Abstract
The Streptomyces phage phiC31 integrase was tested for its ability to excise transgenic DNA from the wheat genome by site-specific recombination. Plants that stably express phiC31 integrase were crossed to plants carrying a target construct bearing the phiC31 recognition sites, attP and attB. In the progeny, phiC31 recombinase mediates recombination between the att sites of the target locus, which results in excision of the intervening DNA. Recombination events could be identified in 34 independent wheat lines by PCR and Southern blot analysis and by sequencing of the excision footprints. Recombinant loci were inherited to the subsequent generation. The results presented here establish the integrase-att system as a tool for catalysing the precise elimination of DNA sequences from wheat chromosomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Site-specific recombination systems have become important tools for the controlled and precise in vivo manipulation of genomes. Unlike homologous recombination, site-specific recombination acts at short specific sequences, which are the recombination sites. The reactions invariably require specialised proteins that recognise the recombination sites, the site-specific recombinases. By arranging the relative position of the recombination sites within the target DNA, the outcome of the recombination process can be influenced. In a cis-configuration, recombination between directly oriented sites leads to a deletion/excision of the sequence (Ow and Medberry 1995), whereas oppositely orientated recombination sites result in an inversion of the intervening DNA (Kilby et al. 1993). Recombination between sites in trans leads to a reciprocal translocation of two linear DNA molecules, or integration, if at least one of the two molecules is circular (Ow 2002). In summary, implementation of site-specific recombination systems can permit flexible, controllable and specifically adapted molecular engineering of genomes.
Site-specific recombinases are generally classified into two subgroups based upon amino acid homology and their distinct biochemical properties (Grindley et al. 2006). Class I comprises the well-characterised members of the tyrosine recombinase family, which is sometimes referred to as the “λ integrase family”. During formation and resolution of a Holliday junction, the recombinases cleave one strand of each of the two DNA molecules involved and then exchange the strands. During the reaction, the target DNA is transiently attached to the enzyme through a phosphotyrosine link. Two members of the tyrosine family, the Cre-lox from Escherichia coli phage P1 and FLP-FRT from the 2-μm plasmid of Saccharomyces cerevisiae (Ow 2002), have been studied in numerous approaches for the genome engineering of Drosophila (Bischof and Basler 2008) and mammalian cells (Wirth et al. 2007). Furthermore, both systems have been widely implemented in plants (reviewed by Gilbertson 2003; Lyznik et al. 2003; Ow 2007) for transgene integration (Albert et al. 1995; Chawla et al. 2006; Vergunst et al. 1998), marker gene excision (Ow 2007), the induction of “genetic switches” that trigger the activation of a gene either by the excision of a sequence that blocks the reading frame (Hoa et al. 2002; Luo et al. 2000; Tungsuchat et al. 2006) or, alternatively, by the reconstitution of a reading frame through flipping of an inverted sequence (Gleba et al. 2004) and for resolving complex transgene integration patterns in order to create plants with single copy transgene insertions (De Buck et al. 2007; Srivastava et al. 1999; Srivastava and Ow 2001). From an operator’s point of view, it is noteworthy that Cre and FLP catalyse a reaction between two identical recombination sequences (lox, FRT). As a consequence, the recombination sites remain unaltered throughout the process, which results in reversible site-specific recombination reactions (Lyznik et al. 2003). Thus, the applicability of such systems is hampered for certain purposes. In particular, integrated DNA is readily re-excised, since intramolecular reactions are kinetically favoured over intermolecular interactions (Ow 2002). In order to reduce the reversibility of the Cre-mediated reaction, Albert et al. (1995) engineered truncated lox sites that resulted in recombination product sites that are inefficiently recognised by the recombinase. Although the truncated site probably still retains some ability to recombine with a second lox site, the reaction is decreased sufficiently to stabilise the recombination products.
The second class of site-specific recombinases is represented by the serine recombinases, which are sometimes referred to as the “invertase/resolvase family” (Smith and Thorpe 2002). Bacteriophage-encoded serine recombinases, such as the 68 kDa single polypeptide phiC31 integrase, cause excision and integration with strictly controlled directionality (Smith and Thorpe 2002; Thorpe and Smith 1998). The native function of phiC31 is to catalyse the integration of the Streptomyces phage phiC31 into the host chromosome. phiC31 integrase brings two non-identical recombination sites attB and attP (bacterial and phage attachment site) together in a synapse and catalyses a concerted, four-strand staggered break and rejoining mechanism, during which a phosphoserine link is formed (Smith et al. 2004). The integration reaction generates the recombinant junctions, attL and attR, as products. These sequences are not substrates for the phiC31 integrase in the absence of bacterial accessory proteins, which renders the recombination reaction irreversible in non-bacterial systems. As a consequence, the process is predicted to result in stable recombination products.
Phage phiC31 integrase has been demonstrated to function in heterologous cellular environments including Xenopus laevis (Allen and Weeks 2006), Drosophila (Bateman et al. 2006; Venken et al. 2006) and mammalian cells, making it an attractive tool for gene therapy, the construction of transgenic organisms, targeted gene-knockout and the manipulation of cell lines (Andreas et al. 2002; Ginsburg and Calos 2005; Wirth et al. 2007). In plants, however, phiC31 integrase has received relatively little attention and has been applied solely in two model species. In N. tabacum, phiC31-mediated plastid transformation (Lutz et al. 2004) and marker gene excision (Kittiwongwattana et al. 2007) were successfully carried out. In A. thaliana, T-DNA deletions were performed (Gils et al. 2008).
Yet, to our knowledge, there is no phiC31 integrase expression system established for genome manipulation in monocotyledonous plants. Recently, a constitutively expressed phiC31 integrase was demonstrated to catalyse site-specific recombination of extrachromosomal DNA in wheat (Rubtsova et al. 2008). Based upon these results, we now show the capability of stably expressed phiC31 integrase to perform site-specific recombination of chromosomal wheat DNA.
For most applications, site-specific recombination should be a controllable process. Temporal or spatial control of the recombinase action can be achieved by genetic crosses, a second round of transformation (transient or stable) or the transcriptional activation of the recombinase using inducible promoters (Gleba et al. 2004; Ow 2002). In the present study, the recombination substrate is combined with the phiC31 integrase by sexual crossing. In 34 independent lines, DNA sequences between att recombination sites were excised from the wheat genome after being exposed to an integrase encoded by a transgene that resides on a different chromosomal locus. We produced recombinant loci that were transmitted to the subsequent generation.
Materials and methods
Design of the constructs
All plasmids used in this study are pBIN19-based binary vectors (Fig. 1). The construction and structure of vectors pICH14313 and pICH13130, which contain the coding sequence for the Streptomyces phiC31 integrase (Thorpe and Smith 1998), were described previously (Rubtsova et al. 2008). The cloning and design of vector pICH27371 was also previously reported (Kempe et al. 2009). Vector pICH27371 harbours N- and C-terminal fragments of a barnase gene from Bacillus amyloliquifaciens that were fused to intein sequences from the Synechocystis sp. gene DnaB. After translation of the fusion proteins, the inteins confer ligation of the protein fragments, thus forming a cytotoxic barnase. The expression is controlled by the tapetum-specific osg6B promoter from rice (Tsuchiya et al. 1995). The barnase-intein fusions are flanked by target sequences for the phiC31 recombinase (attP 1 , attP 2 : GTGCCCCAACTGGGGTAACCTTTGAGTTCTCTCAGTTGGGGGCGTAG; attB: GAGTAGTGCCCCAACTGGGGTAACCTTTGAGTTCTCTCAGTTGGGGGCG TAG).
Genetic structure of the constructs (T-DNA part, not drawn to scale) a The vector pICH27371 carries the sequences attP and attB, which are targets for the Streptomyces phage phiC31 integrase. The integrase is expressed from the construct pICH13130 or pICH14313. When pICH27371 is used to transform wheat plants the integrase catalyses an irreversible site-specific recombination event between an attP and attB site. All vectors were cloned into pBIN19-based binary vectors between the T-DNA left and right borders (LB and RB). b Crossing scheme employed in the study. Not all T0 plants used in the experiments were male-sterile. Note that the split-barnase sterility system is not part of the study presented here. Abbreviations: Bar-N, Bar-C, N- and C-terminal gene fragments from the Bacillus amyloliquifaciens barnase gene; ALS, mutated version of the rice acetolactate synthase gene; DnaB IntN, DnaB IntC, N- and C-terminal intein sequences from the DnaB gene of Synechocystis sp.; attL and attR, hybrid products that originate from the recombination between attP and attB; attP, attB, Streptomyces phage phiC31 recombination sites (attP 1 and attP 2 are identical); Pact, rice actin 1 promoter (McElroy et al. 1990); Ptap, tapetum-specific promoter osg6B from rice (Tsuchiya et al. 1995); Tocs, octopine synthase terminator (Gielen et al. 1984); phiC31, phage phiC31 recombinase (Thorpe and Smith 1998); Pspm, maize spm promoter (Gierl et al. 1985); Pubi, maize ubiquitin promoter (Christensen and Quail 1996); Tnos, nopaline synthase terminator (Jones et al. 1992); intron, sequence derived from an intron of the Petunia hybrida Psk7 gene (GenBank accession number AJ224165); NLS, SV40 T-antigen nuclear localisation signal; amino acids PKKKRKV (Andreas et al. 2002)
Transgenic wheat plants
Spring wheat (Triticum aestivum L., cultivar ‘‘Bobwhite’’) was used throughout this study. Plants were grown under greenhouse conditions with 16 h of light at 20°C and 8 h of darkness at 16°C.
The transformation of wheat by particle bombardment using the vectors pICH14313 and pICH13130 was recently described by Rubtsova et al. (2008). The transformation of pICH27371 by particle bombardment was carried out as reported by Kempe et al. (2009). In addition, pICH27371 was used to generate a further set of transgenic lines by Agrobacterium-mediated transformation which was performed according to Hensel et al. (2009). Plants obtained using pICH27371 were selected through exposure to the herbicides primisulphuronmethyl (PSM) and imazethapyr (IMA) following a modified protocol of Kempe et al. (2009).
Male-sterile plants (transformed with pICH27371) were pollinated by placing pollen-shedding anthers of either untransformed Bobwhite plants or integrase-expressing plants DH13130/DH14313 into the closed flower. The fertility assays were performed as recently described (Kempe et al. 2009). Fertile plants were mechanically emasculated prior to pollination.
Molecular analysis of transgenic plants
For DNA isolation, leaf tissues were harvested, frozen in liquid nitrogen and stored at −80°C. Homogenisation was carried out using a TissueLyser© from Qiagen (Hilden, Germany). We isolated total plant DNA following a modified protocol from Dellaporta et al. (1983).
Polymerase chain reactions were carried out in a thermocycler (DNA-Engine™ PTC-0200, Bio-Rad, Munich, Germany) with 35 cycles (94°C for 30 s; 55–63°C for 30 s; 72°C for 1–2 min). The sequences of the primers used in this study are listed in Table 1. For detection of the N-terminal barnase gene sequence, we used the primers Bar-N-FW and dnaB-intN-REV. Amplification of the C-terminal barnase fragment by PCR was carried out using Bar-C-REV and dnaB-intC-FW. The PCR reactions were performed as a multiplex reaction containing all four primers.
The molecular analysis of site-specific recombination was performed using primers that amplify the excision footprint sequences containing the hybrid recombination products, attL or attR. Their respective positions are shown in Fig. 2a. As standard primer combinations, we used primer pairs Rec-1 and Rec-2 for the detection of ICH27371-N and Rec-3 and Rec-4 for the detection of ICH27371-C. To check for the presence of an intact barnase gene fragment, Rec-2 or Rec-3 was replaced by the primer Ptap in some analyses. Additional nested primers were used for sequencing reactions (Rec-5; Rec-6). By using primers non-Rec 2 and non-Rec 4, a non-recombined target locus ICH27371 could be detected.
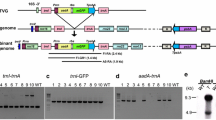
Screen for chromosomal excision events by PCR and Southern blot analyses in T2 and T3 plants a Schematic map of the recombination products ICH27371-N and ICH27371-C, with the positions of the PCR primer binding sites (symbolised by arrows) and the recognition sites for NotI (N) and PmeI (Pme). Note that the PmeI and one of the NotI sites are located outside of the T-DNA region on the vector sequence (symbolised by dashed lines). The illustration describes an “idealised” scenario of a single-copy integration of the vector pICH27371. Blots were hybridised with probes homologous to the ALS gene (Probe ALS) or to the C-terminal part of the barnase gene (Probe Bar-C). Regions of homology are symbolised by rectangles. b PCR reactions conducted on DNA from wildtype (wt), T1-control plants without the phiC31 integrase encoding locus (lanes T 1 ) and T2 plants derived from crosses with lines DH14313 (lanes marked 14) or DH13130, respectively (lanes marked 13). (I) line 27371-987 with primers Rec-1/ Rec-2; (II) line 27371-1199 with primers Rec-3/ Rec-4; (III) line 27371-1104 with primers Rec-4/ Ptap; (IV) line 27371-1051 with primers Rec-1/ Ptap; (V) line 27371-987 with primers non-Rec 2/ non-Rec 4. PCR was conducted on control plants without integrase (T1, wt) and two T2 plants carrying both types of recombination events (13a, 13b). c For Southern blot analysis, total DNA from T1, T2 and T3 plants from line 27371-961 (VI, VIII), from T1 and T2 plants from line 27371-987 (VII) and from wildtype plants (wt) was digested with NotI and hybridised with the probe ALS in order to detect ICH27371-N. For detection of ICH27371-C, the total DNA of T1 and T2 plants from line 27371-987 was digested with NotI and PmeI (IX). The membrane was hybridised with the probe Bar-C. The analysis of the corresponding recombination products by PCR is indicated below the autoradiograms
The presence of ICH13130 was confirmed with primers C31-5′-REV and Ubi1-FW. The presence of ICH14313 was monitored with the primers C31-5′-REV and spmProm-FW.
Southern blot analyses were performed according to Southern (1992). For characterisation of pICH27371 integration, total DNA from wheat (10 μg) was digested with PstI and hybridised with the probe Bar-N (produced with primers Bar-N-FW and dnaB-intein-N-REV, using plasmid pICH27371 as a template) or Bar-C (produced with primers Bar-C-REV and dnaB-intein-C-FW, using plasmid pICH27371 as a template). The restriction digest results in fragments harbouring the homologous vector sequence and a genomic DNA content of unpredictable size. Therefore, the number of integrated plasmid copies can be estimated. For the detection of site-specific recombination products ICH27371-N or ICH27371-C, total DNA from wheat was digested with NotI or with NotI and PmeI (Fig. 2a, c). In the case of C-terminal excision at the target locus, digestion with NotI releases a 2.3 kb fragment that is detected by the probe ALS and contains the hybrid attL sequence. In the presence of unaltered ICH27371, the expected fragment size is 5.5 kb. For N-terminal excision at the target locus, digestion with NotI and PmeI results in a 3.0 kb fragment that is detected by the probe Bar-C and contains the hybrid attR sequence. The presence of ICH27371 would lead to a 4.4 kb-fragment. The primers used for producing the probes are given in Table 1. DNA fragments were separated using 0.8% agarose gels and transferred onto a nylon membrane (Biodyne B; Pall, USA). After blotting, the membranes were hybridised with [32P]-labelled DNA fragments.
For detecting the integrase sequence from pICH13130, total DNA was digested with HindIII, which releases a 969 bp fragment including the integrase sequence that is covered by the probe INT.
For sequence analysis, PCR products were either subjected to direct DNA sequencing or subcloned into pGEM-T vector (Promega, Madison, WI, USA) with subsequent DNA sequencing. DNA sequencing was conducted by the PGRC Sequencing Service of the Leibniz Institute of Plant Genetics and Crop Plant Research (Gatersleben, Germany) or by GATC (Konstanz, Germany).
Results
Design of constructs
For the induction of site-specific recombination, the two components, phiC31 integrase and target-recombination sequences, were combined in one plant via sexual crossing.
Two different constructs were used as the source of phiC31 integrase (site-specific recombinase, Fig. 1a). Integrase expression was controlled either by the maize ubiquitin promoter (pICH13130) or the maize spm promoter (pICH14313). In the case of pICH13130, an intron was also inserted into the integrase coding region. Two independent doubled haploid (DH) plant lines carrying pICH13130 (hereafter designated as DH13130-5 and DH13130-10) and one DH line carrying pICH14313 (denoted as DH14313-1) were deployed for sexual crosses in this study. The production of the integrase DH lines was previously described. According to the results of Rubtsova et al. (2008), lines DH13130-5 and DH13130-10 express integrase more strongly than DH14313-1.
The construct pICH27371 contains attB and attP recombination sites that serve as targets for the phiC31 integrase. The construct also harbours two fragments of a barnase gene, which encodes a cytotoxic extracellular ribonuclease from B. amyloliquifaciens (Mariani et al. 1990). Both gene fragments are expressed under the control of the tapetum-specific promoter osg6B from rice (Tsuchiya et al. 1995), ensuring that the cytotoxic gene product is exclusively expressed in tissue that is essential for pollen development. Complementation of the fragments in the tapetum is facilitated through intein-mediated protein trans-splicing. Consequently, wheat plants carrying a functional pICH27371 vector are male-sterile (Kempe et al. 2009).
By combining the two constructs in one cell, the integrase may catalyse site-specific recombination reactions between an attP and attB site. Two alternative site-specific recombination reactions can lead either to deletion of the C- or N-terminal part of the locus ICH27371, resulting in the formation of the derivative loci ICH27371-N or ICH27371-C (Fig. 2a). In the first case, a hybrid attL sequence is produced via a site-specific recombination reaction between attB and attP 2 , whereas, in the latter case, recombination between attP 1 and attB results in a hybrid attR sequence, including the removal of the selection marker gene ALS. attL and attR are not substrates for the phiC31 integrase in plants. Therefore, the described phiC31 integrase-mediated recombination reactions are irreversible, and the two reactions are mutually exclusive.
For analysing chromosomal site-specific recombination events, we used target lines harbouring pICH27371, regardless if the plants were sterile or fertile.
Production of hybrid plants
The experiments were carried out as described in Fig. 1b. 31 independent plants that have been transformed with the target vector pICH27371 by biolistic bombardment were backcrossed with wildtype plants. The resulting T1 progeny was analysed for the presence of the target locus through PCR (data not shown). Southern blot analyses revealed that all T1 plants carried the transgenic DNA in multiple copies (examples of Southern blot analyses are given in Supplemental Fig. 1). The T1 plants were used as pollen acceptors for crosses with the integrase lines DH13130 (including DH13130-5, DH13130-10) and DH14313. In the hybrid T2, the presence of the integrase coding locus and the target locus was confirmed by PCR.
phiC31 integrase expressed from ICH13130 catalyses chromosomal excision events in 31 independent hybrid wheat lines
Hybrid T2 plants were screened for site-specific excision events at the target locus ICH27371 by PCR (strategy depicted in Fig. 2a). The design of the primers allows for the production of PCR fragments of the predicted size only in the presence of the derivative loci ICH27371-N or ICH27371-C.
We were able to identify site-specific recombination events in T2 progeny plants of all 31 independent target lines that have been crossed to DH13130 (Table 2). The results of PCR experiments are exemplified in Fig. 2b. Plants were examined by PCR at an age of 2–4 weeks and at a later developmental stage by analysing the youngest leaf of three different ears of 3-month-old plants.
We obtained PCR fragments indicating recombination in 96% of hybrid T2 plants (249 of 259). Differences in recombination frequency within the progeny of DH13130-5 or pICH13130-10, respectively, could not be detected. From these findings, we conclude that phiC31 expression from locus ICH13130 is sufficient to foster the excision of either the N- or C-terminal part of a target locus ICH27371 that is present in the same cell on a second chromosome.
In 61 % (153 of 249) of plants displaying recombination events, non-recombined loci (ICH27371) were found (Table 2).
Unlike in the case of ICH13130, the presence of ICH14313 in hybrid T2 plants did not result in site-specific recombination events. We could not detect site-specific recombination in 263 hybrid T2 plants (that were derived from 23 independent T0 plants; including nine target lines that produced recombined loci in hybrid T2 plants carrying ICH13130).
The results of the PCR experiments were confirmed by Southern blot analyses (Fig. 2c). A 2.3-kb NotI-DNA-fragment detected by the probe ALS was released as a result of the excision of the C-terminal vector part (VI, VII, VIII). In the case of excision of the N-terminal vector part, a 3-kb PmeI/ NotI fragment was released that could be detected by the probe Bar-C (IX). The larger bands in the same lanes are caused presumably by additional not-recombined loci or loci that have undergone complex rearrangements. As a control, we used T1 plants that did not carry an integrase coding locus (T1). In those plants, fragments indicating site-specific recombination events were not identified.
Excision footprint sequencing analysis
Amplification products were examined by DNA sequencing. Plants from all 31 independent lines displaying recombination were included in the analysis. At least one PCR fragment covering the recombination junction sites attL and/or attR was sequenced per independent line (for position of primer binding site see Fig. 2a and Supplemental Fig. 2). Overall, 56 fragments derived from PCR using the primers Rec-1 and Rec-2 and 41 fragments derived from PCR with primers Rec-3 and Rec-4 were sequenced either directly or as a subcloned DNA fragment. In order to gain more information about the integrity of the derivative loci, two subcloned 5 kb DNA-fragments that were produced with the primers Ptap and Rec-1 and three subcloned 1.4 kb DNA-fragments that were produced with the primers Ptap and Rec-4 were also sequenced. The footprint of the excision detected within the T2 plants always corresponded to a perfect recombination event. They contained either a sequence of the hybrid attL or the hybrid attR recombination product, which joins the newly linked vector parts (sequence given in Supplemental Fig. 2). From these results, we conclude that: (1) the PCR experiments can reliably monitor phiC31 integrase-catalysed site-specific recombination at the locus ICH27371 and ii) the site-specific recombination that is catalysed by the phiC31 integrase is a precise process that leads to a predictable sequence in wheat.
Recombination between attB and attP 2 occurs more frequently than between attP 1 and attB
In 70% of the T2 plants (180 of 259), we found both N- and C-terminal excision events (Table 2a, b). In the case of plants showing only one type of recombinant locus, we registered a bias toward C-terminal recombination. Twenty-two plants (8%) exclusively showed excision of the N-terminal vector part, whereas 47 plants (18%) exclusively displayed excision of the C-terminal part.
The target locus ICH27371 is stable in the absence of the integrase expressing locus
Plants without integrase coding locus were screened for rearrangements of the target locus ICH27371. All 31 T0- and 64 T1-ancestors of the recombinant T2 plants were screened via PCR for the presence of a recombined locus ICH27371-N or ICH27371-C, respectively. In these plants, recombination events were not found. As the sole exception, the primary transformants and T1 progeny of line 27371-1072 displayed a deletion of the C-terminal vector, as was indicated by a PCR fragment of ~ 700 bp using the primers Rec-1 and Rec-2. However, sequence analysis of the locus revealed an aberrant locus structure (Supplemental Fig. 3).
At total of 332 T2 plants that were derived from backcrossing 14 independent T1 lines displayed no recombination according to the PCR analysis. Additionally, several hybrid lines harbouring both ICH14313 and ICH27371 were screened over three generations without showing any hint of DNA excision in the subsequent generations (data not shown). In summary, the results suggest that the target locus ICH27371 is stable during the absence of the phiC31 recombinase and that spontaneous (recombinase-independent) recombination does not occur at a relevant frequency.
Germinal transmission of the recombined loci
In order to analyse whether the recombination events were transmitted to the next generation, we examined the descendants derived from 51 T2 plants (representing 11 independently transformed lines) for the presence of recombined target DNA via PCR and Southern blot analysis (Fig. 2c). Without exception, all T2 plants transmitted ICH27371-N and/or ICH27371-C to the subsequent generation (Table 3). In most of the T3 progeny (42 of 51), a recombined locus was present in at least 50% of the plants. Altogether, 323 of the 502 investigated T3 plants contained a recombinant locus. Its loss, however, was expected for a proportion of the T3 plants as a result of segregation, since the T2 generation is hemizygous for the target loci. A total of 95 T3 plants lost the integrase through segregation but still carried a recombinant locus. For these cases, it is clear that the phiC31-mediated site-specific recombination events did not emerge during the development of the T3 plants, but rather within the parental T2 plants, and that the recombination products were inherited through the germline. The presence of ICH13130 was examined using PCR analyses (data not shown) and Southern blots (Fig. 3).
Presence of ICH13130 in T3 plants with recombination events. Total wheat DNA from lines 987, 1000, 961 and wildtype (Wt) plants was digested with HindIII (H) and analysed with a probe homologous to the phiC31 integrase sequence (Probe-INT, the region of homology is shown by a rectangle in the schematic illustration). As been shown by PCR, T3 plants displayed N- and/or C-terminal recombination events (indicated below the autoradiogram)
From these results, we conclude that phiC31 can be used to generate integrase-free plants harbouring only the recombined target DNA.
Chromosomal excision events in single-locus plants
Fifty-nine primary transgenic (T0) wheat plants were produced by means of Agrobacterium carrying pICH27371. The plants were analysed for the locus copy number by Southern blot analysis according to the strategy described in Fig. 4, results are given in Supplemental Fig. 1. The majority (53) of the primary transformed plants contained multiple copies of the target locus ICH27371. Six plants carried a single integration of the target locus. After selfing, three of these lines were crossed to wheat plants carrying ICH13130 (Fig. 1b). Table 4 depicts the results of the PCR-based recombination assay. In all three single-locus lines, N- and C-terminal excision events were found in the majority of T2 plants.
Analysis of recombination events in line 1726. Total DNA was digested with PstI (P). The membrane was hybridised with probes homologous to the N-terminal and the C-terminal part of the barnase gene (rectangles in illustration). The results of PCR analyses for monitoring recombination events are given below the autoradiograms. Plants that lost the target-vector by segregation were used as controls (with line numbers given in parentheses)
In case of line 1726, we identified T2 progeny plants derived from three T1 plants (1726-10, -11 and -14), that showed a site-specific recombination of either the N- or the C-terminal fragment of the target locus ICH27371 (examples given in Fig. 4). The particular recombination events correlated with the presence of the expected recombined locus (as been shown by PCR analysis and sequencing of the PCR products). C-terminal excision events apparently resulted in a complete removal of the target-vector part. On the contrary, N-terminal excision events led to a smaller fragment with homology to the probe Bar-N. Still, the “parental” N-terminal fragment found in primary transformants or T1 plants (without integrase construct) was absent in all T2 plants displaying N-terminal recombination.
According to our PCR results, the non-recombined locus ICH27371 was not present in T2 plants that contained an integrase construct, whereas the T0 and T1 control-plants (without integrase construct) displayed the unaltered targeting sequence.
Discussion
For modern plant biotechnology, the design of complex genomic engineering strategies for crop improvement or gene expression studies plays an increasingly important role (Hare and Chua 2002; Ow 2002, 2007). Therefore, multiple molecular tools that promote precise excision and integration of target sequences are required. Due to the lack of efficient homologous recombination systems in plant chromosomal DNA, the development of prokaryotic site-specific recombination systems is an important goal. In the field of agricultural biotechnology, this applies particularly to monocots for several reasons. First, most of the economically important crops belong to this class. Second, unlike for dicotyledonous plants, only modest progress has been made in the development of site-specific recombination systems in monocots.
In this study, we succeeded in using the Streptomyces phage phiC31 integrase for the removal of transgenic DNA from the wheat genome by site-specific recombination in 34 independent wheat lines. To our knowledge, this represents the first application of a serine (invertase/resolvase) recombinase for genome manipulation in a monocotyledonous plant species. Until now, serine recombinases that have been used in plants include the Gin recombinase of phage Mu in tomato (Maeser and Kahmann 1991), the Streptomyces phage phiC31 integrase in N. tabacum (Lutz et al. 2004; Kittiwongwattana et al. 2007) and A. thaliana (Gils et al. 2008) and the β-six recombinase from Streptococcus pyogenes in A. thaliana and N. tabacum (Gronlund et al. 2007).
We have chosen the Streptomyces phiC31 integrase system since it lacks a readily reversible reaction in non-bacterial systems. This might give it a distinct advantage for employing in plant genome engineering, since recombined molecules are predicted to be more stable than those created by the well established reversible site-specific recombination systems like Cre-lox or FRT-FLP. As an example, pICH27371 may be used for the establishment of a hybrid wheat production system, similar to the one recently described for A. thaliana (Gils et al. 2008). The system includes the production of two transgenic loci that express complementary fragments of a protein and are located on isoallelic chromosomal loci in order to facilitate a “repulsion linkage”. This is achieved by a derivatisation of a primary vector using site-specific recombination, and the subsequent combination of the two recombination products in one heterozygote progeny plant that expresses the trait. The use of phiC31 integrase, together with the arrangement of target recombination sites (attP 1 -attB-attP 2 ) would allow the fixation of the recombination event by a “downstream-inactivation” of the target locus after the recombination reaction (attP 1 -attL for ICH27371-N; attR-attP 2 for ICH27371-C; with attL and attR not being substrates for the integrase). In contrast, application of a reversible system with identical recombination sites, such as wildtype Cre-lox, would ultimately lead to the undesirable loss of the complete target locus in many cases.
For the creation of derivative loci ICH27371-N or, respectively ICH27371-C, a possible limitation is the observed bias towards the recombination of the C-terminal locus part of ICH27371 in most of the lines. This is presumably attributed to the proximity of attB to attP 2 (3 kb) compared to that of attP 1 to attB (6.6 kb), which kinetically favours an excision of the C-terminal vector part. This observation is in accordance to studies of Coppoolse et al. (2005). The authors observed that Cre-mediated deletion in somatic tomato cells is less efficient when the lox sites are separated by larger distances. Interestingly, when a construct with equispaced pairs of att sites was used as a target vector for site-specific recombination in A. thaliana, a bias towards N- or C-terminal excision was not found (Gils et al. 2008). For future applications, it may be advantageous to adapt constructs of a similar structure. Alternatively, N- and C-terminal sequences might be flanked by recognition sites of different site-specific recombination systems (Shamay 2005). N- and C-terminal excisions could be selectively induced by crossing sister plants to different recombinase sources, but these systems would be more complex.
In the past, several strategies for “trapping” recombination products by reducing subsequent recombination events have been suggested. They include temporally limited expression or activity of the recombinase or the inactivation of the recombinase as a result of integration (reviewed by Lyznik et al. 2003). As a further important contribution, non-reversible, site-specific recombination systems were created by mutating lox sites into heterospecific versions that can resist the reverse reaction (Albert et al. 1995). Although this has been successfully used for the integration of transgenes in tobacco (Albert et al. 1995), rice (Srivastava et al. 2004) and mouse embryonic stem cells (Araki et al. 1997), an intrinsic challenge of this approach is to modify the lox sites in order to minimise the reverse reaction without limiting the forward reaction too much (Gilbertson 2003).
In summary, all the above strategies have necessitated modifications to the systems to reduce or obviate their reversibility. We believe that naturally occurring unidirectional recombination systems like the phiC31-att can provide a less complex solution with a reduced risk of failure.
In this study, three derivates/versions of transgenic loci are possible in the T2 or T3 plants. First, the recombined loci ICH27371-N or ICH27371-C can be produced. Second, complex DNA integrations may be resolved into unpredictable products by multiple consecutive recombination events that are catalysed by phiC31 integrase, similarly to what has been recently described for Arabidopsis (Gils et al. 2008). In contrast, these integration events may be completely removed if they are flanked by interacting att sequences. Third, as was indicated by PCR and Southern blot analyses, one or more target loci remain unaltered, while others become recombined. We also assume that, in some cases, the ballistic delivery of pICH27371 led to proloci that are rearranged a priori. This can be concluded from the fact that some T2 plants showing no recombination contain no intact locus ICH27371 although the presence of Bar-N and Bar-C was shown (Table 2). Furthermore, in the continued presence of phiC31 integrase, target loci might recombine in different generations, thus leading to genetic chimeras. For plants, condensed chromosomal DNA has been postulated to have a reduced accessibility for enzymes that are involved in recombination processes (Mengiste et al. 1999; Puchta 2003), similarly to what has been described for the Cre-lox system in cultured mammalian cells (Baubonis and Sauer 1993). Hence, variations in the efficiency of site-specific recombination between different targeted loci might be due to their different chromosomal positions.
In this work, phiC31-mediated recombination events were transmitted from all 51 analysed hybrid T2 plants to their progeny, with 42 of these T2 lines (82%) transmitting recombinant loci to at least 50% of the progeny plants. More importantly, 29% of the T3 plants carrying recombinant loci have lost the integrase as a result of segregation, showing that the recombination events must have taken place in the T2 generation before they were transmitted to the T3 plants. These results accomplish the concept of a “genetic switch” that is initiated by crossing in a recombinase and completed when the recombinase is removed again by segregation. An important prerequisite for such a method is the stability of the target locus in the absence of the integrase, which has been shown in our analyses. We assume that the “illicit” loss of the C-terminal vector fragment in the line 27371-1072 was caused by DNA rearrangements during the particle bombardment procedure.
Based on the frequency of inherited recombination events, we speculate that phiC31-mediated excision of transgenes from wheat chromosomes is possible at a rather early state of wheat development. This supposition is supported by the fact that recombinant loci could be identified in young plants. Also, as has been indicated by transient assays, the integrase seems to be particularly active in younger tissue (Rubtsova et al. 2008). The results obtained with line 1726 strengthen this hypothesis. By PCR or Southern blot analysis, we did not find any indication for a chimaeric tissue in the hybrid T2 plants which may consist of sectors containing recombined or non-recombined target sequences. We speculate that, in this line, the “decision” for one of the two (mutually exclusive) recombination events took place at a very early stage of development. Confirmatively, all T2 plants display either recombination of the N- or the C-terminal part, but never both. Still, the time of recombination during plant development might vary between different lines. Furthermore, in case of plants carrying the target locus in multiple copies, additional late events, which cannot be detected separately by PCR, cannot be excluded.
In earlier publications, phenotypic aberrations were correlated to the constitutive expression of Cre recombinase in a species belonging to the Solanaceae family (Coppoolse et al. 2003; Mlynarova and Nap 2003; Que et al. 1998). Similarly, due to phenotypical lesions, the constitutive expression of Gin recombinase was not possible in plants (Maeser and Kahmann 1991). The presence of “pseudo-loxP” sites in mammalian and insect genomes is suspected to cause illegitimate chromosome rearrangements, growth inhibition and phenotypic abnormalities in the presence of Cre (Heidmann and Lehner 2001; Loonstra et al. 2001). With the wheat plants expressing the phiC31 integrase under the control of either the maize ubiquitin promoter (DH13130) or the maize spm promoter (DH14313), we have not observed any detrimental effects on plant development over three generations of growth. Thus, these results strengthen those of Rubtsova et al. (2008) at a larger scale and are in accordance with data reported for the expression of Cre in wheat (Srivastava et al. 1999).
In summary, the constitutive expression of phiC31 integrase in the hemizygous descendants of DH13130-5 and DH13130-10 plants seems to be suitable for the induction of chromosomal site-specific recombination events that are faithfully transmitted to subsequent generations without causing apparent damage to the wheat plants.
In the past, there has been significant interest for site-specific recombinases due to their potential for the targeted integration of transgenes. For such applications, irreversible site-specific recombinases are particularly favourable. They may permit the creation of important tools for complex gene stacking strategies that are not achievable using only freely reversible systems (Ow 2007). Thus, in the future, it will be important to study if phiC31 integrase can also be used for the efficient integration of foreign DNA into the wheat genome.
The availability of various site-specific recombination systems in plants would be beneficial for the design of more complex genomic engineering strategies for crop improvement (Ow 2002). We believe that the results described in this article validate the use of the phiC31 integrase-att system for genome manipulation of the economically important plant species, wheat.
Abbreviations
- ALS:
-
Acetolactate synthase
- attB :
-
Bacterial attachment site, phiC31 target recombination sequence
- attP :
-
Phage attachment site, phiC31 target recombination sequence
- attR, attL:
-
phiC31 integrase recombination products
- DH:
-
Doubled haploid
- pICH14313, pICH13130:
-
Vectors containing a Streptomyces phage phiC31 integrase coding sequence
- ICH14313, ICH13130:
-
Genomic locus containing the Streptomyces phage phiC31 integrase coding sequence
- pICH27371:
-
Vector containing phiC31 integrase target recombination sequences
- ICH27371:
-
Genomic target locus carrying phiC31 integrase target recombination sequences
- ICH27371-N, ICH27371-C:
-
Derivative genomic locus resulting from phiC31 integrase-mediated site-specific recombination
References
Albert H, Dale EC, Lee E, Ow DW (1995) Site-specific integration of DNA into wild-type and mutant lox sites placed in the plant genome. Plant J 7:649–659
Allen BG, Weeks DL (2006) Using phiC31 integrase to make transgenic Xenopus laevis embryos. Nat Protoc 1:1248–1257
Andreas S, Schwenk F, Kuter-Luks B, Faust N, Kuhn R (2002) Enhanced efficiency through nuclear localization signal fusion on phage PhiC31-integrase: activity comparison with Cre and FLPe recombinase in mammalian cells. Nucleic Acids Res 30:2299–2306
Araki K, Araki M, Yamamura K (1997) Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res 25:868–872
Bateman JR, Lee AM, Wu CT (2006) Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics 173:769–777
Baubonis W, Sauer B (1993) Genomic targeting with purified Cre recombinase. Nucleic Acids Res 21:2025–2029
Bischof J, Basler K (2008) Recombinases and their use in gene activation, gene inactivation, and transgenesis. Methods Mol Biol 420:175–195
Chawla R, Ariza-Nieto M, Wilson AJ, Moore SK, Srivastava V (2006) Transgene expression produced by biolistic-mediated, site-specific gene integration is consistently inherited by the subsequent generations. Plant Biotechnol J 4:209–218
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Coppoolse ER, de Vroomen MJ, Roelofs D, Smit J, van Gennip F, Hersmus BJ, Nijkamp HJ, van Haaren MJ (2003) Cre recombinase expression can result in phenotypic aberrations in plants. Plant Mol Biol 51:263–279
Coppoolse ER, de Vroomen MJ, van Gennip F, Hersmus BJ, van Haaren MJ (2005) Size does matter: cre-mediated somatic deletion efficiency depends on the distance between the target lox-sites. Plant Mol Biol 58:687–698
De Buck S, Peck I, De Wilde C, Marjanac G, Nolf J, De Paepe A, Depicker A (2007) Generation of single-copy T-DNA transformants in Arabidopsis by the CRE/loxP recombination-mediated resolution system. Plant Physiol 145:1171–1182
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II Plant Mol Biol. Pep 1:19–29
Gielen J, De Beuckeleer M, Seurinck J, Deboeck F, De Greve H, Lemmers M, Van Montagu M, Schell J (1984) The complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J 3:835–846
Gierl A, Schwarz-Sommer Z, Saedler H (1985) Molecular interactions between the components of the En-I transposable element system of Zea mays. EMBO J 4:579–583
Gilbertson L (2003) Cre-lox recombination: cre-ative tools for plant biotechnology. Trends Biotechnol 21:550–555
Gils M, Marillonnet S, Werner S, Grutzner R, Giritch A, Engler C, Schachschneider R, Klimyuk V, Gleba Y (2008) A novel hybrid seed system for plants. Plant Biotechnol J 6:226–235
Ginsburg DS, Calos MP (2005) Site-specific integration with phiC31 integrase for prolonged expression of therapeutic genes. Adv Genet 54:179–187
Gleba Y, Marillonnet S, Klimyuk V (2004) Design of safe and biologically contained transgenic plants: tools and technologies for controlled transgene flow and expression. Biotechnol Genet Eng Rev 21:325–367
Grindley ND, Whiteson KL, Rice PA (2006) Mechanisms of site-specific recombination. Annu Rev Biochem 75:567–605
Gronlund JT, Stemmer C, Lichota J, Merkle T, Grasser KD (2007) Functionality of the beta/six site-specific recombination system in tobacco and Arabidopsis: a novel tool for genetic engineering of plant genomes. Plant Mol Biol 63:545–556
Hare PD, Chua NH (2002) Excision of selectable marker genes from transgenic plants. Nat Biotechnol 20:575–580
Heidmann D, Lehner CF (2001) Reduction of Cre recombinase toxicity in proliferating Drosophila cells by estrogen-dependent activity regulation. Dev Genes Evol 211:458–465
Hensel G, Kastner C, Oleszczuk S, Riechen J, Kumlehn J (2009) Agrobacterium-mediated gene transfer to cereal crop plants: current protocols for barley, wheat, triticale, and maize. Int J Plant Genomics 2009:1–9
Hoa TT, Bong BB, Huq E, Hodges TK (2002) Cre/ lox site-specific recombination controls the excision of a transgene from the rice genome. Theor Appl Genet 104:518–525
Jones JD, Shlumukov L, Carland F, English J, Scofield SR, Bishop GJ, Harrison K (1992) Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res 1:285–297
Kempe K, Rubtsova M, Gils M (2009) Intein-mediated protein assembly in transgenic wheat: production of active barnase and acetolactate synthase from split genes. Plant Biotechnol J 7:283–297
Kilby NJ, Snaith MR, Murray JA (1993) Site-specific recombinases: tools for genome engineering. Trends Genet 9:413–421
Kittiwongwattana C, Lutz K, Clark M, Maliga P (2007) Plastid marker gene excision by the phiC31 phage site-specific recombinase. Plant Mol Biol 64:137–143
Loonstra A, Vooijs M, Beverloo HB, Al Allak B, van Drunen E, Kanaar R, Berns A, Jonkers J (2001) Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci USA 98:9209–9214
Luo H, Lyznik LA, Gidoni D, Hodges TK (2000) FLP-mediated recombination for use in hybrid plant production. Plant J 23:423–430
Lutz KA, Corneille S, Azhagiri AK, Svab Z, Maliga P (2004) A novel approach to plastid transformation utilizes the phiC31 phage integrase. Plant J 37:906–913
Lyznik LA, Gordon-Kamm WJ, Tao Y (2003) Site-specific recombination for genetic engineering in plants. Plant Cell Rep 21:925–932
Maeser S, Kahmann R (1991) The Gin recombinase of phage Mu can catalyse site-specific recombination in plant protoplasts. Mol Gen Genet 230:170–176
Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature 374:737–741
McElroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
Ow DW, Medberry SL (1995) Genome manipulation through site-specific recombination. CRC Crit Rev Plant Sci 14:239–261
Mengiste T, Revenkova E, Bechtold N, Paszkowski J (1999) An SMC-like protein is required for efficient homologous recombination in Arabidopsis. EMBO J 18:4505–4512
Mlynarova L, Nap JP (2003) A self-excising Cre recombinase allows efficient recombination of multiple ectopic heterospecific lox sites in transgenic tobacco. Transgenic Res 12:45–57
Ow DW (2002) Recombinase-directed plant transformation for the post-genomic era. Plant Mol Biol 48:183–200
Ow DW (2007) GM maize from site-specific recombination technology, what next? Curr Opin Biotechnol 18:115–120
Puchta H (2003) Towards the ideal GMP: homologous recombination and marker gene excision. J Plant Physiol 160:743–754
Que Q, Wang HR, Jorgensen A (1998) Distinct patterns of pigment suppression are produced by allelic sense and antisense chalcone synthase transgenes in petunia flowers. Plant J 13:401–409
Rubtsova M, Kempe K, Gils A, Ismagul A, Weyen J, Gils M (2008) Expression of active Streptomyces phage phiC31 integrase in transgenic wheat plants. Plant Cell Rep 27:1821–1831
Shamay I (2005) Method of producing a male sterile plant by exogenic allelism. US Patent 6852911, US2005066388, WO0116287, EP1209967 FERTISEEDS LTD [IR]
Smith MC, Thorpe HM (2002) Diversity in the serine recombinases. Mol Microbiol 44:299–307
Smith MC, Till R, Brady K, Soultanas P, Thorpe H (2004) Synapsis and DNA cleavage in phiC31 integrase-mediated site-specific recombination. Nucleic Acids Res 32:2607–2617
Southern EM (1992) Detection of specific sequences among DNA fragments separated by gel electrophoresis. 1975. Biotechnology 24:122–139
Srivastava V, Ow DW (2001) Single-copy primary transformants of maize obtained through the co-introduction of a recombinase-expressing construct. Plant Mol Biol 46:561–566
Srivastava V, Anderson OD, Ow DW (1999) Single-copy transgenic wheat generated through the resolution of complex integration patterns. Proc Natl Acad Sci U S A 96:11117–11121
Srivastava V, Ariza-Nieto M, Wilson AJ (2004) Cre-mediated site-specific gene integration for consistent transgene expression in rice. Plant Biotechnol J 2:169–179
Thorpe HM, Smith MC (1998) In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci U S A 95:5505–5510
Tsuchiya T, Toriyama K, Yoshikawa M, Ejiri S, Hinata K (1995) Tapetum-specific expression of the gene for an endo-beta-1, 3-glucanase causes male sterility in transgenic tobacco. Plant Cell Physiol 36:487–494
Tungsuchat T, Kuroda H, Narangajavana J, Maliga P (2006) Gene activation in plastids by the CRE site-specific recombinase. Plant Mol Biol 61:711–718
Venken KJ, He Y, Hoskins RA, Bellen HJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314:1747–1751
Vergunst AC, Jansen LE, Hooykaas PJ (1998) Site-specific integration of Agrobacterium T-DNA in Arabidopsis thaliana mediated by Cre recombinase. Nucleic Acids Res 26:2729–2734
Wirth D, Gama-Norton L, Riemer P, Sandhu U, Schucht R, Hauser H (2007) Road to precision: recombinase-based targeting technologies for genome engineering. Curr Opin Biotechnol 18:411–419
Acknowledgments
The authors are grateful to Dr. Heike Schmuths (Saaten Union Biotec Gatersleben, Germany) for logistic cooperation and Kerstin Denzin, Linda Tillack, Christin Meinhardt and Erika Grützemann for laboratory support and plant care. Furthermore, we acknowledge Wolf v. Rhade, Dr. Ralf Schachschneider (Nordsaat GmbH Böhnshausen, Germany), Dr. Jens Weyen (Saaten Union Biotec, Leopoldshöhe, Germany) and Dr. Frank Wolter (PflanzenInnovationsAgentur; PIA) for constant support. We thank anonymous reviewers for helpful comments. In particular, the authors also wish to thank Dr. Renate Schmidt for her comments on the manuscript and for many stimulating discussions. The research was done at the Leibniz Institut für Pflanzengenetik und Kulturpflanzenforschung (IPK) Gatersleben with funding from the Bundesministerium für Bildung und Forschung (BMBF, GABI-FUTURE grant 0315043A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Katja Kempe and Myroslava Rubtsova contributed equally to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kempe, K., Rubtsova, M., Berger, C. et al. Transgene excision from wheat chromosomes by phage phiC31 integrase. Plant Mol Biol 72, 673–687 (2010). https://doi.org/10.1007/s11103-010-9606-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9606-7