Abstract
Using the Affymetrix poplar genome array, we explored the leaf transcriptome of salt-tolerant Populus euphratica Oliv. and salt-sensitive P. popularis 35-44 (P. popularis) under control and saline conditions. Our objective was to clarify the genomic differences in regulating K+/Na+ and reactive oxygen species (ROS) homeostasis between the two species. Compared to P. popularis, salt-tolerant P. euphratica responses to salinity involved induction of a relatively larger number of probesets after short-term (ST) exposure to 150 mM NaCl (24 h) and relatively fewer probesets after a long-term (LT) exposure to salinity (200 mM NaCl, 28 days). Compared to P. popularis, leaves of the control P. euphratica plants exhibited a higher transcript abundance of genes related to Na+/H+ antiport (Na+/H+ antiporters, H+ pumps) and K+ uptake and transport. Notably, the expression of these genes did not decrease (with a few exceptions) during salt treatment. Regarding ROS homeostasis, P. euphratica exhibited rapid up-regulation of a variety of antioxidant enzymes after exposure to ST salinity, indicating a rapid adaptive response to salt stress. However, the effect of NaCl on transcription in P. popularis leaves was more pronounced after exposure to prolonged salinity. LT-stressed P. popularis up-regulated some genes mediating K+/Na+ homeostasis but decreased transcription of main scavengers of superoxide radicals and H2O2 except for some isoforms of a few scavengers. Mineral and ROS analyses show that NaCl induced a marked increase of leaf Na+ and H2O2 in LT-stressed plants of the two species and the effects were even more pronounced in the salt-sensitive poplar. We place the transcription results in the context of our physiological measurements to infer some implications of NaCl-induced alterations in gene expression related to K+/Na+ and ROS homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is a major adverse environmental factor affecting plant growth and productivity. To deal with salt-induced ionic toxicity, osmotic stress and secondary oxidative stress, salt-tolerant plant species have developed a variety of alterations to survive saline environments, such as regulating ionic homeostasis and detoxifying reactive oxygen species (ROS) (Serrano and Rodriguez 2002; Xiong and Zhu 2002; Zhu 2003).
The entry of Na+ usually reduces K+/Na+ homeostasis in the cytosol because Na+ can compete with K+ for binding sites at the plasma membrane (PM) (e.g., low-affinity non-selective cation channels and high-affinity transporters) (Serrano 1996; Tester and Davenport 2003; Demidchik and Maathuis 2007; Shabala and Cuin 2008). In our previous studies, the salt-resistant poplar species Populus euphratica usually maintained higher K+ nutrition than salt-sensitive species at both the tissue and cellular levels (Chen et al. 2001, 2003a). K+ efflux in roots of NaCl-stressed and salt-sensitive Populus popularis is significantly greater than in P. euphratica, presumably resulting from K+ leakage through depolarisation-activated outward rectifying K+ channels (Chen et al. 2005, 2007; Cuin et al. 2008; Sun et al. 2009a). Species differences in retaining K+ are thought to be associated with gene expression under saline conditions. Using a salt-sensitive poplar, P. × canescens, Escalante-Pérez et al. (2009) found that transcription of transporters mediating K+ uptake (i.e., PtHKT1, putative high-affinity K+ uptake system; KPT1, guard cell K+ uptake channel; PKT1, putative K+ uptake channel) appeared to be down-regulated in leaves, but the expression of ion channels able to release K+ (i.e., PTORK, outward rectifying K+ channel; PTORK2, outward rectifying K+ channel 2; PTK2, K+ channel 2) showed remarkable up-regulation during salt stress in root and shoot organs. However, the molecular basis of the ability of P. euphratica in retaining K+ homeostasis under high soil salt concentrations is largely unknown.
Generally, plants prevent excessive cytosolic Na+ accumulation by reducing Na+ influx, excluding Na+ to the apoplast and/or compartmentalising Na+ into a vacuole via corresponding Na+/H+ antiporters and exchangers (Ward et al. 2003). Selective ion uptake restricts entry of Na+ into plant cells, appearing to occur at least partly through the transporter HKT1 (high-affinity K+ transporter) (Rus et al. 2001; Laurie et al. 2002; Mäser et al. 2002) and nonselective cation channels (Amtmann and Sanders 1999). The cloned transporters HKT1 and LCT1 (low-affinity cation transporter 1) have Na+ permeability when expressed in yeast or oocytes, suggesting that they are Na+ transporters mediating Na+ influx (Rubio et al. 1995; Schachtman et al. 1997). Overexpression of AtSOS1, a PM Na+/H+ antiporter, can confer salt tolerance by retrieving Na+ from the xylem of transgenic plants (Shi et al. 2003). Vacuolar Na+/H+ exchangers (NHXs) have been cloned from glycophytes (Apse et al. 1999; Fukuda et al. 1999; Gaxiola et al. 1999; Darley et al. 2000; Quintero et al. 2000) and overexpression of AtNHX1 improved plant salt tolerance in Arabidopsis (Arabidopsis thaliana) and tomato (Lycopersicon esculentum cv. Moneymaker), suggesting their contributions to vacuolar compartmentation of Na+ (Apse et al. 1999; Zhang and Blumwald 2001). Studies show that P. euphratica plants have a higher capacity for salt exclusion and vacuolar salt compartmentation compared to salt-sensitive poplar species (Chen et al. 2002a, 2003a). Short- (ST) and long-term (LT) stressed P. euphratica roots can extrude Na+ via the Na+/H+ antiport system (Sun et al. 2009b). PM Na+/H+ antiporters in P. euphratica, such as PeNhaD1 (Ottow et al. 2005a) and PeSOS1 (Wu et al. 2007), are thought to be involved in Na+ extrusion under NaCl stress. Overexpression of PeNhaD1 was found to decrease NaCl-induced Na+ accumulation in transgenic P. tomentosa (Chen 2007). However, the expression pattern of Na+/H+ antiporters varies with tissues, species and duration of salt stress. The transcript level of PeNhaD1 remained constant in P. euphratica leaves but decreased in P. × canescens after 24-h NaCl stress (Ottow et al. 2005b). However, in a 2-weeks study, the Na+/H+ antiporter PcNhaD1 was detected in roots, shoots, bark and leaf tissues of P. × canescens and the expression remained unchanged throughout the experiment (Escalante-Pérez et al. 2009). The mRNA level of PeSOS1 in the leaves stayed relatively constant although the level of protein expressed by PeSOS1 in the leaves of P. euphratica was significantly up-regulated after NaCl treatment (Wu et al. 2007). PtSOS1 expression was up-regulated by twofold in P. × canescens roots after 2 weeks of salt stress; the expression in shoot and bark did not change throughout the experiment but declined in leaves (Escalante-Pérez et al. 2009).
H+-ATPase retains electrochemical H+ gradients and provides a driving force for Na+/H+ antiporters to exclude Na+ from the cytoplasm or move Na+ into the vacuoles. Our data have shown that inhibition of PM H+-ATPase by sodium orthovanadate enhanced K+ efflux and reduced Na+/H+ antiport activity (Sun et al. 2009a, b). H+-ATPase properties in the tonoplast and PM vesicles of P. euphratica were characterised and a salt-induced activity of H+-ATPase was usually found in P. euphratica callus (Ma et al. 2002; Yang et al. 2007). The transcriptional regulation of H+-ATPase genes in P. euphratica tissues is largely unknown although the tonoplast and PM H+-ATPase genes revealed a different regulation pattern in response to salt stress in three Italian P. alba clones (Beritognolo et al. 2007).
Salt stress usually leads to increased ROS production in plant cells (Sudhakar et al. 2001; Grene 2002; Xiong and Zhu 2002; Xiong et al. 2002). Plants have developed enzymatic detoxifying systems, such as the ascorbate–glutathione cycle, to reduce the detrimental effects of ROS on macromolecules and cellular structures (Chinnusamy et al. 2005; Apel and Hirt 2004). The importance of oxidative protection in salt tolerance has been confirmed in Arabidopsis pst1. This mutant has significantly higher activity of superoxide dismutase (SOD) and ascorbate peroxidase (APX) than wild-type Arabidopsis when treated with high levels of NaCl (Tsugane et al. 1999). Overexpression of NtGST/GPX (glutathione S-transferase/glutathione peroxidase) improved salt and chilling tolerance by reducing ROS-mediated membrane damage (Roxas et al. 1997, 2000). We found that salinised P. euphratica did not exhibit an oxidative damage over the duration of salinity, partly resulting from up-regulation of antioxidant enzymes after the onset of salt stress (Wang et al. 2007, 2008). In contrast, P. popularis was not able to prevent the harmful effects of ROS although the activity of antioxidant enzymes (APX; catalase, CAT; glutathione reductase, GR) was markedly increased at high levels of soil NaCl (Wang et al. 2008). Transcriptome data on antioxidant enzymes would help us understand the differences in ROS homeostasis regulation between contrasting poplar species under salt stress.
P. euphratica trees grow in semi-arid saline areas and young plants can tolerate 450 mM NaCl under hydroponic conditions (Gu et al. 2004). In this salt-resistant tree model, physiological mechanisms underlying the salinity resistance of P. euphratica have been widely studied in recent years. Our previous studies have shown that salt tolerance in P. euphratica largely depends on its capacity for salt exclusion, effective vacuolar salt compartmentation and up-regulation of antioxidant enzymes after the onset of salt stress (Chen et al. 2001, 2002a, b, 2003a, b; Wang et al. 2007, 2008; Sun et al. 2009a, b).
Investigators have explored the molecular basis of salt tolerance in this species (Gu et al. 2004; Ottow et al. 2005a; Brosché et al. 2005). Based on a suppression subtractive hybridisation approach, Gu et al. (2004) found that transcripts up-regulated by salt stress included ionic and osmotic homeostasis elements, such as the Mg2+ transporter-like protein, syntaxin-like protein, seed imbibition protein and PM intrinsic protein. Brosché et al. (2005) clarified gene expression in adult P. euphratica trees growing in the desert canyon of Ein Avdat in Israel. Their microarray data suggest that several genes are putatively involved in osmoregulation (galactinol synthase), water balance maintenance (aquaporins) and detoxification (aldehyde dehydrogenase and metallothioneins). Ottow et al. (2005a) reported changes in transcript levels of various genes involved in salt-stress signalling and adaptation, including members of calcium (Ca2+)-regulated pathways, redox control, reactive oxygen formation and detoxification. A striking discovery was that the number of P. euphratica genes displaying different transcript levels accounted for only about 1% of the genes on the array of adult trees (Brosché et al. 2005). Moreover, a range of transcripts showed no altered transcript levels in juvenile plants or adult trees of P. euphratica; e.g., (1) in the “classic” enzymes involved in antioxidant defense, including CAT, APX, peroxiredoxin and SOD or (2) in the crucial transporters and channels mediating Na+ and K+ homeostasis (SOS1, NhaD1, KORCs, etc.). One reason is that the cDNA probes derived from the suppression subtractive hybridisation (Gu et al. 2004) or those from arbitrarily selected expressed sequence tags (Ottow et al. 2005a) did not cover the complete genomic information and may not have delineated the overall transcriptional profiles, especially those known to be involved in ionic and ROS homeostasis regulation.
Genome-wide transcriptional analysis offers an overall and improved understanding of genetic variations in salt tolerance exhibited in different poplar species. However, differences in gene expression patterns between salt-tolerant and salt-sensitive poplar species are largely unexplored. We used Affymetrix poplar chips with 65,535 probesets to analyse the gene expression profiles of ST- and LT-stressed P. euphratica leaves. Changes in transcript levels of a salt-sensitive poplar species, P. popularis 35-44, were examined in parallel under ST and LT salinity conditions. Our objective was to clarify the profile differences in ionic and ROS homeostasis between the two species. In this study, we applied real-time PCR to validate the microarray data.
Materials and methods
Plant material
In April 2007, 1-year-old seedlings of Populus euphratica Oliv. (P. euphratica), obtained from the Xinjiang Uygur Autonomous Region of China and hardwood cuttings of Populus popularis 35-44 (P. popularis) from the nursery of Beijing Forestry University (BFU), were planted in individual, 10 L pots containing loam soil and placed in a greenhouse at BFU. Plants in pots were irrigated two to three times per week according to evaporation demand and watered with 1 L full-strength Hoagland’s nutrient solution every 2 weeks. The temperature in the greenhouse ranged from 20 to 25°C with a 16-h photoperiod (7:00–23:00) and 150–1,000 μmol m−2 s−1 of photosynthetically active radiation under natural conditions.
Salt stress treatments
Plants were subjected to a short term (ST, 150 mM NaCl, 24 h) and a long term (LT, 200 mM NaCl, 28 days) salinity stress treatment. For ST stress, potted plants of P. euphratica and P. popularis were subjected to 24 h of increasing NaCl stress by top watering of 2 L of 50, 100 and 150 mM NaCl in a full-strength Hoagland’s nutrient solution every 3 h and then left standing for 18 h after the final concentration of NaCl was applied. Control plants were irrigated with the same amount of water at each treatment time. For LT stress, plants were subjected to 4 weeks of increasing NaCl stress by top watering of 2 L of 50, 100, 150 or 200 mM NaCl in a full-strength Hoagland’s nutrient solution on days 1, 8, 15 and 23. Control plants were kept well watered and fertilised without the addition of NaCl. Upper mature leaves (leaf index numbers 4–20 from the shoot apex) were sampled for RNA preparation after 24 h (ST) or 28 days (LT) of treatment.
RNA preparation
Total leaf RNA was extracted using TRIzol reagent and purified using a RNA purification kit (Qiagen, RNeasy spin column), followed by an on-column DNase treatment. After the RNA quality was assessed by a 1% (w/v) agarose gel analysis, RNA samples were eluted and adjusted to a final concentration of 1 μg μL−1.
Microarray analysis
The Affymetrix Poplar Array (Cat. 90078; Affymetrix; Santa Clara, CA, USA) was used in this experiment. This gene chip contains 65,535 probesets derived from 13 Populus species. The design of this array was based on the contents from the UniGene Build #6 (March 16, 2005) and from GeneBank mRNA and EST database for all poplar species. The chip, consisting of 11 probe pairs (25 bp per oligonucleotide) for each transcript, provides multiple independent measurements for each individual transcript. A 2-μg total RNA from each sample was used in preparation of biotin-tagged cRNA using the MessageAmp™ II aRNA Amplification Kit. These biotin-tagged cRNA probes were then fragmented to strands of 35–200 bp in length and hybridised with the Affymetrix Poplar Array. Hybridisation and scanning were performed at the Laboratory of CapitalBio Corporation in China. Details of the scanning and experimental procedures can be found at the Affymetrix Website (http://www.affymetrix.com/support/downloads/manuals/expression_analysis_technical_manual.pdf). In order to satisfy biological reduplication requirements, three individual seedlings for each treatment (control, ST and LT) were sampled from the two species.
Data processing and statistical analysis
Image analysis was performed using the GeneChip Operating software (GCOS 1.4). The scanned images were first assessed by visual inspection and then analyzed to generate raw data files, saved as CEL files, using the default setting of GCOS 1.4. A global scaling procedure was performed to normalise the different arrays using dChip software. The annotations were made by using the Probe Match tool on the NetAffx™ Analysis Center Website (NetAffx, http://www.affymetrix.com/analysis/index.affx). The probesets of genes presumably functioning in ionic and ROS homeostasis were extracted from the complete data set and are listed in Table S1. Those probesets with signals below 100 were considered as unstable background probesets (the signal of these probesets was adjusted to 100 for further analysis). In a comparative analysis, we applied a two-class Student’s t test method (Mev4.3.02 software, http://mev.tm4.org) (Saeed et al. 2003) to identify significantly differentially expressed genes. Relative expression was calculated as salt stress (ST, LT)/control or control P. euphratica/control P. popularis. Only genes with average signal values pass the threshold ratio 2 or the minus ratio 0.5; their false-positive discovery rates below 0.1 were considered significantly expressed. Probesets were clustered using hierarchical clustering based on Pearson correlation coefficients with Mev software and PCA analysis with pcaMethods software (Stacklies et al. 2007).
Verification of expression profiles by quantitative real-time PCR
To confirm the validity of the microarray analyses, relative quantitative real-time PCR was performed (MJ option2, Bio-Rad Corp.). Several typical genes were selected for confirmation: (1) highly expressed genes in the control P. euphratica compared to P. popularis are HAK1 (Ptp.7291.1.S1_at), ATGPX2 (glutathione peroxidase 2) (Ptp.5821.1.S1_at) and APX (PtpAffx.117027.1.S1_a_at) and (2) up-regulated genes in ST-stressed P. euphratica are POD (PtpAffx.2170.2.S1_s_at) and K+ channel (PtpAffx.46298.1.S1_at). These genes and their primers for real-time PCR are listed in Figure S1. All real-time reactions were performed using a ABI SYBR Green PCR Master Mix. Relative expression ratios were normalised to the actin housekeeping gene and agreement between the microarray analysis and real-time PCR was assessed (Fig. S1). Details of the real-time PCR are as follows: 2 μg of total RNA was reverse transcribed with the SuperScriptII (Invitrogen) using Oligo(dT) primers (Invitrogen) in a total volume of 25 μL. The reverse transcription product was then diluted with 25 μL of sterilised water to a final volume of 50 μL; 1 μL of the dilution was used as the template in the real-time PCR experiments. Primers for the PCR reactions were designed by PrimerSelect 5.00 of DNAStar (DNASTAR Inc) to have a Tm of ~60°C and an optimal annealing temperature of 53 to 55°C, with amplicon lengths between 200 and 300 bp.
Ion analysis
Leaf K+ and Na+ were analysed as described by Chen et al. (2003a). Briefly, leaves sampled from salt-treated and control plants were rinsed with de-ionised water and oven-dried at 65°C for 4 days, then ground to pass a 1-mm sieve for mineral analysis. Two hundred milligrams of leaf material were extracted with 10 mL of 0.1N HNO3 for 30 min and then filtered through filter paper (Whatman Grade 1). Na+ and K+ contents in the solutions were determined by an atomic absorption spectrophotometer (Perkin–Elmer 2280).
H2O2 analysis
Endogenous H2O2 levels were detected by a H2O2-specific fluorescent probe, H2DCF-DA (green) (Molecular Probe, Eugene, OR, USA, prepared in a Mes-KCl buffer, pH 5.7). The excised leaves sampled from control and salinised plants were washed with a buffer solution (BS: 0.5 mM KCl, 0.2 mM CaCl2, 0.1 mM MgCl2, 0.1 mM NaCl, 2.5% sucrose, pH 5.7) and then incubated in the BS containing 50 μM H2DCF-DA for 40 min at room temperature. Leaves were visualised using a Leica SP5 confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) after the excess fluorophore was washed thoroughly with BS. The confocal settings were as follows: excitation = 488 nm, emission = 510–530 nm, frame 512 × 512.
Results
Leaf Na+ and K+
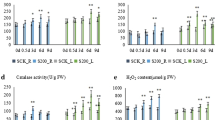
Non-stressed P. euphratica had more Na+ in leaves compared with control P. popularis plants (Fig. 1a). A short-term (ST) stress (150 mM NaCl, 24 h) did not significantly change the Na+ content in either species; however, leaf Na+ of both poplars increased markedly after a long-term (LT) salt treatment (200 mM NaCl, 28 days) (Fig. 1a). In comparison, LT-stressed P. popularis accumulated more Na+ in leaves, which was 2.3 times higher than that of P. euphratica (Fig. 1a).
Leaf Na+, K+ and K+/Na+ in P. euphratica and P. popularis following a short-term (ST) and a long-term (LT) salt treatment. Each column is the mean of four individual plants and bars represent the standard error of the mean. Columns labeled with different letters indicate significant difference between treatments at p < 0.05. NS: no significant difference
K+ was not significantly reduced by NaCl stress but the LT treatment lowered K+/Na+ ratios in the two species, due to the elevation of Na+ (Fig. 1b, c). Compared to P. euphratica, the salt-induced reduction of K+/Na+ was more pronounced in P. popularis leaves (Fig. 1c).
H2O2 production
In control plants of the two species, CLSM (confocal laser scanning microscopy) analysis of leaf surfaces showed that H2O2 (DCF-dependent fluorescence, green) was usually found in cells around the stomata (Fig. 2a). Notably a larger number of DCF-fluorescent cells were observed in control P. euphratica than in P. popularis (Fig. 2a). When subjected to ST stress, P. euphratica leaves exhibited a higher H2O2 production than P. popularis (Fig. 2a). However, an opposite trend was observed after a long period of salt stress. LT-stressed P. popularis showed more pronounced H2O2 production than P. euphratica (Fig. 2a). There was no green fluorescence in any of the negative samples without H2DCF-DA application (Fig. 2a), indicating that green fluorescence was produced by H2O2. Moreover, all leaf samples displayed nearly the same level of orange–yellow colour from the chlorophyll autofluorescence (Fig. 2b). This indicates that the excitation and reception parameters of the CLSM remained stable during fluorescent measurements.
Representative images illustrating the confocal laser scanning microscopy (CLSM) detection of H2O2 in leaves of P. euphratica (E) and P. popularis (P) following a short-term (ST) and a long-term (LT) salt treatment. The detection was carried out using the fluorescent probe H2DCF-DA and the bright green fluorescence corresponded to the detection of H2O2 (Series A). The orange–yellow color is due to the chlorophyll autofluorescence (Series B). In the two species, leaf sections without H2DCF-DA application did not exhibit any green fluorescence (PN, EN). CLSM analysis was performed using four individual seedlings for control, ST and LT treatments of the two species. Bar, 100 μm
Gene transcription
Our microarray data revealed species differences in gene transcription under control conditions. Control P. euphratica plants usually displayed a high abundance of salt tolerance-related genes compared to P. popularis. Furthermore, the two poplar species showed distinctly different tendencies in gene expression under ST and LT treatments. The sample-clustering results reveal that ST salinity strikingly changed the leaf transcriptome of P. euphratica; however, most of the up-regulated genes induced by ST stress returned to control levels after a prolonged exposure to salt treatment (Fig. 3). P. popularis showed an opposite trend in which LT salinity caused a drastic alteration of the transcriptome, whereas ST had only a slight effect on the leaf profile (Fig. 3).
Sample clustering of transcript abundance of genes relating to ionic and ROS homeostasis in P. euphratica (E) and P. popularis (P) following a short-term (ST) and a long-term (LT) salt treatment. Microarray analysis was performed using three individual seedlings for control (CK), ST and LT treatments of the two species
We summarized and compared probesets of differently expressed genes that presumably function in ionic and ROS homeostasis. Our microarray results were validated by real-time PCR with three additional independent biological replicates. The data from real-time PCR confirmed that the tendency of each pair of samples was similar to microarray results although the specific ratios varied between the two methods (Fig. S1). According to the potential function of the encoded enzymes and proteins, we categorised these differentially expressed genes into three groups: K+ homeostasis, Na+/H+ antiport and ROS homeostasis (Figs. 4, 5; Tables 1, 2, 3).
Hierarchical clustering of transcript abundance of genes relating to K+/Na+ homeostasis in P. euphratica (E) and P. popularis (P) leaves following a short-term (ST) and a long-term (LT) salt treatment. Microarray analysis was performed using three individual seedlings for each treatment (control, ST and LT) of the two species. Those probesets with signals below 100 were considered as background and unstable probesets (the signal of these probesets was adjusted to 100). Hierarchical clustering was carried out using the TMeV software package according to the relative expression indicated by the colour scale. Relative expression was calculated as salt stress (ST, LT)/control or control P. euphratica/control P. popularis. Only genes with average signal values passing the threshold of ratio 2 or minus ratio 0.5 and false-positive discovery rates below 0.1 were considered significantly expressed. EST: ST/control (P. euphratica); ELT: LT/control (P. euphratica); PST: ST/control (P. popularis); PLT: LT/control (P. popularis); E/P: Control P. euphratica/control P. popularis
Hierarchical clustering of transcript abundance of genes relating to ROS homeostasis in P. euphratica (E) and P. popularis (P) leaves following a short-term (ST) and a long-term (LT) salt treatment. Microarray analysis was performed using three individual seedlings for each treatment (control, ST and LT) of the two species. Those probesets with signals below 100 were considered as background and unstable probesets (the signal of these probesets was adjusted to 100). Hierarchical clustering was carried out using the TMeV software package according to the relative expression indicated by the colour scale. Relative expression was calculated as salt stress (ST, LT)/control or control P. euphratica/control P. popularis. Only genes with average signal values passing the threshold of ratio 2 or minus ratio 0.5 and false-positive discovery rates below 0.1 were considered significantly expressed. EST: ST/control (P. euphratica), ELT: LT/control (P. euphratica), PST: ST/control (P. popularis), PLT: LT/control (P. popularis), E/P: Control P. euphratica/control P. popularis
Transcript profiling of K+ homeostasis genes
Under no-salt control conditions, a variety of K+ transporter or exchanger proteins, especially KUP11 (K+ uptake permease 11), high-affinity K+ transporters (HAK1, OsHAK14, OsHKT7) and stelar K+ outward rectifier (SKOR) were more strongly expressed in P. euphratica than in P. popularis (Fig. 4; Table 1). After exposure to a ST salt treatment, a high-affinity K+ transporter (OsHKT7) and a K+ channel protein increased expression in P. euphratica and a K+-exchanger-like protein was up-regulated in P. popularis (Fig. 4; Table 1). In LT-stressed P. popularis leaves, a K+ channel protein exhibited down-regulated transcription, while transcript levels of the K+ efflux antiporter KEA2 (K+: H+ antiporter), KUP11 and K+ transporters (especially the high-affinity K+ transporter HAK1) were up-regulated (Fig. 4; Table 1). In contrast, LT-stressed P. euphratica leaves showed no alterations in transcription of K+ channel and transporter proteins, with the exception of a K+-exchanger-like protein (Fig. 4; Table 1).
Transcript profiling of Na+/H+ antiport genes
Control P. euphratica leaves retained higher expression of three members of the Na+/H+ antiporter family (e.g., SOS1 and ATNHD2) and two members of the SLT1 (sodium- and lithium-tolerant 1) family (Fig. 4; Table 2). Meanwhile, H+ pumps, i.e., PM H+-ATPase (very close to Lycopersicon esculentum and Daucus carota orthologs), vacuolar inorganic pyrophosphatase (PPase) and H+-ATPase (116-kDa subunit C) were found highly expressed in P. euphratica (Fig. 4; Table 2). In P. popularis leaves, a putative PM H+-ATPase, showing similarity to the Oryza sativa ortholog, was maintained at high transcript abundance (Fig. 4; Table 2). Notably, a variety of subunits of vacuolar type ATPase (V-ATPase subunits G 2, A and H) were more strongly expressed in P. popularis than in P. euphratica (Fig. 4; Table 2).
After being subjected to ST stress, inorganic PPase (soluble) displayed an increased transcript level in P. euphratica, while SLT1 (Arabidopsis thaliana ortholog) and Na+/H+ antiporter (a Medicago sativa ortholog) showed down-regulation (Fig. 4; Table 2). In contrast, ST-stressed P. popularis increased transcription of SLT1 (Arabidopsis thaliana and Nicotiana tabacum orthologs) but reduced expression of the PM H+-ATPase (Fig. 4; Table 2).
Under LT saline conditions, expressions of SLT1 proteins, tonoplast-bound PPase and V-type ATPase 116-kDa subunit family protein were enhanced in P. popularis, while the transcription of inorganic PPase (soluble) displayed an inconsistent response: one PPase member, highly close to the Papaver rhoeas ortholog, was up-regulated, but the other two members showing similarity to Arabidopsis thaliana and Oryza sativa orthologs displayed decreased transcript levels (Fig. 4; Table 2). In contrast to P. popularis, there were no differently expressed genes relating to Na+/H+ antiport in LT-stressed P. euphratica leaves, except for up-regulation of PM H+-ATPase (Fig. 4; Table 2).
Transcript profiling of ROS homeostasis genes
Among antioxidant enzymes, members of superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), peroxiredoxin (PRX), thioredoxin (TRX), glutaredoxin (GRX) and GST were differently expressed in the two poplar species, regardless whether control or salt treated (ST and LT) (Fig. 5; Table 3).
SOD
SODs are the main scavengers of superoxide radicals, O −.2 . Under non-saline conditions, three members of Cu–Zn SODs were strongly expressed in P. popularis (Fig. 5; Table 3). However, they exhibited a different response to ST and LT treatments: the SOD ortholog of Nelumbo nucifera was up-regulated in ST-stressed P. euphratica, but two members (Nelumbo nucifera and Arabidopsis thaliana orthologs) showed decreased transcript levels in LT-stressed P. popularis (Fig. 5; Table 3).
APX, POD and catalase
The expression of enzymes that detoxify hydrogen peroxide (H2O2) varies under control and saline conditions. One APX isoform (a close ortholog to APX of Arabidopsis thaliana) had higher transcript abundance in control P. euphratica leaves, whereas the other two isoforms (showing similarities to APX of Oryza sativa and Cucurbita cv. Kurokawa Amakuri) exhibited greater expression in control P. popularis leaves (Fig. 5; Table 3). We noted two different responses of OsAPx03 to salt stress: its expression was elevated with ST salinity in both poplar species but declined under LT salinity in P. popularis (Fig. 5; Table 3).
Similar to APX, POD isoforms exhibited divergent expression patterns in the two species. Control P. euphratica leaves retained higher expression levels of POD orthologs of Arabidopsis thaliana (TPX 1 and ATGPX2), Armoracia rusticana, Pisum sativum, Panax ginseng and Petroselinum crispum, whereas P. popularis leaves exhibited higher expression of isoforms similar to those from Arabidopsis thaliana (Atperox P47, Atperox P3, PER64) and Glycine max (Fig. 5; Table 3). Under ST stress, catalase-4 (a Glycine max ortholog) and three POD isoforms (Atperox P3, PER64, as well as a Glycine max ortholog) displayed an increased transcript level in P. euphratica (Fig. 5; Table 3). An anionic POD showed a similar response in ST-stressed P. popularis (Fig. 5; Table 3). After exposure to LT salinity, a large number of PODs exhibited declining expression in P. popularis, with a few exceptions (Fig. 5; Table 3). LT salinity down-regulated transcript abundance of hydroperoxide lyase while catalase-4 was up-regulated in P. popularis (Fig. 5; Table 3). Unlike P. popularis, the majority of PODs in P. euphratica leaves retained stable transcription under the long term treatment. The LT salinity changed the expression of only three members: the POD orthologs of Pisum sativum and Populus kitakamiensis were down-regulated while the ortholog of Glycine max was up-regulated (Fig. 5; Table 3).
PRX, TRX and GRX
The family of redoxins, PRX, TRX and GRX proteins, make an important contribution to retaining the cellular redox state. In control plants, 2-Cys PRX (PtpAffx.25069.1.A1_at) had higher transcript abundance in P. euphratica, but PRX Q and 2-Cys PRX BAS1 (Oryza sativa orthologs) were highly expressed in P. popularis (Fig. 5; Table 3). ST and LT stress had different effects on PRX transcription in the two species. ST salinity enhanced expression of PRX Q in P. euphratica; however, LT stress reduced transcription of PRXs in P. popularis leaves (Fig. 5; Table 3).
In general, H-type TRXs were highly expressed in P. euphratica, whereas M-type TRXs displayed higher transcript abundance in P. popularis under no-salt control conditions (Fig. 5; Table 3). Five types of TRX (including TRX M, TRX m4 and ATTDX) showed increased transcript abundance in ST-stressed P. euphratica, but LT-stressed plants retained TRX expression at a constant level, with the exception of one isoform that declined (TRX-H, a Ricinus communis ortholog) (Fig. 5; Table 3). TRX members displayed an inconsistent response to salt stress in P. popularis leaves: the majority remained unchanged whereas some members showed up- or down-regulation under ST and LT conditions (Fig. 5; Table 3).
There were species differences in the response of GRX to various saline conditions. A GRX family protein (Arabidopsis thaliana) increased transcripts in P. euphratica leaves under ST and LT stress (Fig. 5; Table 3). However, GRX members exhibited the opposite expression pattern in LT-treated P. popularis: LT salinity decreased the expression of Arabidopsis thaliana GRX orthologs (e.g., AtGrxC3), but elevated transcription of GRX members with close similarity to Oryza sativa and Ricinus communis (Fig. 5; Table 3).
GST
As shown in Fig. 5 and Table 3, GST isoforms (including GST 8, GST 14, GST 15 and GST 20) exhibited higher expression levels in control P. euphratica leaves; however, P. popularis leaves had higher transcript abundance of GST 9, GST 10, GST 13, GST 25 and GST U1. ST and LT stress did not change transcription of GSTs of P. euphratica with the exception of up-regulation of GST part C (Nicotiana tabacum, ST) and GST 25 (Glycine max, ST and LT) (Fig. 5; Table 3). ST-stressed P. popularis produced a pattern such as that of P. euphratica (Fig. 5; Table 3). Unlike the ST-stressed plants, LT salinity markedly changed transcription of GSTs in P. popularis. Several (e.g., GST 13, GST 14, GST 15 and GST 20) displayed increased expression while others (e.g., GST U1 and GST 10) showed the opposite response (Fig. 5; Table 3).
Discussion
NaCl-induced alterations of K+/Na+ and H2O2
NaCl caused a marked change of leaf ionic and ROS homeostasis, especially in the salt-sensitive poplar P. popularis (Figs. 1, 2). The salt-induced decline of K+/Na+ was more pronounced in LT-stressed P. popularis, although leaf K+ was not significantly decreased by NaCl stress in the two poplar species (Fig. 1). Na+ uptake in both species was significantly elevated after long-term salt exposure, but there was a large amount of Na+ in P. popularis leaves (Fig. 1). The results suggest that P. euphratica is more efficient in excluding salt than P. popularis (Chen et al. 2002a, 2003a). LT stress caused a drastic increase of H2O2 in P. popularis (Fig. 2), which resulted in oxidative damage to leaf cells (Wang et al. 2006, 2007, 2008). However, H2O2 levels were maintained at a lower level in LT-stressed P. euphratica (Fig. 2). It is interesting to note that ST salinity caused a significant increase of H2O2 in P. euphratica (Fig. 2), indicating that H2O2 acts as a stress signal to mediate plant stress adaptation (Sun et al. 2010). Our microarray data show that the capacity to retain K+/Na+ and ROS homeostasis in P. euphratica is related to the gene expression although transporters and antioxidant enzymes are usually regulated by pH, Ca2+ concentrations and protein modification (e.g., phosphorylation).
Transcription profiles of the two poplar species
By comparing leaf profiling of the two species, we found marked profiling differences between P. euphratica and P. popularis under both normal and saline conditions (ST and LT) (Fig. 3). Compared to P. popularis, control P. euphratica leaves exhibited a higher transcript abundance of genes related to Na+/H+ antiport (Na+/H+ antiporters, H+ pumps) and K+ uptake and transport (Fig. 4; Tables 1, 2). The expression data of control poplar plants are very similar to what has been reported for Arabidopsis and its related halophyte, Thellungiella halophila, in which a number of abiotic stress-inducible genes were expressed in salt cress under normal growing conditions (Taji et al. 2004). Our microarray results also show that a relatively large number of probesets were up-regulated in P. euphratica leaves after ST exposure, whereas in P. popularis leaves, more probesets were altered after LT exposure to a higher salinity (Tables 1, 2 and 3). Similarly, there are species-differences between herbaceous plants in the response to salinity. In Arabidopsis, many genes were induced by NaCl stress (Kreps et al. 2002; Taji et al. 2004) in contrast to Thellungiella ecotypes (Taji et al. 2004; Wong et al. 2006). Zhu (2001) posed the hypothesis that subtle differences in gene expression regulation result in large variations in tolerance or sensitivity between halophytes and glycophytes. Therefore, the differences in transcription profiles between salt-tolerant and salt-sensitive poplar species lead to a better understanding of the molecular basis for salt tolerance in tree plants.
Transcriptional profiles related to leaf K+/Na+ homeostasis under NaCl stress
K+ homeostasis genes
K+ is an essential macronutrient and plant salt tolerance is usually correlated with the capacity for K+ uptake and transport in the face of high Na+ content. A 4-weeks salinity treatment did not significantly decrease leaf K+ in the two species (Fig. 1), partly due to the supply of root-derived K+, displaced by Na+ (Chen and Polle 2010). K+ concentrations in P. euphratica leaves decreased only moderately after prolonged exposure to salt stress (Ottow et al. 2005a) because of the high K+ uptake and K+ concentrations in the xylem sap in the presence of high levels of external sodium (Chen et al. 2003a). In a previous study, we used X-ray microanalysis to examine subcellular K+ compartmentation in salt-tolerant and salt-sensitive poplar species. We found a high K+ level in all examined cell types of P. euphratica leaves (i.e., leaf bundle, vascular parenchyma, mesophyll, adaxial and abaxial epidermis) (Dai et al. 2006). Our microarray data in the current study indicate that the greater ability of P. euphratica to retain K+ is correlated with the transcription abundance of K+ transporters and channels. A variety of K+ transporters, especially high-affinity K+ transporters and stelar K+ outward rectifiers, were more strongly expressed in P. euphratica than in P. popularis (Fig. 4; Table 1). High-affinity K+ transporters assist in the uptake of K+ by P. euphratica cells, especially in the face of high levels of Na+ salinity. SKOR is usually expressed in root stelar tissues and its greater abundance in leaves benefits K+ translocation between vascular bundles and parenchyma. Under ST stress, a K+ channel protein (similar to the vacuolar membrane two-pore K+ channel AtKCO1; Czempinski et al. 2002) and a high-affinity K+ transporter (OsHKT7) increased expression levels in P. euphratica leaves (Fig. 4; Table 1), indicating that P. euphratica enhanced K+ uptake and redistributed intracellular K+ to compensate for losses. Ottow et al. (2005a) suggest that P. euphratica maintains the balance of K+ against Na+ ions by shifting K+ from the vacuoles to the cytoplasm.
Under LT saline conditions, P. euphratica did not change expression levels of proteins mediating K+ homeostasis (Fig. 4; Table 1). In contrast, LT-stressed P. popularis exhibited marked alterations in gene expressions related to K+ uptake and transport, such as a down-regulated K+ channel protein and the up-regulated K+ efflux antiporter KEA2 and K+ transporters, especially high-affinity K+ transporters (Fig. 4; Table 1). The decreased expression of K+ channels may affect the redistribution of K+ between cell compartments and the increased transcript levels of KEA2 would cause a K+ efflux. The high-affinity K+ transporters were up-regulated in P. popularis, presumably stimulated by a K+ shortage in the apoplast space (Dai et al. 2006). In previous work, SIET (the Scanning Ion-selective Electrode Technique) measurement showed increased K+ efflux in LT-stressed P. popularis roots (Sun et al. 2009b), which could restrict the root-to-shoot K+ transport and subsequently reduce K+ levels in leaves. Our microarray data for LT-stressed P. popularis (28 days of NaCl stress) are inconsistent with the RT-PCR data derived from work with the salt-sensitive poplar, P. × canescens (14 days of 50 mM NaCl stress) (Escalante-Pérez et al. 2009). In that study, the authors found that the transcription of transporters mediating K+ uptake (PtHKT1, KPT1, PKT1) was generally inhibited but that salinity did not reduce expression of transporters mediating K+ transport (PTORK, PTORK2, PTK2).
Na+/H+ antiport system
We found that less Na+ was accumulated in P. euphratica leaves than in P. popularis after 4 weeks of treatment (Fig. 1). These results are consistent with our previous reports (Chen et al. 2002a; Wang et al. 2008). The involvement of Na+/H+ antiporters and H+ pumps in Na+ extrusion has been verified at tissue and cellular levels (Shabala 2000; Shabala and Newman 2000; Sun et al. 2009a, b). Compared with the salt-sensitive poplar species, P. euphratica cells have a greater ability to exclude Na+ to the apoplast (Chen et al. 2002a, 2003a; Dai et al. 2006; Ottow et al. 2005a) and/or to compartmentalise Na+ into vacuoles (Chen et al. 2002a, 2003a; Dai et al. 2006). The SIET data reveal that Na+ extrusion exhibited by salt-stressed P. euphratica results from the active Na+/H+ antiport across the PM (Sun et al. 2009a, b). Ottow et al. (2005a) suggested that Na+ export from the cytosol into the apoplast is most likely carried out by an SOS1 homolog and an additional PeNhaD1. Leaf transcriptome data from our study support this hypothesis. Control P. euphratica leaves retained a higher expression of Na+/H+ antiporters like SOS1 and NHD2 (Fig. 4; Table 2). This is in good agreement with the finding observed in model plants. Taji et al. (2004) found that the expression of the SOS1 gene in Thellungiella halophila was higher than that of Arabidopsis in the absence of stress. Notably, the expression of the two Na+/H+ antiporters in P. euphratica did not decline under ST and LT salinity (Fig. 4; Table 2), indicating active transport of Na+ with H+ in stressed cells. Similarly, RT-PCR data showed that P. euphratica maintained the transcriptional level of NhaD1 (Ottow et al. 2005b) and PeSOS1 (Wu et al. 2007) during the period of salt stress (24–48 h). However, expression of Na+/H+ antiporters usually decreased in the salt-sensitive poplar species P. × canescens; for example, PcNhaD1 decreased transcription during a 24-h salt stress (Ottow et al. 2005b) and PtSOS1 expression declined in a 14-days study (Escalante-Pérez et al. 2009).
H+ pumps provide electrochemical H+ gradients and function in driving Na+/H+ exchange across the PM. PM H+-ATPases exhibited higher expression in P. euphratica and did not show transcription level declines under various saline conditions (Fig. 4; Table 2). Similarly, in Thellungiella halophila, the ATPase genes, plasma membrane proton ATPase (PMA) and PM H+-transporting ATPase type 2, are expressed at high levels even in the absence of stress conditions (Taji et al. 2004). Confirming these findings, NaCl-treated cells retained a higher influx of H+ to exchange with cytosol Na+ via the PM Na+/H+ antiporters (Sun et al. 2009b). It is interesting to note that the activity of H+ pumps (assessed by cytochemical staining) is elevated by NaCl in leaf cells of P. euphratica (X. Ma, L. Deng, J. Li, X. Zhou and S. Chen, unpublished data). Evidence by Western blotting proved that the salt-induced PM H+-ATPase in P. euphratica was partly attributable to the increase of protein content (X. Ma, L. Deng, J. Li, X. Zhou and S. Chen, unpublished data). These results suggest that post-transcription modifications of H+-ATPase is involved in acclimatising to NaCl stress in salt-resistant poplar species.
Our SIET data have shown that the Na+/H+ antiport system in salt-stressed P. popularis is insufficient to exclude Na+ at the tissue and cellular levels (Sun et al. 2009a, b), presumably because of its lower transcription of Na+/H+ antiporters. The lower transcript abundance of Na+/H+ antiporters may also restrict the capacity for vacuolar salt compartmentation, although P. popularis displayed a higher expression of vacuolar ATP synthase subunits (Table 2). X-ray microanalysis has revealed that leaf cells of NaCl-stressed P. popularis cannot effectively sequester Na+ into the vacuole (X. Ma, L. Deng, J. Li, X. Zhou and S. Chen, unpublished data).
Transcriptional profiles related to leaf ROS homeostasis under NaCl stress
P. euphratica retained a moderate amount of H2O2 after being subjected to ST and LT salinity (Fig. 2). Antioxidant enzymes play a crucial role in maintening ROS homeostasis; however, activation of genes encoding these enzymes differed markedly between the two species under saline conditions. After exposure to ST salinity, P. euphratica showed strikingly up-regulated transcription of a variety of anti-oxidant enzymes, including Cu–Zn SOD, CAT, PODs, PRX, TRXs, GRX and GSTs (Fig. 5; Table 3). Compared with P. euphratica, fewer antioxidant enzymes (e.g., APX, anionic POD, TRXs and GRX) were up-regulated in ST-stressed P. popularis, implying an insensitivity to salt stress (Fig. 5; Table 3). We have shown that iso-enzymatic and total activity of leaf SOD, APX and POD in P. euphratica were rapidly increased after the onset of salt stress (Wang et al. 2006, 2007, 2008). In this study, increases in transcripts of antioxidant enzymes following ST treatment enabled P. euphratica plants to initiate an effective antioxidant defense to deal with prolonged saline conditions. Unlike ST-stressed plants, expression of antioxidant enzymes in LT-stressed P. euphratica had not significantly altered, with a few exceptions (Fig. 5; Table 3). The transcriptional pattern agrees with the identification by Ottow et al. (2005a), who found that salt shock induced more stress-related genes and their stronger expression, including ROS detoxifying systems (chloroplastic SOD, glutathione peroxidase and polyamine oxidase) in P. euphratica leaves than salt adaptation.
In contrast to LT-stressed P. euphratica, LT salinity significantly altered many more genes of antioxidant enzymes in P. popularis leaves. NaCl decreased transcription of Cu–Zn SODs, H2O2 lyase, APX, PRXs and some members of POD, TRX, GRX and GST, but enhanced expression of CAT and several members of POD, TRX, GRX and GST (Fig. 5; Table 3). These results indicate the member-specific response to salinity in each family of antioxidant enzymes. In one of our previous studies, a prolonged salt exposure did not inhibit SOD activity but enhanced the activity of APX, CAT (both iso-enzymatic activity and total activity) and GR (glutathione reductase) in P. popularis leaves (Wang et al. 2008). However, NaCl caused a drastic H2O2 production in P. popularis leaves (Fig. 2). The occurrence of oxidative damage in this species indicated that the rate of ROS production exceeded the oxygen-scavenging capacity of the antioxidant enzymes (Wang et al. 2008), although some antioxidant enzymes (e.g., POD, TRX, GRX and GST) were up-regulated. In another study using a higher concentration of NaCl, the isoenzymatic activity of SOD (CuZn-SOD I and CuZn-SOD II) and POD in P. popularis decreased, over time, with salt exposure (Wang et al. 2007). The correspondingly increased leaf malondialdehyde content and membrane permeability indicated lipid peroxidation and salt damage (Wang et al. 2007).
Conclusions
Based on expression profiles using the Affymetrix Poplar Array, we found that various genes related to ionic and ROS homeostasis were highly expressed under no-salt conditions in P. euphratica. Our result is largely consistent with Arabidopsis-related halophyte, Thellungiella. Leaf transcriptome data in this study could largely explain our findings that P. euphratica has a great ability to retain ionic and ROS homeostasis under saline conditions. Regarding ionic homeostasis, control P. euphratica exhibited a higher transcript abundance of genes mediating Na+/H+ antiport and K+ uptake and transport, compared to P. popularis. Moreover, salt stress did not significantly reduce the expression of these genes (with a few exceptions). This pattern assists P. euphratica in retaining K+/Na+ homeostasis during salt stress. In terms of ROS homeostasis regulation, P. euphratica leaves rapidly up-regulated transcription of a variety of antioxidant enzymes after exposure to short-term salinity, whereas there were no corresponding changes in P. popularis. This finding accords with our hypothesis that P. euphratica avoids an oxidative burst by rapid enhancement of antioxidant enzymes at low soil salt concentrations. In contrast to P. euphratica, P. popularis exhibited a fundamental change in gene transcription under long-term salinity. This outcome is presumably the result of a damage effect in leaves (e.g., K+ shortage, Na+ buildup and excessive ROS). Given these results, we conclude that the low capacity of P. popularis to deal with ROS production and ion balance at the beginning of salt stress results in an overproduction of ROS in leaf cells, leading to oxidative damage over long lasting salinity.
References
Amtmann A, Sanders D (1999) Mechanisms of Na+ uptake by plant cells. Adv Bot Res 29:75–112
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 55:373–399
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Beritognolo I, Piazzai M, Benucci S, Kuzminsky E, Sabatti M, Mugnozza GS, Muleo R (2007) Functional characterisation of three Italian Populus alba L. genotypes under salinity stress. Trees (Berl) 21:465–477
Brosché M, Vinocur B, Alatalo ER, Lamminmäki A, Teichmann T, Ottow EA, Djilianov D, Afif D, Bogeat-Triboulot M-B, Altman A, Polle A, Dreyer E, Rudd S, Paulin L, Auvinen P, Kangasjärvi J (2005) Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biol 6(R101):1–17
Chen CX (2007). PeNhaD1 gene transformation and salt resistance of transgenic Populus tomentosa. PhD thesis, Beijing Forestry University, Beijing, People’s Republic of China (in Chinese)
Chen S, Polle A (2010) Salinity tolerance of Populus. Plant Biol 12:317–333
Chen S, Li J, Wang S, Hüttermann A, Altman A (2001) Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees (Berl) 15:186–194
Chen S, Li J, Fritz E, Wang S, Hüttermann A (2002a) Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress. For Ecol Manag 168:217–230
Chen S, Li J, Wang T, Wang S, Polle A, Hüttermann A (2002b) Osmotic stress and ion-specific effects on xylem abscisic acid and the relevance to salinity tolerance in poplar. J Plant Growth Regul 21:224–233
Chen S, Li J, Wang S, Fritz E, Hüttermann A, Altman A (2003a) Effects of NaCl on shoot growth, transpiration, ion compartmentation, and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can J For Res 33:967–975
Chen S, Li J, Wang T, Wang S, Polle A, Hüttermann A (2003b) Gas exchange, xylem ions and abscisic acid response to Na+-salts and Cl−-salts in Populus euphratica. Acta Bot Sin 45:561–566
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28:1230–1246
Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabala S (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt stressed barley. Plant Physiol 145:1714–1725
Chinnusamy V, Jagendorf A, Zhu J-K (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008) A root’s ability to retain K+ correlates with salt tolerance in wheat. J Exp Bot 59:2697–2706
Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Mueller-Roeber B (2002) Vacuolar membrane localization of the Arabidopsis ‘two-pore’ K+ channel KCO1. Plant J 29:809–820
Dai S, Chen S, Fritz E, Olbrich A, Kettner C, Polle A, Hüttermann A (2006) Ion compartmentation in leaf cells of Populus euphratica and P. tomentosa under salt stress. J Beijing For Univ 28(Suppl 2):1–5 (in Chinese with English abstract)
Darley CP, van Wuytswinkel OC, van der Woude K, Mager WH, de Boer AH (2000) Arabidopsis thaliana and Saccharomyces cerevisiae NHX1 genes encode amiloride sensitive electroneutral Na+/H+ exchangers. Biochem J 351:241–249
Demidchik V, Maathuis FJM (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175:387–404
Escalante-Pérez M, Lautner S, Nehls U, Selle A, Teuber M, Schnitzler JP, Teichmann T, Fayyaz P, Hartung W, Polle A, Fromm J, Hedrich R, Ache P (2009) Salt stress affects xylem differentiation of grey poplar (Populus × canescens). Planta 229:299–309
Fukuda A, Nakamura A, Tanaka Y (1999) Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim Biophys Acta 1446:149–155
Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96:1480–1485
Grene (2002) Oxidative stress and acclimation mechanisms in plants. The Arabidopsis Book (Special revue), The American Society of Plant Biologists, Rockville, MD, USA, pp 1–20
Gu R, Fonseca S, Puskás LG, Hackler L Jr, Zvara Á, Dudits D, Pais MS (2004) Transcript identification and profiling during salt stress and recovery of Populus euphratica. Tree Physiol 24:265–276
Kreps JA, Wu Y, Chang H-S, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130:2129–2141
Laurie S, Feeney KA, Maathuis FJ, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32:139–149
Ma T, Liu Q, Li Z, Zhang X (2002) Tonoplast H+-ATPase in response to salt stress in Populus euphratica cell suspensions. Plant Sci 163:499–505
Mäser P, Gierth M, Schroder J (2002) Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 247:43–54
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M, Kangasjärvi J, Jiang X, Polle A (2005a) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139:1762–1772
Ottow EA, Polle A, Brosché M, Kangasjärvi J, Dibrov P, Zörb C, Teichmann T (2005b) Molecular characterization of PeNhaD1: the first member of the NhaD Na+/H+ antiporter family of plant origin. Plant Mol Biol 58:73–86
Quintero FJ, Blatt MR, Pardo JM (2000) Functional conservation between yeast and plant endosomal Na+/H+ antiporters. FEBS Lett 471:224–228
Roxas VP, Smith RK Jr, Allen ER, Allen RD (1997) Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15:988–991
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270:1660–1663
Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA 98:14150–14155
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378
Schachtman DP, Kumar R, Schroeder JI, Marsh EL (1997) Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proc Natl Acad Sci USA 94:11079–11084
Serrano R (1996) Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int Rev Cytol 165:1–52
Serrano R, Rodriguez PL (2002) Plants, genes and ions: workshop on the molecular basis of ionic homeostasis and salt tolerance in plants. EMBO Rep 3:116–119
Shabala S (2000) Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ 23:825–837
Shabala S, Cuin TA (2008) Cellular mechanisms of potassium transport in plants. Physiol Plant 133:651–669
Shabala S, Newman IA (2000) Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: masking role of the cell wall. Ann Bot 85:681–686
Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Stacklies W, Redestig H, Scholz M, Walther D, Selbig J (2007) pcaMethods—a bioconductor package providing PCA methods for incomplete data. Bioinformatics 23:1164–1167
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Sun J, Dai S, Wang R, Chen S, Li N, Zhou X, Lu C, Shen X, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009a) Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol 29:1175–1186
Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, Zhang Z, Song J, Xu Y (2009b) NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149:1141–1153
Sun J, Wang M, Ding M, Deng S, Liu M, Lu C, Zhou X, Shen X, Zheng X, Zhang Z, Song J, Hu Z, Xu Y, Chen S (2010) H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ (doi: 10.1111/j.1365-3040.2010.02118.x)
Taji T, Seki M, Satou M, Sakurai T, Kobayashi M, Ishiyama K, Narusaka Y, Narusaka M, Zhu J-K, Shinozaki K (2004) Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol 135:1697–1709
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195–1206
Wang R, Chen S, Ma H, Liu L, Li H, Weng H, Hao Z, Yang S (2006) Genotypic differences in anti-oxidative stress and salt tolerance of three poplars under saline conditions. Front For China 1:82–88
Wang R, Chen S, Deng L, Fritz E, Hüttermann A, Polle A (2007) Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees 21:581–591
Wang R, Chen S, Zhou X, Shen X, Deng L, Zhu H, Shao J, Shi Y, Dai S, Fritz E, Hüttermann A, Polle A (2008) Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol 28:947–957
Ward JM, Hirschi KD, Sze H (2003) Plants pass the salt. Trends Plant Sci 8:200–201
Wong CE, Li Y, Labbe A, Guevara D, Nuin P, Whitty B, Diaz C, Golding GB, Gray GR, Weretilnyk EA, Griffith M, Moffatt BA (2006) Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in Thellungiella, a close relative of Arabidopsis. Plant Physiol 140:1437–1450
Wu Y, Ding N, Zhao X, Zhao M, Chang Z, Liu J, Zhang L (2007) Molecular characterization of PeSOS1: the putative Na+/H+ antiporter of Populus euphratica. Plant Mol Biol 65:1–11
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139
Xiong L, Schumaker KS, Zhu J-K (2002) Cell Signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Yang Y, Zhang F, Zhao M, An L, Zhang L, Chen N (2007) Properties of plasma membrane H+-ATPase in salt-treated Populus euphratica callus. Plant Cell Rep 26:229–235
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:1–5
Acknowledgments
The research was supported jointly by the HI-TECH Research and Development Programme of China (863 Programme, grant number 2006AA10Z131), the National Natural Science Foundation of China (grant numbers 30430430, 30872005), the Foundation for Supervisors of Beijing Excellent Doctoral Dissertations (grant number YB20081002201), the Foundation for Authors of the National Excellent Doctoral Dissertation of PR China (grant number 200152), the Teaching and Research Award Programme for Outstanding Young Teachers at Higher Education Institutions of the Ministry of Education (MOE), PRC (grant number 2002-323), the Key project of the MOE, PRC (grant number 2009-84) and the Natural Science Foundation of Hubei Province (grant number 2007ABB003). We thank Prof. Dr. Tom Hazenberg (Faculty of Forestry and the Forest Environment, Lakehead University, Thunder Bay, ON, Canada) for his English corrections. We acknowledge the offer for the use of the confocal miscroscope by the Platform of Large Instruments and Equipment at Beijing Forestry University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mingquan Ding, Peichen Hou, Xin Shen, Meijuan Wang and Shurong Deng contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ding, M., Hou, P., Shen, X. et al. Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol Biol 73, 251–269 (2010). https://doi.org/10.1007/s11103-010-9612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9612-9