Abstract
Background Data on the effectiveness of oral ibuprofen treatment for patent ductus arteriosus are limited, and the factors affecting its effectiveness remain unclear. Objective The aim was to identify the potential factors affecting the clinical effectiveness of oral ibuprofen in preterm infants. Setting Neonatal intensive care unit in a prefecture-level maternal and child healthcare hospital in China. Method Over a 5-years period, the medical records of 327 preterm infants with patent ductus arteriosus who were admitted to the neonatal intensive care unit of our hospital to receive a single course of oral ibuprofen were retrospectively reviewed. Main outcome measures The prevalence of risk factors affecting the effectiveness of oral ibuprofen. Results In total, 201 (61.47%) preterm infants were considered to have undergone “effective therapy” and classified accordingly, whereas 11 (3.36%) showed certain adverse events. Factors affecting therapeutic effectiveness were postnatal age at the initiation of treatment and Day 1/Day 0 ratio of urine output/fluid intake during the treatment course, with odds ratios of 0.892 (95% CI: 0.835–0.953; P = 0.001) and 0.473 (95% CI 0.265–0.845; P = 0.011), respectively. Conclusion A single course of oral ibuprofen for patent ductus arteriosus closure among preterm infants is effective and safe. Preterm infants with postnatal age of ≤ 9 days at the initiation of treatment and Day 1/Day 0 ratio of ≤ 0.708 of the urine output/fluid intake during the treatment course can be considered predictors of effectiveness of patent ductus arteriosus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on practice
-
These study findings provide another perspective on the possible impact of gestational age and birth weight on the effectiveness of ibuprofen on patent ductus arteriosus. Postnatal age at the initiation of oral ibuprofen treatment seems to be another crucial factor. This data could assist pharmacists and physicians in optimizing strategies for the clinical treatment of preterm infants with patent ductus.
-
The study proves that oral ibuprofen is effective in the treatment of patent ductus arteriosus in preterm infants. However, treatment with this drug was not effective for all preterm infants with patent ductus arteriosus.
-
For instance, the Day 1/Day 0 ratio of > 0.708 of urine output/fluid intake during the treatment course may face treatment failure. Thus, these parameters are crucial for the monitored use of oral ibuprofen in clinical practice.
Introduction
Patent ductus arteriosus (PDA) is one of the most common complications among preterm infants which is associated with increased mortality and morbidities such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and intraventricular hemorrhage (IVH) [1]. Many studies have shown that treatment with cyclooxygenase inhibitors (such as indomethacin and ibuprofen) [2, 3] and surgical closure [4] are effective in closing ductus arteriosus in preterm infants. However, the debate regarding selection of the appropriate treatment method is ongoing, and there is lack of definite evidence supporting the benefit of these PDA treatments in contrast to watchful waiting with supportive care [5,6,7,8]. Emerging concerns of morbidity associated with surgical ligation were identified, such as increased risk of chronic lung disease, retinopathy of prematurity, and neurodevelopmental impairment, particularly in the most immature babies and during the 1st week of life [9, 10]. Recently, ibuprofen has gained increased attention. Ibuprofen is as effective as indomethacin in PDA closure [2, 11]; however, it is considered superior to indomethacin because it decreases the mesenteric and renal blood circulation to a lesser extent and is accompanied by fewer renal complications [12].
Although PDA is treated with ibuprofen in current clinical practice, the closure rate of PDA is significantly different in some reports and there are some controversies surrounding the clinical rationale, administration and optimal timing of PDA treatment. Yoo et al. [13] suggested that PDA treatment can be delayed until the clinical symptoms become prominent. Moreover, definite evidence supporting the benefits of PDA treatment in contrast to watchful waiting with supportive care is necessary [14, 15]. These studies revealed other factors affecting ibuprofen effectiveness in PDA treatment. Furthermore, ibuprofen is similar to all nonsteroidal anti-inflammatory drugs (NSAIDs) and functions by blocking cyclooxygenases, which are key enzymes in the first rate-limiting step in the conversion of arachidonic acid into prostaglandins (PGs), which may be associated with adverse effects on multiple organ systems such as thrombocytopenia, impaired renal function, and gastrointestinal symptoms [16, 17]. More notably, PG E2 signaling was related with the enhanced expression of germ cell survival genes in human fetal ovaries [18], and Leverrier-Penna et al. [19] reported that ibuprofen had deleterious effects on the development of first trimester human fetal ovary ex vivo. Therefore, it is unclear whether it affects the germ cells in preterm infants. These findings suggest that ibuprofen should be used rationally and carefully to treat PDA, and evaluation of factors influencing its effectiveness for PDA can help identify eligible patients. Recently, only a few reports evaluated factors associated with ibuprofen effectiveness in PDA treatment [17, 20]. Currently available data suggest that factors including gestational age, birth weight, and perinatal risk factors increased the risk of PDA [21,22,23]. Unfortunately, the correlations between these factors and treatment outcomes have not been clearly explored.
Ethics approval
The study protocol was approved by the Medical Ethics Committee of Ningbo Women and Children’s Hospital (registration no: EC2020-034). Analysis was performed using anonymized data.
Method
Subjects
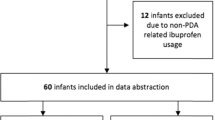
We retrospectively reviewed preterm infants admitted to the neonatal intensive care unit (NICU) of our hospital from January 2014 to October 2019 who presented with PDA and were receiving OIBU treatment. All patients were started on a recommended loading dose at 10 mg/kg of OIBU via a nasogastric tube and followed up with two additional doses of OIBU (5 mg/kg), which were administered at 24-h intervals [5, 21]. The medical records of 327/440 preterm infants admitted to the NICU presenting with PDA were retrospectively reviewed (Fig. 1).
For diagnosis and follow-up, echocardiography and Doppler ultrasonography were performed for PDA. In our unit, infants were examined daily during the initial weeks for changes in respiratory support and clinical symptoms/signs related to PDA (e.g., cardiac murmur, bounding pulses, hyperdynamic precordium, and/or significant respiratory distress). Treatment was necessary when the PDA appeared to be ≥ 1 mm in size on color Doppler echocardiography, with dominant left-to-right ductal shunting. Patients with the following factors at the initiation of treatment were excluded from our analysis: the presence of major congenital anomalies, grade 3 intraventricular hemorrhage, hemorrhage tendency, hyperbilirubinemia requiring blood exchange, and NEC or suspected NEC or in patients with serum creatinine level of > 141.44 μmol/L, blood urea nitrogen (BUN) level of > 17.8 mmol/L, and platelet count of < 60 × 106/mL. As shown in Fig. 1, 113 infants were excluded from the analyses.
Data collection and outcome measurement
Data collected on the basic clinical characteristics of patients treated with OIBU included the following: sex, gestational age, birth weight, 1- and 5-min Apgar scores, small for gestational age, mode of delivery, chorioamnionitis, antenatal steroid use, postnatal age at the initiation of OIBU, body weight at the initiation of OIBU, presence/absence of oxygen support, BUN and serum creatinine levels, and the use of inotropic drugs (dopamine and dobutamine) and diuretics for ≥ 24 h during OIBU treatment.
Data on the therapeutic effectiveness and adverse events during the treatment were collated. The patients were assessed clinically and echocardiographically at 24 h after the third dose. They were divided into the “effective” and “ineffective” groups based on whether the clinical signs improved and PDA flow disappeared or the ductal shunt was minimal on echocardiography. All infants whose condition did not meet this criterion were included in the ineffective group. IBU-administered patients were observed for renal, gastrointestinal, and cerebral adverse events. Serum BUN, creatinine and platelet counts were evaluated before and after treatment. During treatment, adverse outcomes of OIBU treatment were considered as increase in serum creatinine level to > 141.44 μmol/L or BUN to > 17.8 mmol/L and decrease in urine output to < 1 mL/kg/h and platelet count to < 60 × 106/mL.
Outcome measures included death before discharge, BPD (defined as the need for supplemental oxygen and/or positive pressure to maintain oxygen saturation of > 90% at 28 days after birth), IVH (grade ≥ 3), periventricular leukomalacia, NEC (> bell stage IIb), and retinopathy of prematurity (stage ≥ 3). Long-term neurodevelopmental outcome data were not collected in this study.
Statistical analysis
Enumeration and categorical data were expressed as mean ± standard deviation and numbers, respectively. Nominal variables were analyzed using Pearson’s Chi square test or Fisher’s exact test. Moreover, univariate crude odds ratio (OR) with 95% confidence interval (CI) for significant factors associated with the therapeutic effectiveness of PDA treatment were calculated using multivariate logistic regression analysis. A receiver operating characteristic (ROC) curve for factors influencing the outcomes of PDA treatment was created, with area under the curve (AUC) recorded, to determine cutoff values. All data were analyzed using statistical package for social sciences (SPSS) version 19.0. P values of < 0.05 were considered statistically significant.
Results
A total of 327 preterm infants (181 males, 146 females) were included in this study. Their mean gestational age was 31.17 ± 3.06 weeks (range 23.71–36.57 weeks) and birth weight was 1632 ± 617 g (range 700–3200 g). Ninety-four (28.75%) of the infants were very-low-birth-weight or extremely-low-birth-weight infants. Based on the aforementioned criteria, 201 (61.47%) preterm infants were classified as effective and 126 (38.53%) as ineffective, of which 26 infants received a second course of OIBU treatment. Table 1 shows the clinical characteristics of both groups. At the initiation of OIBU treatment, the effectiveness significantly differed for birth weight (P < 0.001), gestational age (P < 0.001), presence of respiratory distress syndrome (P = 0.003), 1-min Apgar score (P = 0.006), 5-min Apgar score (P < 0.001), number of sepsis events (P = 0.043), age at first diagnosis of PDA (P = 0.027), postnatal age (P < 0.001), body weight (P < 0.001), and mean heart rate (P < 0.001).
Eleven preterm infants showed some adverse events, including thrombocytopenia (n = 2) and renal function impairment (n = 9). Among preterm infants with renal impairment, serum creatinine levels of four were > 141.44 μmol/L, BUN level of one was > 17.8 mmol/L, and four had urine output of < 1 mL/kg/h. These were considered complications of OIBU treatment, and all complications were resolved within 1 week after discontinuation of the drug.
Fluid intake and urine output were evaluated before and after the course of OIBU treatment. The average urine output/fluid intake ratio was significantly different before and after the course of OIBU treatment among patients (Table 2). The urine output/fluid intake ratio was significantly different between responders and nonresponders before the treatment (Table 2). Notably, the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment in the effective group was significantly lower than that in the ineffective group (Table 2).
No significant differences were found in mean serum creatinine and BUN levels before and after the treatment course. However, it was noteworthy that platelet counts were higher after the treatment course (as opposed to falling) (Table 3).
Table 4 shows that no significant differences were found in the mortality using univariate analysis; however, the incidence of adverse outcomes including that of NEC, PVL, and BPD was higher in the ineffective group than in the effective group. Furthermore, the length of hospital stay was shorter in the effective group than in the ineffective group.
Postnatal age at the initiation of OIBU and the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment were found to act as predictive factors of the effectiveness of OIBU treatment for PDA, with ORs of 0.892 (95% CI 0.835–0.953; P = 0.001) and 0.473 (95% CI 0.265–0.845; P = 0.011), respectively (Table 5). Postnatal age at the initiation of OIBU treatment was the most significant predictive factor of effectiveness with an AUC of 0.690 (95% CI 0.632–0.748; P < 0.001; Fig. 2). The postnatal age cutoff value of 9 days at the initiation of OIBU treatment had a specificity and sensitivity of 77.6% and 58.8%, respectively, in predicting the effectiveness of PDA treatment. ROC curve obtained for the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment also showed significance in predicting the effectiveness of PDA treatment, with an AUC of 0.629 (95% CI 0.567–0.690; P < 0.001; Fig. 2). The cutoff value of 0.708 for this predictive factor had a specificity and sensitivity of 45.8% and 76.2%, respectively, in predicting the effectiveness of PDA treatment.
Receiver operating characteristic curve for predicting effectiveness of OIBU in treating PDA based on postnatal age at the initiation of OIBU and the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment. 1: Black, postnatal age at the initiation of OIBU treatment. 2: Blue, the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment. (N = 327). PDA patent ductus arteriosus, OIBU oral ibuprofen, AUC area under the curve
Discussion
Appropriate management of PDA, particularly in very preterm infants, is an ongoing challenge [6]. The risk of morbidities such as NEC, IVH, and BPD may increase because of later closure of PDA with prolonged left-to-right ductal shunting in very preterm infants [24, 25]. There is a significant amount of published data on the effectiveness of ibuprofen for closure of PDA [2, 5, 11]; however, to our knowledge, factors influencing effectiveness of OIBU treatment for PDA have not been explored in detail. This study demonstrated that single-course OIBU treatment is effective for the treatment of infants with PDA in NICU settings. We further evaluated the clinical presentations of study subjects and identified risk factors influencing the effectiveness of OIBU administered at a recommended dose for closure of PDA in preterm infants.
In our study, the PDA closure rate was observed in 61.47% of preterm infants (201/327), which was essentially consistent with the findings in the first course treatment of relevant reports [5, 17]. Furthermore, we found that the incidence of NEC, PVL, and BPD decreased significantly and the total hospitalization duration was shorter in the early PDA closure group. Gestational age has been reported to be an independent protective factor [17, 20]. We also found that the effectiveness of OIBU treatment increased with gestational age and birth weight in preterm infants, which may be related to the pharmacokinetic changes of ibuprofen among preterm infants with different maturity. Moreover, the maturity of preterm infants is positively correlated with that of the ductal constriction mechanism, possibly promoting the effective closure of PDA [26].
Sharma et al. [20] reported that the 1st week after birth is recognized as a sensitive period for drug treatment: when a preterm infant is administered the drug within 40 h of birth, the PDA closure rate is 90%, and when administered 40 h after birth, the PDA closure rate is only 68%. This may be attributable to the maturation of cytochrome P450 proteins with increasing age, leading to a significant increase in ibuprofen clearance and a rapidly shortened half-life of the drug. van der Lugt et al. [17] reported a positive correlation between the postnatal age at the start of the first IBU course and the need for a second IBU course. The closure rate in patients who received the first course during the first 4 postnatal days was found to be significantly higher [17]. It is noteworthy that our study indicated such a relationship, i.e., the time of first course of ibuprofen after birth was significantly earlier in the effective group than in the ineffective group. Results of multivariate logistic regression analyses reveled that postnatal age at the initiation of OIBU treatment significantly predicted its clinical effectiveness. ROC curve revealed the cutoff for postnatal age at the initiation of OIBU treatment of 9 days. Therefore, timely PDA treatment is significant to improve the closure rate. Moreover, Bell et al. [27] found that PDA morbidity could be significantly reduced if fluid intake was strictly controlled in preterm infants within 5 days after birth. Sung et al. [6] reported that the use of diuretics and fluid restriction during treatment compared with mandatory closure with indomethacin for PDA in extremely-low-birth-weight infants yields more positive outcomes. To a certain extent, these studies have revealed the effect of fluid imbalance during PDA treatment. In our study, infants were managed with judicious fluid restriction and pro re nata diuretics with respiratory support as required. Notably, the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment acted as a predictive factor for the effectiveness of ibuprofen treatment of PDA, with an OR of 0.473. The ROC curve indicated a cutoff value of 0.708 for this factor had a specificity of 45.8% and a sensitivity of 76.2% in predicting the effectiveness of PDA treatment. The underlying reasons may be related to the sensitivity to ibuprofen among different infants, i.e., the higher the sensitivity, the better the therapeutic effect. These findings suggest that the effectiveness of OIBU treatment for PDA is partially dependent on postnatal age at the initiation of OIBU treatment of ≤ 9 days and the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment of ≤ 0.708. This may benefit some of patients who failed the treatment using a recommended dose, for instance, timely administration or dosage adjustment.
The rates of the complications of IBU in preterm infants have significantly differed. van der Lugt et al. [17] reported that 15 patients (9.15%) had OIBU-related complications, whereas Olgun et al. [5] reported only 3 patients (3.2%). In our study, OIBU-related complications were observed in 11 patients (3.36%) and none of the complications observed were severe; all complications resolved after drug discontinuation.
As a retrospective review of medical records, this study has certain limitations. OIBU was determined by clinician’s preference rather than randomization. Thus, the definition of “effectiveness” is not sufficiently objective. There may be unmeasured confounders associated with our observation, such as individual difference in attending physicians, nursing care, and medical devices. Moreover, other confounding variables were variations in clinical management policies including respiratory management, fluid administration, and the use of diuretics. Further, due to the limited conditions, neurodevelopmental outcomes and long-term benefits and risks of OIBU for PDA in preterm infants were not studied. Therefore, future prospective double-blind trials are needed to clarify these issues.
Conclusion
We investigated the clinical features of preterm infants who received OIBU treatment for PDA and identified factors affecting effectiveness and safety. Our data suggested that a single course of OIBU is effective and safe in PDA closure; furthermore, preterm infants with PDA and postnatal age at the initiation of OIBU of ≤ 9 days and the Day 1/Day 0 ratio of urine output/fluid intake during the course of OIBU treatment of ≤ 0.708 may be an appropriate intervention for PDA. These findings warrant a prospective study to determine factors associated with the short- and long-term benefits and risks of OIBU for PDA in preterm infants.
References
Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–44.
Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2020;2:CD003481.
Sivanandan S, Bali V, Soraisham AS, Harabor A, Kamaluddeen M. Effectiveness and safety of indomethacin versus ibuprofen for the treatment of patent ductus arteriosus in preterm infants. Am J Perinatol. 2013;30(9):745–50.
Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150(3):229–34.
Olgun H, Ceviz N, Kartal I, Caner I, Karacan M, Tastekin A, et al. Repeated courses of oral ibuprofen in premature infants with patent ductus arteriosus: efficacy and safety. Pediatr Neonatol. 2017;58(1):29–35.
Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, et al. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr. 2016;177:66–71.
Clyman RI. The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):102–7.
Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, Aziz K, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012;161(4):689–94.
Sehgal A, Paul E, Menahem S. Functional echocardiography in staging for ductal disease severity: role in predicting outcomes. Eur J Pediatr. 2013;172(2):179–84.
Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007;119(6):1165–74.
Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA. 2018;319(12):1221–38.
Thomas RL, Parker GC, Van Overmeire B, Aranda JV. A meta-analysis of ibuprofen versus indomethacin for closure of patent ductus arteriosus. Eur J Pediatr. 2005;164(3):135–40.
Yoo H, Lee JA, Oh S, Jung YH, Sohn JA, Shin SH, et al. Comparison of the mortality and in-hospital outcomes of preterm infants treated with ibuprofen for patent ductus arteriosus with or without clinical symptoms attributable to the patent ductus arteriosus at the time of ibuprofen treatment. J Korean Med Sci. 2017;32(1):115–23.
Richards J, Johnson A, Fox G, Campbell M. A second course of ibuprofen is effective in the closure of a clinically significant PDA in ELBW infants. Pediatrics. 2009;124(2):e287–93.
Prescott S, Keim-Malpass J. Patent ductus arteriosus in the preterm infant: diagnostic and treatment options. Adv Neonatal Care. 2017;17(1):10–8.
Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17(6):275–342.
van der Lugt NM, Lopriore E, Bokenkamp R, Smits-Wintjens VE, Steggerda SJ, Walther FJ. Repeated courses of ibuprofen are effective in closure of a patent ductus arteriosus. Eur J Pediatr. 2012;171(11):1673–7.
Bayne RA, Eddie SL, Collins CS, Childs AJ, Jabbour HN, Anderson RA. Prostaglandin E2 as a regulator of germ cells during ovarian development. J Clin Endocrinol Metab. 2009;94(10):4053–60.
Leverrier-Penna S, Mitchell RT, Becker E, Lecante L, Ben Maamar M, Homer N, et al. Ibuprofen is deleterious for the development of first trimester human fetal ovary ex vivo. Hum Reprod. 2018;33(3):482–93.
Sharma PK, Garg SK, Narang A. Pharmacokinetics of oral ibuprofen in premature infants. J Clin Pharmacol. 2003;43(9):968–73.
Heyman E, Morag I, Batash D, Keidar R, Baram S, Berkovitch M. Closure of patent ductus arteriosus with oral ibuprofen suspension in premature newborns: a pilot study. Pediatrics. 2003;112(5):e354.
Supapannachart S, Limrungsikul A, Khowsathit P. Oral ibuprofen and indomethacin for treatment of patent ductus arteriosus in premature infants: a randomized trial at Ramathibodi Hospital. J Med Assoc Thai. 2002;85(Suppl 4):S1252–8.
Hammoud MS, Elsori HA, Hanafi EA, Shalabi AA, Fouda IA, Devarajan LV. Incidence and risk factors associated with the patency of ductus arteriosus in preterm infants with respiratory distress syndrome in Kuwait. Saudi Med J. 2003;24(9):982–5.
Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G. What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants? J Perinatol. 2012;32(5):344–8.
Morris IP, Goel N, Chakraborty M. Efficacy and safety of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: a systematic review and meta-analysis. Eur J Pediatr. 2019;178(8):1171–84.
Liu H, Manganiello V, Waleh N, Clyman RI. Expression, activity, and function of phosphodiesterases in the mature and immature ductus arteriosus. Pediatr Res. 2008;64(5):477–81.
Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2014;12:503.
Acknowledgements
The authors would like to acknowledge the pediatricians and nurses at our NICU for supporting this study.
Funding
This study was funded by the Natural Science Foundation of Ningbo (2018A610245 to C.Y.), Zhejiang Province Public Welfare Technology Application Research Project (2017C35010 to Q.L.), and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2020KY279 to Q.L.).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, C., Lyu, Q., Jiang, L. et al. Factors affecting the effectiveness of oral ibuprofen in the treatment of patent ductus arteriosus in preterm infants. Int J Clin Pharm 43, 1074–1081 (2021). https://doi.org/10.1007/s11096-020-01219-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-020-01219-6