Abstract
Background Cisplatin-induced nephrotoxicity still occurs despite the intensive hydration approach adapted to prevent its occurrence. Objective Evaluation of the effect of acetazolamide (ACTZ) on minimizing cisplatin-induced nephrotoxicity compared to mannitol when added to hydration regimen. Setting Nasser Institute Cancer Center (NICC), Cairo, Egypt. Method A total of 35 patients planned to receive cisplatin were divided into two groups: 20 patients received mannitol and 15 patients received ACTZ. Both groups received standard hydration measures as well for prevention of cisplatin-induced nephrotoxicity. Main outcome measure Patients’ kidney function was assessed using serum creatinine, creatinine clearance and blood urea nitrogen. Kidney injury was assessed using RIFLE criteria. Patients’ liver function tests and hematological parameters were also monitored. Results Patients in the mannitol group showed higher risk of developing kidney injury (30%) whereas those in the ACTZ group showed lower risk (8.9%), relative risk (RR) 0.269, 95% CI 0.108–0.815. No statistically significant difference occurred between the two groups concerning liver function tests or hematological parameters. Conclusion Use of ACTZ in addition to intensive hydration may have more beneficial effect on minimizing cisplatin-induced nephrotoxicity compared to mannitol plus intensive hydration approach. A large multicenter randomized clinical trials is recommended to confirm study results and to assess effect of ACTZ on tumor response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on practice
-
Acetazolamide seems to be efficace and safe for reducing cisplatin induced nephrotoxicity, when added to a standard hydration regimen.
-
To minimize cisplatin-induced nephrotoxicity, acetazolamide is more effective than mannitol.
Introduction
Cisplatin is an important anti-cancer medication used for the treatment of a variety of malignant tumors [1]. While cisplatin toxicities include ototoxicity, myelosuppression, and allergic reactions, the main dose-limiting side effect of cisplatin is nephrotoxicity [2,3,4,5].
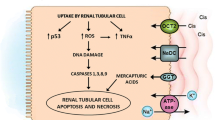
Cisplatin nephrotoxicity is the composite result of the transport of cisplatin into renal epithelial cells, injury to nuclear and mitochondrial DNA, activation of a multiple cell death and survival pathways and initiation of a robust inflammatory response [6, 7].Volume expansion and the administration of intravenous saline to induce diuresis remains the main approach for prevention of cisplatin-induced nephrotoxicity [8, 9]. However, Cisplatin-induced nephrotoxicity still occurs despite intensive hydration. This is shown by reduction in glomerular filtration which occurs in about 21–32% of patients. The risk increases with higher doses of cisplatin, frequency of administration, cumulative dose of cisplatin, underlying kidney damage, concurrent treatment with other potentially nephrotoxic agents, such as aminoglycosides, nonsteroidal anti-inflammatory agents, or iodinated contrast media, cardiac disease, female sex, older age, smoking and hypoalbuminemia [10,11,12,13,14].
Mannitol plus hydration has been used for several years to alleviate toxicity associated with cisplatin therapy. However, the data for mannitol administration is still controversial [15]. Different strategies have been suggested as add-on to saline hydration other than mannitol to diminish or prevent nephrotoxicity of cisplatin [16]. Among those strategies are sulphur-containing compounds which have shown to reduce the nephrotoxicity of cisplatin without inhibiting or decreasing its antitumor effect in patients with non-small cell lung cancer, metastatic breast cancer, ovarian cancer and metastatic colon cancer [4].
Amifostine [2-(3-aminopropylamino) ethylsulfanyl phosphonic acid] is a pharmacologically active free sulfhydryl which binds to and detoxifies cytotoxic platinum-containing metabolites of cisplatin and scavenges free radicals induced by the drug. It is approved by the U.S. Food and Drug Administration for reduction of cumulative nephrotoxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer. However, its higher treatment costs, logistical issues and significant toxicities associated with intravenous administration made amifostine difficult to incorporate into clinical practice [17].
Acetazolamide (ACTZ), a carbonic anhydrase inhibitor, is a sulphur containing compound that may assist in ameliorating cisplatin-induced nephrotoxicity [18]. It is an organic acid which has structural similarity to amifostine and may competitively decrease tubular reabsorption of cisplatin [18]. It has shown promising results in decreasing cisplatin-induced nephrotoxicity in animal models [19, 20]. Moreover, it is available as oral preparation with low cost.
The routinely used protocol at Nasser Institute Cancer Center (NICC), Cairo, Egypt, is mannitol plus standard hydration. However, some patients still suffer from cisplatin-induced nephrotoxicity despite following the recommended hydration protocol. Moreover, no studies to date evaluated the effect of ACTZ in reducing cisplatin-induced nephrotoxicity in humans.
Aim of the study
To compare the safety and efficacy of ACTZ plus standard saline hydration with mannitol plus standard saline hydration in reducing cisplatin induced nephrotoxicity in cancer patients.
Ethics approval
The study was approved by the Ethical committee of Egyptian Ministry of Health and Population then from ethical committee at Nasser Institute Hospital. All patients signed an informed consent (approved by both committees) and every detail was explained to them. Study protocol is registered through clinicaltrials.gov with ID number NCT02760901.
Methods
This study was a prospective, controlled pilot study. It was conducted in Nasser Institute Cancer Center (NICC), Cairo, Egypt. All adult cancer patients (18–65 years) admitted to NICC from November 2013 to October 2015 were assessed for inclusion into the study. Only patients who were planned to receive cisplatin-based chemotherapy protocol were assessed for eligibility to the current study. Patients with existing renal impairment [creatinine clearance (CrCl) < 30 ml/min], severe hepatic impairment (Child-Pugh score C), hypersensitivity to sulphur compounds and/or chronic non-congestive angle-closure glaucoma were excluded. Out of 90 patients, only 35 patients met the inclusion criteria and completed the duration of the current study. All patients received standard saline hydration measures for prevention of cisplatin-induced nephrotoxicity.
Patient allocation
Patients were allocated into two groups. The first group was the mannitol group which comprised 20 patients who received mannitol 20% 100 ml half an hour before cisplatin and saline hydration. The second group was the ACTZ group which comprised 15 patients who received ACTZ 250 mg (safe FDA approved doses for human) half an hour before cisplatin and saline hydration. To ensure compliance of physicians and nurses with the study protocol, patients admitted on Saturdays were allocated to ACTZ group while patients admitted on Mondays were allocated to mannitol group.
Patients’ demographics, comorbidities, concurrent medications, liver function tests: aspartate aminotransferase (AST), alanine aminotransferase (ALT), hematological parameters: hemoglobin (Hg), total leucocytes count (TLC), and platelets were documented at baseline and throughout the study period (i.e. after chemotherapy cycle for three cycles). In addition, adverse drug effects were monitored throughout the study period.
Primary outcomes: Occurrence of nephrotoxicity was evaluated by measuring the effect of cisplatin on kidney function using serum creatinine (Scr), creatinine clearance (CrCl), and blood urea nitrogen (BUN).
Secondary outcomes: Occurrence of hematological toxicity, hepatotoxicity, and/or any other adverse effect.
Assessment of kidney function and injury
Serum creatinine, CrCl, and BUN were evaluated at baseline and after each cycle for three cycles of cisplatin separated by 21 days. Creatinine clearance was calculated according to Cockroft-Gault equation using ideal body weight (IBW) [21]. For low body weight patients and obese patients with weight greater than 30% over IBW, actual body weight and adjusted body weight were used instead of IBW, respectively [22]. RIFLE (risk, injury, failure, loss, and end-stage kidney disease) criteria were calculated for all patients as follows [23]:
-
Risk: 25% reduction in GFR
-
Injury: 50% reduction in GFR
-
Failure: 75% reduction in GFR.
Dose modification for cisplatin in renal impairment [24]
-
CrCl 10–50 mL/min: 75% of dose was administered.
Statistical analysis
Data management and analysis were done using Statistical Software Package for Social Sciences (SPSS) version 22. Graphics were generated utilizing Microsoft Excel 2010. All continuous data were expressed as mean ± SD while categorical data were expressed as frequency in tables. Comparisons between groups with respect to normally distributed numerical data at baseline were done using Independent student t-test. For comparing results of repeated measurements, ANOVA (analysis of variance) with repeated measures procedures was employed. Comparisons between nominal data were done using Chi square test. P value ≤ 0.05 was considered significant and 95% confidence interval used. Kaplan–Meier analysis was used for assessing the risk of developing kidney injury after every cycle of cisplatin chemotherapy.
Results
This study included a total of 35 patients who were assigned to one of the following two groups: Mannitol or ACTZ. Patients’ demographics and clinical characteristics in the two groups are represented in Table 1. Kidney and liver function tests were comparable in the two groups at baseline as shown in Table 2. In addition, patients in the two groups suffered from different types of cancer with various treatment protocols and different comorbidities as shown in Table 3. All of the drug regimens used with cisplatin were not nephrotoxic except gemcitabine which was similar in both groups.
-
1.
Biochemical tests
Biochemical tests were measured after each cycle of chemotherapy containing cisplatin separated by 21 days and for three cycles as represented in Table 4. Creatinine clearance decreased in mannitol group by time, while it increased by time in ACTZ group with P values 0.887 at baseline, 0.014 after the first cycle, 0.001 after the second cycle, and 0.18 after the third cycle as shown in Fig. 1. In addition, BUN level increased in mannitol group by time, but it was stable in ACTZ group with P values 0.089 at baseline, 0.011 after the first cycle, 0.001 after the second cycle, and 0.119 after the third cycle as shown in Fig. 1. However, no significant change between groups occurred in liver function tests and hematological parameters, P>0.05.
-
2.
RIFLE criteria before and after treatment in the two groups
By doing Kaplan–Meier analysis considering developing the risk of kidney injury (defined as 25% or more reduction in GFR) as the event of interest, there was a statistically significant difference between the two groups with P value 0.012 as represented in Fig. 2. Patients in the mannitol group showed a higher risk of developing kidney injury (30%) whereas those in the ACTZ group showed a lower risk (8.9%), relative risk (RR) 0.269, 95% CI 0.108–0.815. It is worth mentioning that no patient in both groups suffered from 50% or more reduction in GFR.
-
3.
Patients requiring dose reduction of cisplatin before and after treatment in the two groups
By doing Kaplan–Meier analysis considering 25% dose reduction as the event of interest, there was a statistically significant difference between the two groups with P value 0.001. Out of the 60 cisplatin doses in mannitol group, 11 needed dose reduction whereas no dose reduction was needed in any of the 45 doses of the ACTZ group with relative risk (RR) 1.918, 95% CI 1.581–2.328. However, it is noteworthy that not all dose reduction was due to nephrotoxicity. Two patients required dose reduction in mannitol group after the second cycle, one due to ototoxicity and the other due to neuropathy.
-
4.
Adverse drug effects
Total adverse effects other than nephrotoxicity were 15 in mannitol group and 7 in ACTZ group. The difference in adverse effects was not statistically significant, P value 0.385 as represented in Fig. 3. In ACTZ group, 1 patient suffered from severe diarrhea; his treatment protocol was cisplatin, docetaxel, and fluorouracil for treatment of gastric cancer. However, this patient completed his ordered cycles without a dose reduction of cisplatin. On the other hand, two patients in mannitol group suffered from an elevation in liver enzymes, one after the first cycle and the other after the third cycle. The latter 2 patients did not need cisplatin dose reduction. However, 2 patients required cisplatin dose reduction in mannitol group, one due to neuropathy and the other due to ototoxicity.
Adverse effects in the two groups, thrombocytopenia defined by platelets count < 100 × 103 per µL, Leucopenia defined by TLC < 4 × 103 per µL, Elevated liver function tests defined by values more than the upper limit of normal range (normal AST is reported between 10 and 40 units per liter and ALT between 7 and 56 units per liter), severe diarrhea defined by more than ten loose watery stools per day
-
Shifting to another chemotherapy
Only one patient died in ACTZ group (6.67%) without shifting to another chemotherapy protocol. Similarly, in mannitol group, only 1 patient died (5%). However, five patients in mannitol group were shifted to another chemotherapy protocol (25%); 1 patient due to peripheral neuropathy, 1 patient due to disease progression, 1 patient due to ototoxicity and 2 patients due to nephrotoxicity. The relative risk (RR) of shifting to another chemotherapy when using mannitol was 1.33, 95% CI 1.035–1.717.
Discussion
Cisplatin is a strong cellular toxin and nephrotoxicity is one of the most im-portant complications of this drug [16, 25]. Its nephrotoxicity is manifested by acute kidney injury which is cumulative, dose-dependent and often necessitates dose reduction or withdrawal [26].The standard approach for prevention of cisplatin nephrotoxicity is the administration of lower doses of cisplatin in combination with full intravenous hydration prior and after cisplatin administration [16]. However, evidence-based recommendations on specific supplementation strategies added to hydration regimens to reduce cisplatin nephrotoxicity are limited.
In the present study, Scr, BUN, CrCl and RIFLE criteria were used for monitoring kidney function in patients receiving cisplatin. The study showed a lower incidence of nephrotoxicity in patients receiving saline hydration with ACTZ (8.9%) compared to those receiving saline hydration with mannitol (30%) based on CrCl. This difference was more prominent after repeated administration of cisplatin. Moreover, mannitol group showed a higher incidence of other adverse effects as neurotoxicity and leucopenia. The increase in the incidence of nephrotoxicity by repeated administration of cisplatin is in accordance with other studies which reported similar results despite preventive measures such as hydration [26, 27]. In addition, many studies used Scr, CrCl and BUN as indicators of cisplatin-induced nephrotoxicity [19, 27,28,29]. Studies on animals have shown promising results when ACTZ was used to decrease cisplatin nephrotoxicity [18, 28,29,30]. However, no studies to date were conducted in humans to evaluate the efficacy and safety of ACTZ in reducing the incidence of nephrotoxicity caused by cisplatin. Osman et al. [30] study in 1983 showed that when ACTZ was given to rats before cisplatin, it resulted in lower nephrotoxicity. Also, renal cisplatin concentration in rats receiving ACTZ was lower than that in those treated with cisplatin alone. In 1985, Heidemann et al. found that ACTZ and furosemide at ceiling diuretic doses achieved protective effect against cisplatin-induced nephrotoxicity when administered to rats before cisplatin but ACTZ produced more significant protection than furosemide [19]. In 1990, Heidemann et al. [29] study on rats showed that ACTZ and sodium chloride protected the kidney from cisplatin nephrotoxicity and improved creatinine clearance. In 1996 another study on rats found that administration of vitamin E and ACTZ reduced cisplatin-induced nephrotoxicity [18].
On the other hand, the effect of mannitol plus hydration on decreasing the incidence of cisplatin-induced nephrotoxicity has been evaluated in different animal and human studies [31,32,33,34,35,36]. Clinically, mannitol reduces the urine concentration of cisplatin, and this effect is considered to be the mechanism underlying the amelioration of renal toxicity [28]. Studies have shown that the incidence of cisplatin-induced nephrotoxicity using mannitol as a protective measure ranged from about 28% to about 34% [33, 37]. This is in accordance with the present study which showed a 30% incidence of nephrotoxicity in the mannitol group. However, the incidence of nephrotoxicity in ACTZ group in the present study was significantly lower than mannitol group (8.9%).
Whether mannitol has a superior protective effect against cisplatin-induced nephrotoxicity compared to other strategies is conflicting [15, 31,32,33, 38]. In a randomized clinical trial comparing mannitol versus furosemide, nephrotoxicity incidence was 19% with furosemide versus 28% with mannitol [37]. Similarly, in another study, hydration plus mannitol was associated with more cisplatin nephrotoxicity compared to saline hydration only or saline plus furosemide [31]. Also, by assessing the effect of mannitol on the urinary excretion of N-acetyl-β-D-glucosaminidase (NAG) and alanine aminopeptidase (AAP) which indicate cisplatin-induced tubular injury, it was found that mannitol infusion did not affect NAG or AAP concentration [39]. Morgan et al. [15] conducted a review of literature from 1945 till 2011 evaluating the effect of mannitol on nephrotoxicity caused by cisplatin and concluded that there are no compelling data that the addition of mannitol provides more protection from nephrotoxicity caused by cisplatin than the use of hydration alone. In 2008, The European Society of Clinical Pharmacy Special Interest Group on Cancer Care recommended not using either mannitol or furosemide in cisplatin hydration protocol [40].A recent systematic review suggested that mannitol may be beneficial only for high dose cisplatin exceeding 100 mg/m2 and/or patients with preexisting hypertension [41]. However, it is worthy to mention that Himmelstein K.J. et al tested the effect of mannitol on cisplatin plasma concentration, and found that it did not have an effect on cisplatin plasma levels or cisplatin clearance [42]. The latter finding may represent an advantage of mannitol over ACTZ as the effect of ACTZ on cisplatin plasma levels has never been tested.
By searching the Pubmed, only one study was found to compare mannitol and ACTZ in reducing cisplatin nephrotoxicity in animal models [28]. The latter study was done on rats and showed that ACTZ administration before cisplatin reduced the cisplatin-induced nephrotoxicity compared to mannitol using uremia as an indicator of nephrotoxicity. This is in accordance with the current study where ACTZ group showed a stable level of uremia compared to the increased level in mannitol group.
In this study, there was no statistically significant difference regarding hepatic function tests or hematological parameters which confers safety of ACTZ compared to mannitol. Although there was no statistically significant difference between the two groups regarding adverse effects, leucopenia and peripheral neuropathy were higher in mannitol group. However, these adverse effects may be related to chemotherapy regimens used and not related to either mannitol or ACTZ.
Conclusion
The present pilot study showed that ACTZ may provide more superior nephroprotective effect than mannitol. This effect appears after repeated administration of cisplatin doses. No significant difference existed between patients who improved or shifted to another chemotherapy in either ACTZ or mannitol group. However, cisplatin therapeutic efficacy couldn’t be measured because not all data for RECIST criteria and investigations were available in patient files. Also, neither cisplatin plasma concentration nor NAG was measured in this study. Accordingly, a large multicenter randomized controlled clinical trial is recommended to confirm this study results and to assess the effect of ACTZ on tumor response and cisplatin plasma concentration. Moreover, newer biomarkers as Kidney injury molecule-1 and Neutrophil gelatinase-associated lipocalin can be used for further assessment of kidney injury due to cisplatin. Also, as cisplatin nephrotoxicity is cumulative, following patients for more than the three cycles monitored in the present study may provide more definitive results.
References
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–518.
Hartmann JT, Fels LM, Knop S, Stolt H, Kanz L, Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. Invest New Drugs. 2000;18(3):281–9.
Thomas Hartmann J, Lipp H-P. Toxicity of platinum compounds. Exp Opin Pharmacother. 2003;4(6):889–901.
Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–24.
Sastry J, Kellie SJ. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol. 2005;22(5):441–5.
Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86(4):396–402.
Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278(5):F726–36.
Portilla D, Safar AM, Shannon ML, Penson RT. Cisplatin nephrotoxicity Uptodate (2015).
Cvitkovic E, Spaulding J, Bethune V, Martin J, Whitmore WF. Improvement of cis-dichlorodiammineplatinum (NSC 119875): therapeutic index in an animal model. Cancer. 1977;39(4):1357–61.
Kidera Y, Kawakami H, Sakiyama T, Okamoto K, Tanaka K, Takeda M, et al. Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal protection. PLoS ONE. 2014;9(7):e101902.
Moon HH, Seo KW, Yoon KY, Shin YM, Choi KH, Lee SH. Prediction of nephrotoxicity induced by cisplatin combination chemotherapy in gastric cancer patients. World J Gastroenterol. 2011;17(30):3510–7.
Sato K, Watanabe S, Ohtsubo A, Shoji S, Ishikawa D, Tanaka T, et al. Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer. 2016;16:222.
De Jongh FE, Van Veen RN, Veltman SJ, De Wit R, Van der Burg ME, Van den Bent MJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88(8):1199–206.
Faig J, Haughton M, Taylor RC, D’Agostino RB, Whelen MJ, Porosnicu Rodriguez KA, et al. Retrospective analysis of cisplatin nephrotoxicity in patients with head and neck cancer receiving outpatient treatment with concurrent high-dose cisplatin and radiotherapy. Am J Clin Oncol. 2016;41(5):432–40.
Morgan KP, Buie LW, Savage SW. The Role of Mannitol as a Nephroprotectant in Patients Receiving Cisplatin Therapy. Ann Pharmacother. 2012;46(2):276–81.
Hayati F, Hossainzadeh M, Shayanpour S, Abedi-Gheshlaghi Z, Beladi Mousavi SS. Prevention of cisplatin nephrotoxicity. J Nephropharmacol. 2016;5(1):57–60.
Block KI, Gyllenhaal C. Commentary: the pharmacological antioxidant amifostine—implications of recent research for integrative cancer care. Integr Cancer Therapies. 2005;4(4):329–51.
Gopalakrishnan R, Murugesan A, Babu E, Sakthisekaran D. Protective role of vitamin E and acetazolamide in cisplatin-induced changes in lipid peroxidation and antioxidant enzyme levels in albino rats. J Clin Biochem Nutr. 1996;20(3):203–10.
Heidemann H, Gerkens J, Jackson E, Branch R. Attenuation of cisplatinum-induced nephrotoxicity in the rat by high salt diet, furosemide and acetazolamide. Naunyn-Schmiedeberg’s Arch Pharmacol. 1985;329(2):201–5.
Heidemann HT, Gjessing L, Brune KH, Ohnhaus EE. The effect of acetazolamide and furosemide on lithium clearance and cisplatin nephrotoxicity in the rat. In: Bach PH, Lock EA, editors. Nephrotoxicity. Springer: US; 1989. p. 367–70.
Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Bauer L. In: McGraw H, editor. Applied clinical pharmacokinetics. New York: Edical Publishing Division; 2001. p. 93–179.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):R204–12.
Aronoff G, Bennett W, Berns J, Brier M, Kasbekar N, Mueller B, et al. Drug prescribing in renal failure dosing guidelines for adults and children. 5th ed. Philadelphia: American College of Physicians; 2007.
Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34(10):1522–34.
Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:17.
Prasaja Y, Sutandyo N, Andrajati R. Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pac J Cancer Prev APJCP. 2015;16(3):1117–22.
Osman NM, Copley MP, Litterst CL. Effects of the diuretics mannitol or acetazolamide on nephrotoxicity and physiological disposition of cisplatin in rats. Cancer Chemother Pharmacol. 1984;13(1):58–62.
Heidemann HT, Hoffmann K, Inselmann G. Long-term effects of acetazolamide and sodium chloride loading on cisplatin nephrotoxicity in the rat. Eur J Clin Invest. 1990;20(2):214–8.
Osman N, Copley M, Litterst C. Amelioration of cisplatin-induced nephrotoxicity by the diuretic acetazolamide in F344 rats. Cancer Treat Rep. 1983;68(7–8):999–1004.
Santoso JT, Lucci JA, Coleman RL, Schafer I, Hannigan E. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol. 2003;52(1):13–8.
Pingle SC, Mishra S, Marcuzzi A, Bhat SG, Sekino Y, Rybak LP, et al. Osmotic diuretics induce adenosine A1 receptor expression and protect renal proximal tubular epithelial cells against cisplatin-mediated apoptosis. J Biol Chem. 2004;279(41):43157–67.
Morgan KP, Snavely AC, Wind LS, Buie LW, Grilley-Olson J, Walko CM, et al. Rates of renal toxicity in cancer patients receiving cisplatin with and without mannitol. Ann Pharmacother. 2014;48(7):863–9.
Muraki K, Koyama R, Honma Y, Yagishita S, Shukuya T, Ohashi R, et al. Hydration with magnesium and mannitol without furosemide prevents the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non-small cell lung cancer. J Thorac Dis. 2012;4(6):562–8.
Al-Sarraf M, Fletcher W, Oishi N, Pugh R, Hewlett JS, Balducci L, et al. Cisplatin hydration with and without mannitol diuresis in refractory disseminated malignant melanoma: a southwest oncology group study. Cancer Treat Rep. 1982;66(1):31–5.
Lehane D, Winston A, Gray R, Daskal Y. The effect of diuretic pre-treatment on clinical, morphological and ultrastructural cis-platinum induced nephrotoxicity. Int J Radiat Oncol Biol Phys. 1979;5(8):1393–9.
Ostrow S, Egorin MJ, Hahn D, Markus S, Aisner J, Chang P, et al. High-dose cisplatin therapy using mannitol versus furosemide diuresis: comparative pharmacokinetics and toxicity. Cancer Treat Rep. 1981;65(1–2):73–8.
Hayes DM, Cvitkovic E, Golbey RB, Scheiner E, Helson L, Krakoff IH. High dose Cis-platinum diammine dichloride. Amelioration of renal toxicity by mannitol diuresis. Cancer. 1977;39(4):1372–81.
Goren MP, Wright RK, Horowitz ME. Cumulative renal tubular damage associated with cisplatin nephrotoxicity. Cancer Chemother Pharmacol. 1986;18(1):69–73.
Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European society of clinical pharmacy special interest group on cancer care. Cancer Chemother Pharmacol. 2008;61(6):903–9.
Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist. 2017;22(5):609–19.
Himmelstein KJ, Patton TF, Belt RJ, Taylor S, Repta AJ, Sternson LA. Clinical kinetics of intact cisplatin and some related species. Clin Pharmacol Ther. 1981;29(5):658–64.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Funding
None.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Hamamsy, M., Kamal, N., Bazan, N.S. et al. Evaluation of the effect of acetazolamide versus mannitol on cisplatin-induced nephrotoxicity, a pilot study. Int J Clin Pharm 40, 1539–1547 (2018). https://doi.org/10.1007/s11096-018-0677-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0677-x