weeks. c Photosynthetic pigment content of 2-week-old wild type, vac1 and ispH seedlings. Levels of chlorophylls and carotenoids were low in the vac1 albino plants and almost undetectable in the ispH albino mutants. d PSII maximum quantum yield (F v /F m ), measured by chlorophyll fluorescence analysis of wild type, vac1 and ispH plants. Chlorophyll fluorescence of ispH albino plants was not detectable (ND). Values shown are means of six samples ± SE
Ultrastructures of vac1 mutant chloroplasts
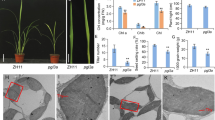
Transmission electron microscopic analysis of vac1 leaf sections revealed that the development of chloroplast is impaired in the mutant. In contrast to wild-type chloroplasts, which are lens-shaped containing well-organized inner thylakoid membrane systems, the morphology of vac1 mutant chloroplasts range from round- or amoeboid-shape and completely lack of thylakoids to lens-shaped with the development of few stacking and non-stacking thylakoids (Fig. 2). These various types of mutant chloroplasts may exist in the same or different cells. The mutant chloroplasts without thylakoids are usually filled with large vesicles. In addition, densely stained globule aggregates are frequently observed in the vac1 mutant chloroplasts. These observations are consistent with the phenotypes that the vac1 mutants still retain certain amounts of photosynthetic pigments and some photosynthetic activity.
Transmission electron micrographs of wild type (WT) and vac1 mutant chloroplasts. Sections are from the first leaves of 2-week-old Arabidopsis plants grown in tissue culture. Various mutant chloroplasts containing large vesicles, densely stained globule aggregates and thylakoid-like structures were observed in the mesophyll cells of vac1 albino plants. Scale bars are 500 nm
Molecular characterization of the vac1 locus
Genetic analysis of the vac1 mutants has confirmed that the albino phenotype co-segregates with the T-DNA insertion. To identify the vac1 locus, we used thermal asymmetric interlaced (TAIL)-PCR to analyze the genomic flanking sequence of the vac1 T-DNA mutant. Sequence analysis of the TAIL-PCR products revealed that the T-DNA insertion is located in the open reading frame of At1g15510 (Fig. 3a). RNA gel blot analysis confirmed that ~2.6 kb transcripts of the predicted At1g15510 gene were detected in the wild type but not in the vac1 albino plants (Fig. 3b). Since the RNA gel blot analysis cannot detect very low abundant transcripts, we conducted RT-PCR analysis to show that there is no trace of VAC1 transcripts left in the mutant (Fig. 3c). These results suggest that vac1 is a null allele of the At1g15510 gene. To further prove that we have identified the allele responsible for the vac1 albino mutant, we introduced a 3.3 kb genomic clone encompassing the predicted open reading frame of the VAC1 gene and its putative promoter into the mutant. The VAC1 gene driven by its own native promoter was able to complement the vac1 albino mutant. The phenotype and genotype of a representative complementation line are shown in Fig. 3d and e, respectively. These results confirm that the vac1 albino phenotype is caused by a loss-of-function mutation in the VAC1 gene.
Molecular characterization of vac1 locus. a Schematic diagram of Arabidopsis VAC1 gene. Arrows indicate Xho I restriction sites. The black box indicates the only exon of VAC1. The T-DNA (white triangle) is not drawn to scale. b RNA gel blot analysis. Forty micrograms of total RNA extracted from 2 weeks old wild type (WT) and vac1 albino plants were used for RNA gel blot analysis to detect the transcripts of VAC1. c RT-PCR analysis of VAC1 and ubiquitin 10 (UBQ10) mRNAs in 2-week-old wild type and vac1. d Seven-day-old Arabidopsis wild-type (WT), vac1 albino mutant and VAC1 complemented (Com) seedlings. e Genomic Southern blot analysis (Xho I digested). The arrow indicates the vac1 mutant allele and the arrowhead indicates the VAC1 transgenic allele in a complemented (Com) line
VAC1 encodes a chloroplast-localized PPR-DYW protein
ArabidopsisVAC1 is an intronless gene that encodes a PPR protein (Fig. 4a). The amino acid sequence and domain composition of VAC1 PPR protein are most similar to those in the DYW subgroup (O’Toole et al. 2008). However, the VAC1 PPR protein has a truncated DYW domain. The other reported PPR-DYW proteins end with a conserved DYW or DFW tri-peptide (Supplemental Fig. S1). The VAC1 PPR protein is predicted to localize to the chloroplast (http://www.cbs.dtu.dk/services/ChloroP/). To verify the prediction, we fused the first 72 amino acids of VAC1 protein, which encompass the putative transit peptide, to the N-terminus of a green fluorescent protein (GFP). The VAC1-GFP fusion construct driven by a cauliflower mosaic virus (CaMV) 35S promoter was transformed into Arabidopsis protoplasts. Confocal microscopy was used to observe the fluorescent signals 24 h after transformation. The green fluorescent signals derived from the VAC1-GFP fusion protein co-localized with the auto-fluorescent signals of chlorophylls in the chloroplasts (Fig. 4b). These results suggest that the Arabidopsis VAC1 PPR protein is localized to the chloroplast.
a Schematic diagram of domain composition of Arabidopsis VAC1 protein. P PPR motif, S shorter variant of PPR motif, L longer variant of PPR motif, E, E+ and DYW motifs are also indicated above the diagram. b Chloroplast localization of Arabidopsis VAC1 PPR protein. Arabidopsis protoplasts were transformed with a 35S:VAC1-GFP construct, which encodes the first N-terminal 72 amino acids of VAC1 fused to GFP. Chloroplasts were visualized by red chlorophyll autofluorescence. The green fluorescent signals of VAC1-GFP co-localized with the red fluorescent signals of chlorophylls (merge). Scale bar is 10 μm. c RT-PCR analysis of VAC1 and ubiquitin 10 (UBQ10) mRNAs in 6-week-old wild type Arabidopsis plants grown in soil. R roots, L leaves, St stems, F flowers, Si siliques. d RT-PCR analysis of VAC1 and ubiquitin 10 (UBQ10) mRNAs in 3-day-old etiolated seedlings exposed to light for 0–4 h. The expression of VAC1 is rapidly induced by light
Light induction of Arabidopsis VAC1 gene
We used semi-quantitative RT-PCR analysis to examine the expression patterns of VAC1 in 6-week-old wild type Arabidopsis plants grown in soil. The expression of VAC1 is ubiquitous as the VAC1 transcripts were detected in all organs examined. Levels of VAC1 transcripts are low in roots and high in leaves, stems, flowers and siliques (Fig. 4c). To test the effects of light on the expression of VAC1, we treated 3-day-old Arabidopsis etiolated seedlings with light for 0, 1, 2 and 4 h. Total RNA extracted from these samples was analyzed by semi-quantitative RT-PCR to detect the expression levels of VAC1. Low levels of VAC1 mRNA were detected in 3-day-old etiolated Arabidopsis seedlings (Fig. 4d, 0 h light). Exposure to light for 1 h rapidly increased the accumulation of VAC1 transcripts and higher levels of VAC1 mRNA were detected in seedlings treated by light for 2 and 4 h (Fig. 4d).
Chloroplast gene expression profiles of wild type, vac1 and ispH albino mutants
Many chloroplast protein-coding genes are organized as clusters and are co-transcribed as polycistronic messages. The expression of these polycistronic genes has to go through extensive posttranscriptional processing. To further identify vac1-specific molecular phenotypes, we used RNA gel blot analysis to compare the abundance and banding patterns of chloroplast transcripts in wild type, vac1 and ispH mutants. The psb genes encode subunits of the photosystem II protein complex and the pet genes encode subunits of the photoelectron transport cytochrome b6f protein complex. Compared to those of wild type, steady-state levels of psbA, psbB-psbH-petB-petD, psbD-psbC, psbE-psbF-psbL-psbJ, psbK-psbI, psbM and psbZ mRNAs are significantly decreased in the vac1 mutant (Fig. 5a). These effects are specific to the vac1 albino mutants, because the expression of most of the psb genes is only slightly affected in the ispH albino plants (Fig. 5a). In addition to the difference in transcript levels, the banding patterns of psbE-psbF-psbL-psbJ, psbK-psbI and psbM transcripts in vac1 are different from those of wild type and ispH. The intensity of each individual band of the psbE-psbF-psbL-psbJ transcripts is differentially regulated in vac1 compared to those of wild type and ispH. Although the abundance of psbK-psbI transcripts is significantly decreased in vac1, at least three unique high molecular weight psbK-psbI transcripts were specifically detected in vac1 but not in wild type and ispH. These results suggest that the abundance and expression patterns of chloroplast psb transcripts are significantly and specifically affected in the vac1 albino mutant.
RNA gel blot analysis of chloroplast genes in wild type (wt), vac1 (v) and ispH (i) mutants. a Steady-state levels of psbA, psbB-psbH-petB-petD, psbD-psbC, psbE-psbF-psbL-psbJ, psbK-psbI, psbM and psbZ transcripts are significantly decreased in vac1. A replicate gel was used to detect the expression levels of nuclear 18S rRNA as a loading control. b Steady-state levels of psaA-psaB-rps14 transcripts are significantly decreased in both vac1 and ispH albino mutants. Transcripts of psaC-ndhD and psaJ are differentially regulated in vac1 and ispH. The expression patterns of psaI are similar between vac1 and ispH. c Differential regulation of pet genes in vac1 and ispH. Steady-state levels of petN transcripts are significantly decreased in vac1. d In atpB-atpE operon, the PEP-transcribed ~2.6 kb transcripts are almost undetectable, whereas the NEP-transcribed ~2.4 kb transcripts are increased in vac1. e Steady-state levels of rpoA, rpoB, rpoC1, rpoC2 and clpP transcripts are increased in vac1. f Up-regulation of 5′rps12-rpl20, 3′rps12-rps7, rps11, rps15, rpl32, ycf1 and ycf2 transcripts in vac1. Arrowheads indicate high molecular weight transcripts that specifically appear in vac1. g Steady-state levels of accD transcripts in wild type, vac1 and ispH
The psa genes encode subunits of the photosystem I protein complex. The psaA, psaB and rps14 genes are co-transcribed in the same operon. Compared to those of wild type, steady-state levels of psaA-psaB-rps14 transcripts are significantly decreased in both vac1 and ispH albino mutants (Fig. 5b). These results suggest that the down-regulation of psaA-psaB-rps14 transcripts is not specific to the vac1 mutant. The ndh genes encode subunits of the NADH dehydrogenase. The psaC and ndhD genes are located in the same operon. Compared to those of wild type and ispH, steady-state levels of high molecular weight precursor transcripts of psaC-ndhD are specifically up-regulated in the vac1 albino mutant (Fig. 5b). In contrast to ndhD, the expression patterns of most ndh genes are similar between the vac1 and ispH albino mutants (Supplemental Fig. S2). Compared to those of wild type, levels of psaI transcripts are slightly decreased in both vac1 and ispH mutants (Fig. 5b). By contrast, steady-state levels of psaJ mRNAs are specifically down-regulated in the vac1 mutant (Fig. 5b). Whereas the abundance and expression patterns of petA, petG and petL transcripts are similar between vac1 and ispH albino mutants, steady-state levels of petN mRNA are significantly decreased in vac1, compared to those of wild type and ispH (Fig. 5c).
The chloroplast atp genes are organized into two transcriptional units in Arabidopsis. One cluster of genes has the order atpB-atpE and the other cluster has the order atpI-atpH-atpF-atpA. We designed probes corresponding to the coding regions of these chloroplast-encoded ATP synthase subunit genes to detect the abundance and banding patterns of these polycistronic transcripts by RNA gel blot analyses. We found that the expression patterns of atpB-atpE transcripts are specifically affected in the vac1 mutant (Fig. 5d). In the atpB-atpE operon, 2.6 and 2.4 kb transcripts are transcribed by PEP and NEP, respectively (Swiatecka-Hagenbruch et al. 2007). In the vac1 mutant, steady-state levels of NEP-transcribed 2.4 kb atpB-atpE transcripts are increased compared to those of wild type and ispH, whereas the PEP-transcribed 2.6 kb atpB-atpE transcripts are almost undetectable (Fig. 5d). Moreover, there are at least two additional high molecular weight atpB-atpE transcripts that specifically accumulate in the vac1 mutant (Fig. 5d). We used sense and antisense probes located in the atpB-atpE operon and its adjacent gene rbcL to demonstrate that these high molecular weight transcripts in vac1 have the same orientation as atpB-atpE transcripts (Supplemental Fig. S3). In contrast to the atpB-atpE transcripts, the expression patterns of atpI-atpH-atpF-atpA in vac1 are similar to those of ispH (Supplemental Fig. S2). Compared to those of wild type, steady-state levels of atpI transcripts are significantly decreased in both vac1 and ispH albino mutants, whereas the abundance of atpH, atpF and atpA transcripts is not affected (Supplemental Fig. S2).
The plastid-encoded RNA polymerase consists α, β, β′ and β″ subunits, which are encoded by the rpoA, rpoB, rpoC1 and rpoC2 genes, respectively. Compared to those of wild type and ispH, steady-state levels of rpoA, rpoB, rpoC1 and rpoC2 transcripts are increased in the vac1 mutant (Fig. 5e). Similarly, steady-state levels of clpP transcripts are specifically up-regulated in the vac1 mutant (Fig. 5e). In addition, a novel low molecular weight clpP transcript was specifically detected in the vac1 mutant (Fig. 5e). The rps and rpl genes encode ribosomal proteins of small and large subunits, respectively. The Arabidopsis rps12 gene is split into two parts, which belong to two different operons in the chloroplast genome. The 5′ and 3′ rps12 transcripts have to go through trans-splicing to form mature transcripts. Compared to those of wild type and ispH, steady-state levels of 5′rps12-rpl20, 3′rps12-rps7, rps11, rps15 and rpl32 are significantly increased in the vac1 mutant (Fig. 5f). The rpl33 and psaJ genes are located in the same operon. Similar to that of psaJ, steady-state levels of rpl33 transcripts are specifically down-regulated in the vac1 mutant (Supplemental Fig. S2). The expression levels of the other rps and rpl genes are similar between the vac1 and ispH albino mutants (Supplemental Fig. S2). The ycf genes encode proteins of unknown functions in the chloroplast. Compared to those of wild type and ispH, steady-state levels of ycf1 and ycf2 transcripts are specifically increased in the vac1 mutant (Fig. 5f). Moreover, the expression patterns and the intensity of each individual band of ycf3 and ycf5 transcripts in vac1 are significantly different from those of wild type and ispH (Supplemental Fig. S2). The accD gene encodes the β subunit of acetyl-CoA carboxylase. Steady-state levels of accD transcripts are decreased in both vac1 and ispH (Fig. 5g).
Levels of chloroplast rRNA are significantly reduced in vac1
The chloroplast rRNA genes are organized as a 16S-23S-4.5S-5S operon similar to that of prokaryotes (Fig. 6a). Ethidium bromide staining of total RNA extracted from 2 weeks old vac1 albino plants reveals that levels of chloroplast ribosomal RNA are significantly reduced in the vac1 albino mutant compared to that of wild type and ispH (Fig. 6b bottom). We used RNA gel blot analysis to examine the expression patterns and steady-state levels of 16S, 23S, 4.5S and 5S rRNAs in wild type, vac1 and ispH mutants. Compared to those of wild type and ispH, steady-state levels of 16S, 23S, 4.5S and 5S rRNAs are significantly decreased in the vac1 mutant (Fig. 6b, c). Despite the dramatic difference in transcript abundance, the banding patterns of chloroplast 4.5S and 23S rRNAs in vac1 are similar to those of wild type and ispH (Fig. 6c). These results suggest that steady-state levels of chloroplast rRNAs are specifically affected in the vac1 mutant but not in the ispH albino plants. In addition to rRNAs, we also used RNA gel blot analysis to examine the expression of chloroplast tRNA genes. It has been suggested that the expression of chloroplast trnE gene is dependent on PEP and tRNAGlu can specifically repress the activity of NEP during the late phase of chloroplast development (Hanaoka et al. 2005). Compared to those of wild type, steady-state levels of trnE transcripts are slightly decreased in ispH and dramatically reduced in vac1 (Fig. 6d). Similarly, steady-state levels of trnH, trnQ, trnR, trnT, trnW and trnY transcripts are also significantly decreased in vac1 (Fig. 6d). By contrast, steady-state levels of trnC, trnF, trnI, trnL, trnfM, trnN, trnP and trnV transcripts are decreased to similar levels in both vac1 and ispH compared to those of wild type (Supplemental Fig. S2).
RNA gel blot analysis of chloroplast rRNA genes in wild type (WT), vac1 and ispH. a Schematic diagram of chloroplast rRNA genes. b RNA gel blot analysis to detect levels of 16S and 5S rRNA. 23S* indicates breakdown products of 23S rRNA. c RNA gel blot analysis to detect levels of 4.5S and 23S rRNA. The structures and sizes of detected transcripts are indicated on the right. d Steady-state levels of trnE, trnH, trnQ, trnR, trnT, trnW and trnY transcripts are significantly decreased in vac1
Distinct regulation of nucleus-encoded photosynthetic genes in vac1 and ispH
Ribulose-bisphosphate carboxylase (Rubisco), the key enzyme of photosynthetic CO2 fixation, is composed of large and small subunits, which are encoded by rbcL in the chloroplast and rbcS in the nucleus, respectively. Compared to those of wild type and ispH, steady-state levels of rbcL transcripts are significantly decreased in vac1 (Fig. 7). By contrast, steady-state levels of rbcS transcripts are not affected in vac1 but are significantly decreased in ispH (Fig. 7). Similarly, compared to those of wild type, steady-state levels of CAB transcripts are only slightly reduced in vac1 but are dramatically decreased in ispH (Fig. 7). The vac1 mutants are albino lethal, which only retain limited ability in photosynthesis (Fig. 1). It is unexpected to observe that the amounts of rbcS and CAB transcripts are not or only slightly affected in the vac1 albino plants. We further examined the expression levels of several nucleus-encoded psa and psb genes in wild type, vac1 and ispH mutants by northern blot analysis. Interestingly, the results are very similar to those of rbcS and CAB. Compared to those of wild type, steady-state levels of psaD, psaE, psaF, psaL, psbO, psbP, psbR, psbS, psbW, psbX and psbY transcripts are not or only slightly affected in vac1 but are significantly decreased in ispH (Fig. 7).
RNA gel blot analysis of chloroplast and nuclear genes in wild type (WT), vac1 and ispH. Compared to those of wild type, steady-state levels of chloroplast-encoded rbcL transcripts are significantly decreased, whereas nucleus-encoded rbcS, CAB, psaD, psaE, psaF, psaL, psbO, psbP, psbR, psbS, psbW, psbX and psbY transcripts are not or only slightly decreased in vac1
The VAC1 PPR protein is involved in retrograde regulation of ZAT10
Norflurazon (NF) is a phytoene desaturase inhibitor that will block the synthesis of carotenoids. The signaling pathways (e.g. gun mutants) involved in NF-initiated plastid-to-nucleus retrograde regulation of CAB and rbcS genes have been established (Susek et al. 1993; Koussevitzky et al. 2007). To examine if the VAC1 PPR protein is involved in plastid-to-nucleus retrograde regulation, we used NF to treat wild type, vac1 and ispH albino plants. Six days old wild-type Arabidopsis, vac1 and ispH plants grown under normal condition were transferred to medium with or without NF for 6 days (Supplemental Fig. S4). Total RNA extracted from these samples was subject to northern blot analysis to examine the expression of retrograde-regulated genes, CAB, rbcS, ZAT10 and ZAT12 (Susek et al. 1993; Pogson et al. 2008). After NF treatment, steady-state levels of ZAT10 mRNA are decreased in wild type and ispH (Fig. 8). Interestingly, this NF-initiated retrograde regulation of ZAT10 does not exist in vac1. Instead of down-regulation, steady-state mRNA levels of ZAT10 are significantly increased in vac1 after NF treatment (Fig. 8). By contrast, steady-state levels of CAB, rbcS and ZAT12 mRNAs are further decreased in both vac1 and ispH albino mutants after NF treatment (Fig. 8). These results suggest that the VAC1 PPR protein may be required for the specific retrograde regulation of ZAT10 initiated by NF treatment.
The vac1 mutant is defective in accD and ndhF RNA editing
We used RT-PCR and bulk sequencing of the amplified cDNAs to examine all 34 chloroplast RNA editing sites in 2-week-old wild type, vac1 and ispH mutants. We found that the editing of two sites in accD and one site in ndhF was significantly reduced in vac1 (Fig. 9a). One of the accD editing sites is located at the 794th nucleotide from the AUG of the accD mRNA. C-to-U editing at this position results in an S265L amino acid change. The other accD editing site is located in the 3′UTR. In ndhF, C-to-U editing at the 290th nucleotide from the AUG leads to an S97L amino acid change. To determine the editing efficiency of these sites, we cloned the RT-PCR products and randomly sequenced 100 independent clones from each sample. Compared to that of wild type, the editing efficiency of these three sites was significantly decreased in vac1 and was only slightly reduced in ispH (Fig. 9a, b). The accD (S265L) editing site is 100%, 62% and 81% edited in wild type, vac1 and ispH, respectively (Fig. 9a, b). The editing of accD (3′UTR) site is partial in wild type (55%) and ispH (48%), but is dramatically reduced in vac1 (8%; Fig. 9a, b). Similarly, the editing efficiency of ndhF (S97L) site is 95%, 49% and 87% in wild type, vac1 and ispH, respectively (Fig. 9a, b).
RNA editing analysis in wild type Columbia (Col), vac1 and ispH. a Editing of accD and ndhF transcripts is specifically affected in vac1. b Editing efficiency of accD and ndhF transcripts in wild type, vac1 and ispH. c Editing of atpF, clpP, psbE and rpl23 transcripts is affected in ispH. d Editing of ndhG, petL, rpoA and rpoC1 is affected in both vac1 and ispH. Sequence chromatograms of PCR amplified genomic DNA (gDNA) or cDNA of the codon encompassing the editing sites are shown. The position of RNA editing indicated on the top represents the name of transcripts and the resulting amino acid change in wild type. Black arrows indicate the editing sites
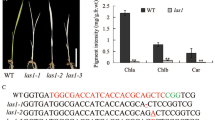
Finding obtained here that the editing of accD (S265L) site is partially impaired in vac1 is different from its allelic mutant ecb2 (Yu et al. 2009). We obtained the ecb2 (SALK_112251) line from the Arabidopsis Biological Resource Center (ABRC) and examined the editing of accD (S265L), accD (3′UTR) and ndhF (S97L) sites in the albino plants. Our results indicate that the editing profiles of these sites in ecb2 are very similar to those of vac1 (Fig. 9a, compare vac1 to ecb2).
By comparing the editing profiles among WT, vac1 and ispH, we have identified several RNA editing sites that are specifically affected in the ispH albino mutant. These RNA editing sites are located in ClpP (H187Y), atpF (P31L), psbE (P72S), and rpl23 (S30L) transcripts (Fig. 9c). In addition, the editing of ndhG (S17F), petL (P2L), rpoA (S67F) and rpoC1 (S170L) is reduced to similar levels in both vac1 and ispH albino mutants (Fig. 9d). The profiles of the other RNA editing sites are similar among wild type, vac1 and ispH (Supplemental Fig. S5).
Discussion
Editing of accD and ndhF is partially reduced, but not completely lost in vac1
The DYW proteins have been suggested to be involved in chloroplast RNA editing (Salone et al. 2007). We have examined all 34 known chloroplast RNA editing sites in wild type, vac1 and ispH. This comparison allows us to identify editing defects that are specific to vac1 or ispH albino mutants. We found that the editing of accD (S265L), accD (3′UTR) and ndhF (S97L) is specifically reduced, but not completely abolished in vac1. In contrast, it has been shown that the editing of accD (S265L) is completely abolished in a mutant (e.g. ecb2) that is allelic to vac1 (Yu et al. 2009). To solve the discrepancy, we obtained the ecb2 (SALK_112251) line from ABRC and analyzed the editing of accD and ndhF transcripts in the mutant. We found that the results are similar to those of vac1. The editing of accD (S265L) is partially reduced rather than completely abolished in ecb2. Thus the conclusion that the albino phenotype of ecb2 is caused by totally impaired in editing of accD (S265L) is incorrect. Moreover, it has been shown that a complete loss of accD (S265L) editing does not affect chloroplast development and the appearance of Arabidopsis (Robbins et al. 2009).
The accD gene encodes the β-carboxyl transferase subunit of acetyl-CoA carboxylase, which is important for fatty acid synthesis. It has been shown that the accD gene is essential and accD knockout plants are pale in tobacco (Kode et al. 2005). The editing of accD (3′UTR) is dramatically reduced in vac1. It is possible that the editing defect in the 3′UTR of accD mRNA may affect its stability or translation, which in turn may affect chloroplast development and function in vac1. Nonetheless, further experiments are required to test this possibility.
Similarity between vac1 and PEP-related mutants
Steady-state mRNA levels of NEP-transcribed genes (e.g. rpoA, rpoB, rpoC1, rpoC2, clpP, ycf1, ycf2 and some ribosomal protein genes) are increased, whereas the abundance of PEP-transcribed transcripts (e.g. rbcL, psaA, psaB and psb genes) is decreased in vac1. This type of molecular phenotypes has been observed in ys1 (Zhou et al. 2009), clb19 (Chateigner-Boutin et al. 2008), dg1 (Chi et al. 2008), ptac (Pfalz et al. 2006), rpo and mutants that do not accumulate PEP (Hess et al. 1993; Allison et al. 1996; Hajdukiewicz et al. 1997; Silhavy and Maliga 1998; De Santis-Maciossek et al. 1999; Krause et al. 2000; Legen et al. 2002). In addition, very long transcripts detected by probes derived from several chloroplast genes were present in vac1, which is similar to those observed in clb19 and PEP mutants (Legen et al. 2002; Chateigner-Boutin et al. 2008). For instance, high molecular weight atpB-atpE transcripts similar to those observed in clb19 were detected in vac1. However, our analyses reveal that the orientation of these high molecular weight transcripts in vac1 is different from those in clb19. The majority of Arabidopsis chloroplast genes are transcribed from multiple NEP and PEP promoters (Swiatecka-Hagenbruch et al. 2007). Those very long transcripts may be transcribed from cryptic NEP promoters. The complex chloroplast gene expression patterns observed in vac1 may be derived from selective usage of PEP and NEP promoters, and/or defects in RNA metabolism.
Glutamyl-tRNA has been proposed to mediate a switch in RNA polymerase use during chloroplast biogenesis (Hanaoka et al. 2005). The plastid-encoded glutamyl-tRNA can bind to and inhibit the transcriptional activity of NEP in vitro (Hanaoka et al. 2005). We used RNA gel blot analyses to examine the steady-state levels of trn transcripts in wild type, vac1 and ispH. These analyses allow us to detect polycistronic and unspliced transcripts of most trn genes. The banding patterns of trn transcripts in vac1 are similar to those of wild type and no obvious defects in tRNA splicing were observed. Interestingly, steady-state levels of some trn transcripts, including those of trnE, are specifically decreased in vac1. Low levels of glutamyl-tRNA in vac1 may relieve the inhibition of NEP, which may in part explain the up-regulation of NEP-transcribed transcripts in vac1.
Most of the molecular phenotypes of vac1 are similar to those of PEP-related mutants. One of the exceptions is the expression of accD gene. It has been shown that the abundance of NEP-transcribed accD transcripts is increased in clb19 and ys1 mutants (Chateigner-Boutin et al. 2008; Zhou et al. 2009). Interestingly, steady-state levels of accD transcripts are slightly decreased in the vac1 mutant. Unlike the clb19 and ys1 mutants, the editing of accD transcripts is specifically affected in vac1. It is possible that these editing defects may affect the stability of accD mRNA or expression accD gene.
Editing defects in rpoA and rpoB transcripts may affect PEP activity and cause profound effects on chloroplast gene expression (Zhou et al. 2009; Chateigner-Boutin et al. 2008). We did not observe specific editing defects in transcripts of rpoA, rpoB and rpoC1 in vac1. The efficiency of rpoA RNA editing is reduced to about 50% in both vac1 and ispH. Similarly, we did not observe significant difference in the editing of rpoB and rpoC1 transcripts between vac1 and ispH. Thus the incomplete editing of rpoA, rpoB and rpoC1 cannot be accounted for the albino phenotype of vac1. Still, it is possible that the rpoA, rpoB, rpoC1 and rpoC2 transcripts are not properly edited in vac1 at a site that has not been reported yet. We used RT-PCR to amplify cDNAs covering the entire coding regions and parts of the 5′ and 3′UTR of rpoA, rpoB, rpoC1 and rpoC2 in wild type and vac1. We compared the amplified cDNA sequences to genomic DNA and did not find new editing sites in these genes (data not shown). It is unlikely that editing defects in rpoA, rpoB, rpoC1 or rpoC2 transcripts are responsible for the vac1 phenotypes. However, we cannot exclude the possibility that the VAC1 protein may be involved in regulating PEP activity via mechanisms other than RNA editing.
Common and distinct molecular phenotypes between two albino mutants
The primary defect of ispH is in the plastid isoprenoid biosynthetic pathway. We found that a defect in a key biosynthetic pathway such as the ispH mutant can have many indirect effects on chloroplast gene expression and RNA editing. Similarly, we expect to see many secondary effects in the vac1 albino mutant. We tried to rule out the indirect or nonspecific effects in vac1 by comparing to another albino mutant ispH. This comparison allows us to distinguish common and distinct molecular phenotypes between two different albino mutants. For instance, a dramatic reduction in the abundance of psaA-psaB transcripts is common in both vac1 and ispH (Fig. 5b). It is likely that the down-regulation of these genes is due to some common secondary effects occurring in both albino mutants. This type of non-specific effects cannot be found by only comparing the vac1 mutant to wild type.
By contrast, we have uncovered several molecular phenotypes that are specific to vac1 or ispH. For example, the abundance and expression patterns of many chloroplast psb transcripts are only slightly affected in ispH but are dramatically affected in vac1. The abundance of 23S, 4.5S and 5S rRNAs and the editing of accD and ndhF transcripts are specifically reduced in vac1. These vac1-specific molecular phenotypes can be attributed to the loss of VAC1 protein rather than the general photo-oxidative defects commonly occur in albino plants.
In addition to common photo-oxidative damages occurred in albino mutants, it is possible that different albino mutants may have specific redox and/or oxidative stress signals. These common and specific signals may be involved in regulating the expression of chloroplast and nuclear genes. For instance, the specific defects in chloroplast psb gene expression in vac1 may be caused by signals specifically generated in vac1 but not in ispH. Another example is the expression of nucleus-encoded psa and psb genes in vac1. It is well known that the functional state of chloroplasts may signal changes in the expression of nuclear genes (Nott et al. 2006; Woodson and Chory 2008). Chloroplast development and function are impaired in vac1. It is expected that the expression of nucleus-encoded photosynthetic genes will be down-regulated in vac1. Interestingly, compared to those of wild type, steady-state mRNA levels of CAB, rbcS and many nucleus-encoded psa and psb genes are significantly decreased in ispH but not or are only slightly decreased in vac1. These results suggest that distinct plastid-to-nucleus retrograde signaling pathways may exist in vac1 and ispH, respectively.
It is likely that multiple plastid-to-nucleus signaling pathways may exist in plants. One of the examples is the GUN signaling pathway involved in NF-initiated retrograde regulation of CAB and rbcS (Susek et al. 1993; Mochizuki et al. 2001; Larkin et al. 2003; Koussevitzky et al. 2007). The expression of several non-photosynthetic genes including ZAT10 and ZAT12 is also subject to plastid-to-nucleus retrograde regulation (Pogson et al. 2008). The ZAT10 gene encodes a zinc finger transcription factor and its expression is regulated by drought, salt, cold and high light (Sakamoto et al. 2000, 2004; Gong et al. 2001; Lee et al. 2002; Rossel et al. 2007). We found that NF treatment significantly represses the expression of ZAT10 in wild type. This NF effect still exists in ispH but not in vac1. By contrast, the NF effect on the repression of CAB and rbcS is not affected in vac1. These results suggest that the VAC1 protein is not involved in the GUN signaling pathway. Instead, the VAC1 PPR protein may be specifically involved in an unknown retrograde regulation of ZAT10 initiated by NF. It will be interesting to further examine if VAC1 is directly or indirectly involved in specific retrograde signaling pathways.
Possible roles of the VAC1 PPR protein
The VAC1 PPR protein has a truncated DYW domain, whereas the other PPR-DYW proteins end with a very conserved tri-peptide DYW or DFW. This suggests that VAC1 could act differently from the other PPR-DYW proteins. The vac1 knockout mutant has global defects in chloroplast gene expression. It remains unclear if the phenotypes of vac1 are caused by a severe editing defect in accD 3′UTR. The molecular phenotypes in vac1 could be explained by defects in translation or PEP activity. Since the accumulation of chloroplast rRNAs is significantly reduced, the function of chloroplast ribosome may be compromised in vac1. A defect in ribosome will directly affect the translation of chloroplast proteins and causes a global effect on chloroplast gene expression. Nevertheless, more studies are required to unravel the exact molecular mechanism of VAC1.
References
Allison LA, Simon LD, Maliga P (1996) Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J 15:2802–2809
Barkan A, Walker M, Nolasco M, Johnson D (1994) A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J 13:3170–3181
Beick S, Schmitz-Linneweber C, Williams-Carrier R, Jensen B, Barkan A (2008) The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol Cell Biol 28:5337–5347
Cai W, Ji D, Peng L, Guo J, Ma J, Zou M, Lu C, Zhang L (2009) LPA66 is required for editing psbF chloroplast transcripts in Arabidopsis. Plant Physiol 150:1260–1271
Chateigner-Boutin AL, Small I (2007) A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res 35:e114
Chateigner-Boutin AL, Ramos-Vega M, Guevara-García A, Andrés C, de la Luz Gutiérrez-Nava M, Cantero A, Delannoy E, Jiménez LF, Lurin C, Small I, León P (2008) CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J 56:590–602
Chi W, Ma J, Zhang D, Guo J, Chen F, Lu C, Zhang L (2008) The pentratricopeptide repeat protein DELAYED GREENING1 is involved in the regulation of early chloroplast development and chloroplast gene expression in Arabidopsis. Plant Physiol 147:573–584
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
de Longevialle AF, Meyer EH, Andrés C, Taylor NL, Lurin C, Millar AH, Small ID (2007) The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 Intron 1 in Arabidopsis thaliana. Plant Cell 19:3256–3265
de Longevialle AF, Hendrickson L, Taylor NL, Delannoy E, Lurin C, Badger M, Millar AH, Small I (2008) The pentatricopeptide repeat gene OTP51 with two LAGLIDADG motifs is required for the cis-splicing of plastid ycf3 intron 2 in Arabidopsis thaliana. Plant J 56:157–168
De Santis-MacIossek G, Kofer W, Bock A, Schoch S, Maier RM, Wanner G, Rudiger W, Koop HU, Herrmann RG (1999) Targeted disruption of the plastid RNA polymerase genes rpoA, B and C1: molecular biology, biochemistry and ultrastructure. Plant J 18:477–489
Delannoy E, Stanley WA, Bond CS, Small ID (2007) Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem Soc Trans 35:1643–1647
Fisk DG, Walker MB, Barkan A (1999) Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J 18:2621–2630
Gong Z, Koiwa H, Cushman MA, Ray A, Bufford D, Kore-eda S, Matsumoto TK, Zhu J, Cushman JC, Bressan RA, Hasegawa PM (2001) Genes that are uniquely stress regulated in salt overly sensitive (sos) mutants. Plant Physiol 126:363–375
Hajdukiewicz PT, Allison LA, Maliga P (1997) The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J 16:4041–4048
Hammani K, Okuda K, Tanz SK, Chateigner-Boutin AL, Shikanai T, Small I (2009) A study of new Arabidopsis chloroplast RNA editing mutants reveals general features of editing factors and their target sites. Plant Cell 21:3686–3699
Hanaoka M, Kanamaru K, Fujiwara M, Takahashi H, Tanaka K (2005) Glutamyl-tRNA mediates a switch in RNA polymerase use during chloroplast biogenesis. EMBO Rep 6:545–550
Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 6:541–549
Hattori M, Miyake H, Sugita M (2007) A pentatricopeptide repeat protein is required for RNA processing of clpP Pre-mRNA in moss chloroplasts. J Biol Chem 282:10773–10782
Hedtke B, Börner T, Weihe A (1997) Mitochondrial and chloroplast phage type RNA polymerases in Arabidopsis. Science 277:809–811
Hess WR, Börner T (1999) Organellar RNA polymerases of higher plants. Int Rev Cytol 190:1–59
Hess WR, Prombona A, Fieder B, Subramanian AR, Börner T (1993) Chloroplast rps15 and the rpoB/C1/C2 gene cluster are strongly transcribed in ribosome-deficient plastids: evidence for a functioning non-chloroplast-encoded RNA polymerase. EMBO J 12:563–571
Hsieh MH, Goodman HM (2002) Molecular characterization of a novel gene family encoding ACT domain repeat proteins in Arabidopsis. Plant Physiol 130:1797–1806
Hsieh MH, Goodman HM (2005) The Arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol 138:641–653
Hu J, Bogorad L (1990) Maize chloroplast RNA polymerase: The 180-, 120-, and 38-kilodalton polypeptides are encoded in chloroplast genes. Proc Natl Acad Sci USA 87:1531–1535
Igloi GL, Kössel H (1992) The transcriptional apparatus of chloroplast. CRC Crit Rev Plant Sci 10:525–558
Jackson AO, Larkins BA (1976) Influence of ionic strength, pH, and chelation of divalent metals on isolation of polyribosomes from tobacco leaves. Plant Physiol 57:5–10
Kishine M, Takabayashi A, Munekage Y, Shikanai T, Endo T, Sato F (2004) Ribosomal RNA processing and an RNase R family member in chloroplasts of Arabidopsis. Plant Mol Biol 55:595–606
Kode V, Mudd EA, Iamtham S, Day A (2005) The tobacco plastid accD gene is essential and is required for leaf development. Plant J 44:237–244
Kotera E, Tasaka M, Shikanai T (2005) A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433:326–330
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316:715–719
Krause K, Maier RM, Kofer W, Krupinska K, Herrmann RG (2000) Disruption of plastid-encoded RNA polymerase genes in tobacco: expression of only a distinct set of genes is not based on selective transcription of the plastid chromosome. Mol Gen Genet 263:1022–1030
Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299:902–906
Lee H, Guo Y, Ohta M, Xiong LM, Stevenson B, Zhu JK (2002) LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO J 21:2692–2702
Legen J, Kemp S, Krause K, Profanter B, Herrmann RG, Maier RM (2002) Comparative analysis of plastid transcription profiles of entire plastid chromosomes from tobacco attributed to wild-type and PEP-deficient transcription machineries. Plant J 31:171–188
Leon P, Arroyo A, Mackenzie S (1998) Nuclear control of plastid and mitochondrial development in higher plants. Annu Rev Plant Physiol Plant Mol Biol 49:453–480
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Liere K, Maliga P (1999) In vitro characterization of the tobacco rpoB promoter reveals a core sequence motif conserved between phage-type plastid and plant mitochondrial promoters. EMBO J 18:249–257
Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8:457–463
Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B et al (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16:2089–2103
Maier RM, Zeltz P, Kössel H, Bonnard G, Gualberto JM, Grienenberger JM (1996) RNA editing in plant mitochondria and chloroplasts. Plant Mol Biol 32:343–365
Meierhoff K, Felder S, Nakamura T, Bechtold N, Schuster G (2003) HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 15:1480–1495
Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98:2053–2058
Mullet JE (1988) Chloroplast development and gene expression. Annu Rev Plant Physiol Plant Mol Biol 39:475–502
Nott A, Jung HS, Koussevitzky S, Chory J (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57:739–759
O’Toole N, Hattori M, Andres C, Iida K, Lurin C, Schmitz-Linneweber C, Sugita M, Small I (2008) On the expansion of the pentatricopeptide repeat gene family in plants. Mol Biol Evol 25:1120–1128
Okuda K, Myouga F, Motohashi R, Shinozaki K, Shikanai T (2007) Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc Natl Acad Sci USA 104:8178–8183
Okuda K, Habata Y, Kobayashi Y, Shikanai T (2008) Amino acid sequence variations in Nicotiana CRR4 orthologs determine the species-specific efficiency of RNA editing in plastids. Nucleic Acids Res 36:6155–6164
Okuda K, Chateigner-Boutin AL, Nakamura T, Delannoy E, Sugita M, Myouga F, Motohashi R, Shinozaki K, Small I, Shikanai T (2009) Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21:146–156
Pfalz J, Liere K, Kandlbinder A, Dietz KJ, Oelmüller R (2006) pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell 18:176–197
Pfalz J, Bayraktar OA, Prikryl J, Barkan A (2009) Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J 28:2042–2052
Pogson BJ, Woo NS, Förster B, Small ID (2008) Plastid signaling to the nucleus and beyond. Trends Plant Sci 13:602–609
Robbins JC, Heller WP, Hanson MR (2009) A comparative genomics approach identifies a PPR-DYW protein that is essential for C-to-U editing of the Arabidopsis chloroplast accD transcript. RNA 15:1142–1153
Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ (2007) Systemic and intracellular response to photooxidative stress in Arabidopsis. Plant Cell 19:4091–4110
Sakamoto H, Araki T, Meshi T, Iwabuchi M (2000) Expression of a subset of the Arabidopsis Cys(2)/His(2)-type zinc-finger protein gene family under water stress. Gene 248:23–32
Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol 136:2734–2746
Salone V, Rüdinger M, Polsakiewicz M, Hoffmann B, Groth-Malonek M, Szurek B, Small I, Knoop V, Lurin C (2007) A hypothesis on the identification of the editing enzyme in plant organelles. FEBS Lett 581:4132–4138
Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S (1999) Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res 6:283–290
Schmitz-Linneweber C, Small I (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci 13:663–670
Schmitz-Linneweber C, Williams-Carrier R, Barkan A (2005) RNA immunoprecipitation and microarray analysis show a chloroplast pentatricopeptide repeat protein to be associated with the 5′ region of mRNAs whose translation it activates. Plant Cell 17:2791–2804
Schmitz-Linneweber C, Williams-Carrier RE, Williams-Voelker PM, Kroeger TS, Vichas A, Barkan A (2006) A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell 18:2650–2663
Silhavy D, Maliga P (1998) Mapping of promoters for the nucleus-encoded plastid RNA polymerase (NEP) in the iojap maize mutant. Curr Genet 33:340–344
Small ID, Peeters N (2000) The PPR motif—a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25:46–47
Sugita M, Sugiura M (1996) Regulation of gene expression in chloroplasts of higher plants. Plant Mol Biol 32:315–326
Sugiura M, Hirose T, Sugita M (1998) Evolution and mechanism of translation in chloroplasts. Annu Rev Genet 32:437–459
Susek RE, Ausubel FM, Chory J (1993) Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74:787–799
Swiatecka-Hagenbruch M, Liere K, Börner T (2007) High diversity of plastidial promoters in Arabidopsis thaliana. Mol Genet Genomics 277:725–734
Swiatecka-Hagenbruch M, Emanuel C, Hedtke B, Liere K, Börner T (2008) Impaired function of the phage-type RNA polymerase RpoTp in transcription of chloroplast genes is compensated by a second phage-type RNA polymerase. Nucleic Acids Res 36:785–792
Williams PM, Barkan A (2003) A chloroplast-localized PPR protein required for plastid ribosome accumulation. Plant J 36:675–686
Woodson JD, Chory J (2008) Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet 9:383–395
Yamazaki H, Tasaka M, Shikanai T (2004) PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression in Arabidopsis. Plant J 38:152–163
Yu QB, Jiang Y, Chong K, Yang ZN (2009) AtECB2, a pentatricopeptide repeat protein, is required for chloroplast transcript accD RNA editing and early chloroplast biogenesis in Arabidopsis thaliana. Plant J 59:1011–1023
Zhou W, Cheng Y, Yap A, Chateigner-Boutin AL, Delannoy E, Hammani K, Small I, Huang J (2009) The Arabidopsis gene YS1 encoding a DYW protein is required for editing of rpoB transcripts and the rapid development of chloroplasts during early growth. Plant J 58:82–96
Acknowledgments
We thank Dr. J. Sheen for the GFP vector, T.Y. Chung for technical assistance and M.J. Fang for assistance in confocal microscopy. This work was supported by grants to M.-H. H. from National Science Council and Academia Sinica of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tseng, CC., Sung, TY., Li, YC. et al. Editing of accD and ndhF chloroplast transcripts is partially affected in the Arabidopsis vanilla cream1 mutant. Plant Mol Biol 73, 309–323 (2010). https://doi.org/10.1007/s11103-010-9616-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9616-5