Abstract
Purpose
Application of external heat using a heating pad over buprenorphine transdermal system, Butrans® has been shown to increase systemic levels of buprenorphine in human volunteers. The purpose of this study was to perform in vitro permeation studies at normal as well as elevated temperature conditions to evaluate the correlation of in vitro data with the existing in vivo data.
Methods
In vitro permeation tests (IVPT) were performed on human skin from four donors. The IVPT study design was harmonized to a previously published clinical study design and skin temperature was maintained at either 32 ± 1 °C or 42 ± 1 °C to mimic normal and elevated skin temperature conditions, respectively.
Results
IVPT studies on human skin were able to demonstrate heat induced enhancement in flux and cumulative amount of drug permeated from Butrans® which was reasonably consistent with the corresponding enhancement observed in vivo. Level A in vitro—in vivo correlation (IVIVC) was established using unit impulse response (UIR) based deconvolution method for both baseline and heat arms of the study. The percent prediction error (%PE) calculated for AUC and Cmax values was less than 20%.
Conclusions
The studies indicated that IVPT studies performed under the same conditions as those of interest in vivo may be useful for comparative evaluation of the effect of external heat on transdermal delivery system (TDS). Further research may be warranted to evaluate factors, beyond cutaneous bioavailability (BA) assessed using an IVPT study, that can influence plasma exposure in vivo for a given drug product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An average person can be exposed to several heat sources in daily life that can result in a local increase in skin temperature. Exposure to heating pads for pain relief, electric blankets for warmth, intense exercise, or saunas at the gym can elevate skin temperature. A local increase in skin temperature at the application site of a topically applied formulation can potentially alter the drug delivery profile resulting in increased bioavailability (BA) from these formulations. Application of controlled external heat over nicotine transdermal delivery systems (TDS) applied on human volunteers resulted in an increased delivery of nicotine from the TDS [1, 2]. Increased delivery of fentanyl into the systemic circulation can be life-threatening. A case of fentanyl overdose in an elderly patient was reported when a heating pad was accidently in contact with the fentanyl TDS [3]. A study on human volunteers with controlled heat application over a fentanyl TDS showed elevated systemic levels of fentanyl [4]. Heat application in vivo can increase systemic levels of nitroglycerin and glyceryl trinitrate from their respective TDS formulations [5, 6]. The ability of heat to enhance drug delivery across skin has been utilized by designing CHADD (controlled heat-aided drug delivery) systems for lidocaine, testosterone, and fentanyl [7,8,9]. Enhanced heat induced drug delivery from dermally applied formulations can be attributed to increased drug release from formulations, increased drug diffusion across skin, increased cutaneous perfusion, and increased dermal clearance [10].
Buprenorphine is lipophilic and a weakly acidic drug (logP = 4.98 and pKa = 8.31). It is a partial mu receptor agonist. It is an opioid indicated for the management of chronic pain requiring long-term opioid treatment and for which alternative treatment options are inadequate. It is also used for treatment of opioid addiction. Lower doses are used for pain management while treatment of opioid addiction requires higher doses. The abuse potential is greater at higher doses of buprenorphine especially in individuals who have a lower level of physical dependence on other opioid drugs [11, 12]. Use of heat application on buprenorphine TDSs can be employed to abuse the drug. Hence, it is important to have an understanding of the effect of heat on Butrans®.
Effect of internal and external heat application on the pharmacokinetics (PK) of Butrans® was previously reported [14]. Briefly, the effect of an elevated internal body temperature on buprenorphine absorption from Butrans® was evaluated in 22 healthy volunteers. After application of Butrans® 10 mg/h system in all volunteers, either an endotoxin or a placebo without the endotoxin was administered on day 2. No significant differences in primary PK parameters (Cmax and AUC) were observed between the endotoxin and placebo treatment groups. Two separate studies were conducted to evaluate the effect of external heat on Butrans®. The first study looked at 28 healthy volunteers who wore Butrans® 5 mg/h system for three sequential applications. On the third week after removal of Butrans®, local heat at 38 °C was applied for 3 h. No increase in Cmax or AUC was observed during heat application. In the second study with 20 healthy volunteers, external heat was applied using a heating pad for three 2 h periods on day 2 and day 4 post application of Butrans® 10 mg/h system (over 7 h on each day). On day 2, heat was applied from 24 h – 26 h, 26.5 – 28.5 h, and 29 h – 30 h. On day 4, heat was applied from 72 h – 74 h, 74.5 h – 76.5 h, and 77 h – 79 h [13]. Increased plasma levels of buprenorphine were observed during the 7 h of intermittent heat application and up to 5 h after heat removal. Thus a 12 h heat effect interval/window was observed. Increase in mean plasma buprenorphine concentrations (26–55%) were observed compared to the volunteers with no heating pad application. An increase in opioid-related adverse effects was also noted in volunteers receiving external heat application [14]. Thus, application of external heat over the Butrans® demonstrates a potential scenario where heat exposure results in an altered PK profile.
In vitro permeation tests have been used to evaluate the effect of heat on dermally applied formulations of diclofenac (TDS, gel, and solution) [15] and fentanyl and nicotine TDSs [16, 17]. Caffeine, methyl paraben, butyl paraben, and acyclovir have shown an increase in flux following exposure to increased temperature conditions [18, 19]. Several IVIVC studies have demonstrated the ability of IVPT studies to adequately predict in vivo results [20, 21]. Harmonization of in vitro and in vivo study designs are vital to the ability of IVPT studies to be able to predict in vivo BA and develop IVIVC models [21]. Such harmonized study designs have been employed by our group to adequately predict heat induced increase in BA from TDS and an IVIVC was established for nicotine and fentanyl TDS [1, 22]. The objective of this study was to investigate the effect of heat on buprenorphine delivery (skin permeation) in vitro by using Butrans® and to evaluate the ability of IVPT studies to correlate with and be predictive of the potential heat-enhanced drug delivery in vivo. Use of IVPT as a tool to evaluate the comparative rate and extent of drug permeation from test and reference products following the application of heat in vitro would be of considerable value. Such in vitro studies require fewer resources, are rapid, and minimize exposure of healthy volunteers to drugs. Development of IVIVC may facilitate development of generic TDS.
Materials and Methods
Materials
Butrans® 5 µg/h TDSs (lot # 3040485AA, exp 09/2017) were purchased from Cardinal Health™ (Dublin, OH). Potassium phosphate monobasic and dibasic salts, methanol, acetonitrile, Brij™ 98, and gentamicin sulfate were purchased from Fisher Scientific Inc. (Fair Lawn, NJ). Buprenorphine hydrochloride salt (≥ 98%) was obtained from Sigma Aldrich (St Louis, MO). All reagents used were of analytical grade. Water from Milli-Q® system (EMD Millipore; Billerica, MA) was used for preparing solutions and buffers. Human abdominal skin tissue was sourced from NCI Cooperative Human Tissue Network (CHTN) skin repository (Charlottesville, VA).
In Vitro Permeation Test (IVPT)
Dermatomed abdominal human skin with a mean thickness of 297 ± 35 µm retaining the topmost layers and part of the dermis was stored at -20 °C. On the day of the experiment, skin was thawed and cut into 4.84 cm2 square pieces. PermeGear flow-through In-Line diffusion cells (Hellertown, PA) were used for the IVPT studies. The skin pieces were positioned between the donor and receiver chamber of the diffusion cell with the dermis-side facing the receiver chamber. The skin was supported by a cross bar membrane support of the In-Line diffusion cell. Transepidermal water loss (TEWL) readings were recorded using a cyberDerm RG-1 open chamber evaporimeter (cyberDERM, Inc.; Broomall, PA) and skin pieces with readings higher than 15.0 g/m2/h, were considered damaged and replaced. Once barrier integrity of the skin was evaluated, a 0.97 cm2 circular disc of the Butrans® was applied on the stratum corneum-side of the skin covering the entire 0.95 cm2 permeation area of the diffusion cell. A 4.84 cm2 square piece of polypropylene knitted mesh (0.15 mm monofilament, 3.0 × 2.8 mm pores, 47 GSM; SurgicalMesh™ Division of Textile Development Associates, Inc.; Brookfield, CT) was placed on top of the Butrans® disc to ensure complete adhesion to the skin. Isotonic potassium phosphate solution at pH 7.4 ± 1.0 containing 0.1% Brij™ 98 and 0.08% gentamicin sulfate was used as the receiver solution maintained at 37 °C in a water bath. Receiver solution was collected at predetermined time points and analyzed using a validated high performance liquid chromatography (HPLC) method.
The in vitro study design was harmonized to the previously published clinical study, which had two study arms [14]. First, the baseline study arm where skin temperature was maintained at 32 ± 1 °C to mimic normal baseline skin temperature for 168 h. Second, the heat study arm where the skin was maintained at 42 ± 1 °C over a duration of 7 h on day 2 and day 4 to mimic elevated skin temperature conditions attained due to the heating pad used in the clinical study [14]. On day 2, heat was applied from 24 h – 26 h, 26.5 – 28.5 h, and 29 h – 30 h. On day 4, heat was applied from 72 h – 74 h, 74.5 h – 76.5 h, and 77 h – 79 h. In both study arms, Butrans® was removed from the skin after 168 h and receiver solution was collected through 174 h. Dermatomed human skin from four donors indicated as donor 1, 2, 3, and 4 was used for each study arm. Four replicates per donor were used for each study arm.
Skin Temperature Control and Monitoring
During the IVPT studies, the diffusion cells rest on a water-jacketed arm. A circulating water bath was used to modulate the temperature of the diffusion cells and hence control the temperature of the skin surface. A Traceable® infrared thermometer (Fisher Scientific; 15–077-966) was used to monitor the temperature of the skin surface during the human skin IVPT studies. To mimic early or late heat application, pre-heated water was added into the circulating water bath to raise the skin temperature to 42 ± 1 °C within 10 min. The skin temperature was maintained at 42 ± 1 °C throughout the specified heating duration. To mimic the removal of heat application period, ice was added into the water bath to lower the skin temperature to 32 ± 1 °C within 10 min.
HPLC Analysis of IVPT Samples
HPLC system consisting of a Waters® Alliance e2695 separations module and a Waters® 2489 dual-wavelength absorbance detector with Waters Empower™ software (Milford, MA) was used. Samples were injected by an autosampler onto an Agilent Zorbax® 300SB-C8 column (3.5 μm, 4.6 × 150 mm) with Phenomenex SecurityGuard™ C8 cartridge (5 μm, 4 × 3.0 mm). Gradient elution was employed with mobile phase consisting of acetonitrile (A) and 50 mM potassium phosphate buffer pH = 5.7 (B) at a flow rate of 1 mL/min to elute buprenorphine at 6.7 min. The initial gradient conditions were 35% (A) for 8 min, increased to 65% for 9 min and then lowered to 35% for 3 min. The higher organic percentage after 9 min of the run elutes surfactant with each run thus preventing column accumulation. The wavelength for UV detection of buprenorphine was set at 210 nm. Receiver solution was diluted with mobile phase in a 1:1 (v/v) ratio. The diluted sample (20 µL) was injected onto the column. The calibration standard samples ranged from 0.05 to 10 μg/mL. The method was precise with intra- and inter-day variation of less than 8% and with accuracy between 96 to 105% for all quality control samples and for lower limit of quantification.
IVIVC Approaches
Two approaches were used to evaluate IVIVC. First approach towards IVIVC was to compare the heat induced enhancement ratios between in vitro and in vivo data [1, 22]. Heat ratios were calculated three different ways resulting in three sets of ratios. First set of heat ratio was calculated by dividing the peak value (flux in vitro with Cmax in vivo) for heat arm with corresponding value (at the same time point) in baseline arm. Second set of heat ratio was calculated using partial cumulative amount permeated (in vitro) or partial AUC (in vivo) in the heat study arm divided by the baseline arm value calculated for the same time period (24 h – 36 h on day 2 and 72 h – 84 h on day 4). Third set of heat ratio was calculated by dividing the peak value (flux in vitro with Cmax in vivo) for heat arm with value prior to application of heat in the same study arm (at 24 h on day 2 and at 72 h on day 4). Heat effect window was defined as time period spanning from the beginning of heat application to the time point in which elevated plasma drug levels returned back to baseline plasma levels. Even though heat was applied over a period of 7 h both on day 2 and day 4 in vivo, the heat effect window was defined to be 12 h for both heat application periods, spanning 24 h – 36 h on day 2 and 72 h – 84 h on day 4. For the first and second set of heat ratio, donor 2 from the in vitro study was excluded from the calculation because of the higher flux observed for baseline arm.
Second approach towards IVIVC was to obtain a point-to-point prediction (Level A IVIVC) of the entire plasma concentration profile for both the baseline and heat study arms using the UIR based deconvolution method [1, 20, 29]. Intravenous (IV) PK data for buprenorphine was obtained from literature using a plot digitizer tool and used to calculate unit impulse response (UIR) values [29]. Transdermal PK data for the baseline study arm was obtained from Clinical Pharmacology and Biopharmaceutics Review(s) document for Butrans® using a plot digitizer tool [14]. This transdermal PK data was deconvoluted using the calculated UIR values to obtain in vivo fraction absorbed into systemic circulation (Fa) based on the total drug amount present in the Butrans®. Published IV PK data for buprenorphine seen in Fig. 3 was best described by a two-compartment PK model using the following bi-exponential equation:
Following are the parameter estimates for the above equation:
-
A = 161.82 (units: ng/mL)
-
B = 45.50 (units: ng/mL)
-
Alpha = 5.93 (units: h.−1)
-
Beta = 0.38 (units: h.−1)
Based on these parameter estimates, UIR values were calculated as follows:
-
A1 = A/stripping dose (units: mL.−1)
-
A2 = B/stripping dose (units: mL.−1)
-
Alpha1 = Alpha (units: h.−1)
-
Alpha2 = Beta (units: h.−1)
Stripping dose is the IV bolus dose of buprenorphine (12 mg). Fa was obtained from numerical deconvolution. Fraction permeated in vitro (Fp) for each donor was calculated by dividing the cumulative amount of drug permeated at time (t) by the total amount of drug present in the Butrans® section used in a single diffusion cell. Mean Fp from four donors was obtained. Fa and Fp data were used to construct an IVIVC model. The correlation between Fa and Fp was developed. The correlation was best described by the following polynomial equation with a R2 value of 0.9995 (Fig. 3).
An internal validation was conducted using the above established correlation. To perform the internal validation of the established correlation, Fa was predicted using the correlation, then convoluted using numerical UIR based method to obtain predicted baseline plasma drug concentrations. Two heat enhancement factors (Hv: heat factor obtained from in vivo data (equation # 2) and Hr: heat factor obtained from in vitro data (equation # 3) were introduced into the calculations to obtain predicted concentration following application of transient heat [1]. The following relationships were used:
where, n = 19 or 20 volunteers for in vivo study
where, n = 4 replicates per donor for in vitro study. Hr was calculated for each donor separately after which, mean Hr for four donors (n = 4) was obtained. For donor 2, the flux values during the early heat window were divided by the flux value at 24 h for each study arm. Hr for donor 2 was then calculated. This approach was followed to normalize for the increased flux in the baseline arm. The predicted baseline concentration was multiplied by either mean Hr or Hv to obtain predicted heat study arm concentration. The use of heat enhancement factor was restricted to the time points within the heat effect window only.
The %PE was calculated for predicted AUC and Cmax values using the following formula [28]:
Data Analysis
Statistical analysis was performed using GraphPad Prism® software version 7.0 (La Jolla, CA). Differences in mean flux and cumulative amount were compared using Student’s t-test. Statistical significance was declared at p < 0.05. Phoenix WinNonlin® software version 7.0 (Princeton, NJ) was used to obtain UIR values from IV bolus PK data for buprenorphine and to perform numerical deconvolution and convolution of data. GraphClick, a graph digitizer software (Arizona Software; LA, California) was used to digitize the in vivo concentration versus time profiles for Butrans® and IV bolus dose.
Results
IVPT on Human Skin
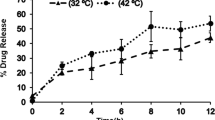
Dermatomed human skin from four different donors was used to perform IVPT. Mean flux profile for the four donors is shown in Fig. 1A. The baseline study arm demonstrates a pseudo zero order release profile of buprenorphine. In the heat study arm, the skin was maintained at 42 ± 1 °C over a duration of 7 h on day 2 and day 4 to mimic elevated skin temperature conditions attained due to the heating pad used in the clinical study. The heat study arm shows an increase in flux values during the duration of heat application on day 2 and day 4. This increase in flux induced by elevated temperature persists even after removal of heat for another 5 h on both day 2 and day 4. The 7 h of heat application plus 5 h of enhancement observed post heat removal constitutes 12 h of observed heat effect and was defined as the heat effect window. At elevated skin temperature, an initial rapid rise in flux is followed by a slow decline after heat removal and flux levels falls below respective baseline profiles.
(A) Flux profile for Butrans® (mean ± SEM (n = 4 human skin donors, 4 replicates/donor) from IVPT data. (B) In vivo concentration versus time profile obtained from the Clinical Pharmacology and Biopharmaceutics Review document for Butrans® available at Drugs@FDA (n = 19 or 20 volunteers) [14]
In vitro flux at steady state (Jss) can be compared to in vivo steady state concentration (Css) using the following equation:
where Css is the predicted steady state concentration (pg/mL), Jss is the steady state flux from each donor (at 72 h), A is area of the TDS (12.5 cm2), and CL is the population total body clearance of buprenorphine (55 L/h) obtained from the product label [13]. Estimated mean Css from IVPT is 84.09 ± 32.46 pg/mL. Observed mean Css in vivo is 154 pg/mL obtained from concentration at 72 h for baseline profile in Fig. 1B. High lipophilicity of buprenorphine along with the inherent variability in human skin might contribute to the lower flux values in vitro. Another factor to consider is the CL value used is the population clearance and may not represent the actual clearance for the study population.
IVIVC
In vitro parameters Jmax, and cumulative amount and in vivo parameters Cmax and partial AUC were calculated for the period spanning the heat effect window. For approach one, Table I summarizes the three sets of calculated heat induced enhancement ratios in vitro and in vivo on day 2 (early heat) and day 4 (late heat). The p-values were obtained from comparison of values in the heat arm and baseline arm for either early or late heat effect window. The mean Jmax values observed during the early and late heat effect window were significantly higher than the corresponding baseline values. This observation stands true for both the first and third sets heat ratios. Mean cumulative amount of drug permeated during the early heat effect window is significantly higher than the corresponding value in the baseline arm while no significant difference was found between the values during the late heat window. The enhancement in Jmax and cumulative amount of drug permeated during the early heat is significantly greater compared to the late heat window (Fig. 2). The in vitro data from four donors showed an 89% and 25% increase in Jmax during early and late heat window, respectively. It showed a 49% and 4% increase for amount of drug permeated during early and late heat window, respectively. With the in vivo study, 100% (early heat window) and 37% (late heat window) increase in peak levels and 63% (early heat window) and 19% (late heat window) increase in AUC were observed (Fig. 3).
Comparison of in vitro heat enhancement ratios for each individual donor calculated as Set I, n = 3 donors (A) Set II, n = 3 donors (B), and Set III, n = 4 donors (C) during early versus late heat window. Each ratio per donor was calculated and then averaged across n = 3 or 4 donors. Donor 2 values were not included for calculation of p value for Set I and II due to differences in flux values between baseline and heat arm before 24 h time point
(A) In vivo concentration versus time profile obtained from Huestis et al. [29]. Intravenous Buprenorphine and Norbuprenorphine Pharmacokinetics in Humans for 12 mg IV bolus dose of buprenorphine (n = 5 volunteers). (B) Level A correlation plot for Fa versus Fp
Approach two for IVIVC using deconvolution method was able to achieve a point-to-point prediction of plasma concentrations for baseline study arm as shown in Fig. 4A. Figure 4B shows the predicted concentrations for heat study arm using both Hv and Hr. Table II describes the predicted Cmax and AUC values and the calculated %PE. The IVIVC model was able to adequately predict the in vivo Cmax and AUC values shown in Table II with each individual %PE being less than 20%. Table III describes the predicted partial AUC values and the calculated %PE for heat arm. The partial AUCs for heat effect window corresponding to 7 h of intermittent heat application (AUC24-31 and AUC72-79) and 5 h of post heat application period (AUC31-36 and AUC79-84) were adequately predicted when IVIVC model was used with Hv.
Discussion
The effect of an elevated external temperature at the skin surface on buprenorphine absorption from Butrans® was evaluated in healthy volunteers as part of the manufacturer’s drug development process. The heat application resulted in an increase in systemic concentrations of buprenorphine. A warning against exposure of Butrans® to a heating pad or to other external heat sources, resulting in a potential increase in the BA of buprenorphine and hence a possibility of overdose and death, was incorporated into the product label [14].
Such clinical trials are expensive, time consuming, and also put human volunteers at risk of exposure to higher drug levels. Hence it is necessary to explore other surrogate methods that may be helpful for comparative evaluation of the effect of external heat on TDS. Ex vivo human abdominal skin model when used for IVPT studies have been shown to retain most of its barrier integrity over an eight-day period unlike mouse and snakeskin models [23, 24]. IVPT experiments have been shown to be predictive of in vivo PK data for dermally applied formulations. Successful demonstration of IVIVC was seen in several cases. Importance of study design harmonization between in vitro and in vivo studies has been emphasized for establishing good IVIVC [1, 22, 25]. Our IVPT study design was harmonized to match the clinical study design with respect to duration of heat application and sampling time points [14]. When exposed to an elevated temperature in vitro, under conditions that closely matched the in vivo study conditions, Butrans® exhibited an increase in the rate and extent of drug delivery relative to its baseline drug delivery at normal (32 ± 2 °C) skin temperature conditions. This increase in rate and extent of drug delivery is reflected in the increase in flux (Jmax) and cumulative amount of drug permeated. The in vitro enhancement in Jmax and cumulative amount of drug permeated observed during early and late heat effect window for each of the four human skin donors is predictive of mean enhancement in Cmax and AUC seen in vivo (Table I). Donor 2 was excluded from calculation of early heat ratio Set I and II in Table I due to differences in flux values between baseline and heat arm before heat application at 24 h time point. Such differences in flux values within the same skin donor may be caused by the inherent variability in human skin. This difference is expected to significantly influence the calculation of ratio Sets I and II where, the baseline flux values are used to divide the heat arm flux values, thereby confounding the analysis for heat induced enhancement ratios in this small dataset within the scope of the research study.
The elevated rate of buprenorphine delivery through the skin did not return to baseline levels until several hours after the external heat source was removed (Fig. 1). This observation was made in vivo as well as in vitro. It seems that the effect of heat on drug delivery persisted even after removal of applied heat for a total duration of 12 h. This prolonged heat effect might be a result of the following contributing factors; buprenorphine is a highly lipophilic compound with a log P > 4 and lipophilic compounds tend to form a reservoir in the skin from which they are slowly cleared [26]. Lipids in skin undergo phase transition at around 40 °C which contributes to enhanced permeation of the compound [27]. The duration of intermittent heat application in this study spans a total of 7 h, which is longer than other studies that evaluated effect of transient heat application TDSs [1, 17, 22]. Extended exposure to heat together with the lipophilic nature of buprenorphine and the skin lipid changes during heat exposure may have caused prolongation of flux enhancement post heat removal.
At the end of early heat effect window, buprenorphine levels reach baseline levels after which, it continues to fall further than corresponding baseline levels (at the same time point). This dip in the profile post early heat effect window is not drastic and soon reaches steady levels and is captured in the IVPT flux profile as well. The drug levels increase to and then pass baseline levels only after application of heat on day 4. As a result, the heat induced enhancement in Jmax or Cmax and AUC or cumulative amount of drug permeated calculated relative to the baseline during the late heat effect window is significantly lower than that during early heat effect window (Fig. 2). In this study, where both early and late heat exposure was on the same skin, it can be concluded that application of heat early during the wear period of Butrans® results in greater heat induced enhancement in buprenorphine permeation. The flux result from the late heat in this study could be influenced by the early or first heat application. However, in a case where early and late heat exposure were on two separate skin, the earlier conclusion might not stand true. In a previous study by our group, the effect of controlled heat on two nicotine TDSs was evaluated by early and late heat exposure on two separate donor’s skin and no difference in heat induced enhancement in Cmax and AUC was observed between early versus late heat exposure [1].
Level A IVIVC allows point-to-point prediction of the PK profile. Current literature has limited examples of Level A IVIVC for TDSs. Level A IVIVC for estradiol TDS was reported but only under normal skin temperature conditions [20]. Two different approaches were considered towards establishing Level A IVIVC for nicotine TDSs under exposure to external heat source [1]. IVIVC for three fentanyl TDSs was explored using multiple approaches to evaluate the effects of heat on enhancement of fentanyl BA [22]. In this study, IVIVC was achieved using a numerical deconvolution approach under both baseline as well as elevated skin temperature conditions. The %PE in Cmax and AUC values for the baseline study arm was less than 20% (Table II). Two different heat factors were used for prediction of concentrations for the heat study arm. The in vivo heat factor gives more accurate prediction for Cmax values than the in vitro heat factor. This can be attributed to the inherent differences in in vitro versus in vivo experimental conditions such as method of skin temperature manipulation, thermoregulation, and vasodilation. The partial AUCs for heat effect window corresponding to 7 h of intermittent heat application (AUC24-31 and AUC72-79) and 5 h of post heat application period (AUC31-36 and AUC79-84) were adequately predicted when IVIVC model was used with in vivo heat factor (Table III). The use of heat enhancement factor (Hv and Hr) was restricted to the time points within the 12 h heat effect window only. After the 12 h heat effect window, our IVIVC approach assumes that the heat arm profile is similar to baseline profile. Hence the dip in observed heat arm profile seen after the 12 h heat effect window is not captured in the predicted profiles and is a limitation of our IVIVC approach for heat arm. This causes an over-prediction of partial AUC36-72 and AUC84-168 (Table III).
Current guidance for industry for development of a Level A IVIVC for extended-release oral dosage forms recommends evaluation of both internal and external predictability of IVIVC for complete evaluation and full application of the IVIVC [28]. The %PE was only estimated internally within the scope of the current work to evaluate how well the model describes the data used to define the IVIVC. There are no available guidelines to establish IVIVC for formulations applied to the skin. Guidelines for oral formulations require the individual %PE to be less than 20% [28]. Accounting for variability between the in vitro and in vivo study populations, the in vivo plasma PK profile of buprenorphine predicted based upon our IVPT study results compares well with the observed in vivo results and thus demonstrates good predictability internally. This indicates that an IVIVC can be established for Butrans®, both, under normal temperature conditions and when Butrans® is exposed to an elevated temperature. However, we acknowledge the limitation of our current analysis in estimation of %PE externally which relates to how well the model predicts data when data sets are used that differ from those used to define the correlation.
Conclusions
Exposure to external sources of heat can increase the rate and extent of drug delivery from Butrans®. Delivery of buprenorphine increased throughout the period of heat application and remained elevated for 5 h after removal of heat both in vitro and in vivo. Heat induced enhancement in buprenorphine delivery observed in vivo can be mimicked in vitro when IVPT study designs are harmonized to the in vivo clinical study design. Predictions of Cmax and AUC under both baseline as well as elevated temperature conditions were possible based on a Level A IVIVC established using both in vitro and in vivo data. The results suggest that IVPT studies performed under the same conditions as those of interest in vivo may be useful for comparative evaluation of the effect of external heat on TDS. Further research may be warranted to evaluate factors, beyond cutaneous BA assessed using an IVPT study, that can influence plasma exposure in vivo for a given drug product.
Abbreviations
- AUC:
-
Area under curve
- BA:
-
Bioavailability
- CHADD:
-
Controlled heat-aided drug delivery
- CHTN:
-
NCI Cooperative Human Tissue Network
- Cmax :
-
Maximum concentration
- Fa:
-
Fraction absorbed
- Fp:
-
Fraction permeated
- HPLC:
-
High performance liquid chromatography
- Hr:
-
In vitro Heat factor
- Hv:
-
In vivo Heat factor
- IV:
-
Intravenous
- IVIVC:
-
In vitro – In vivo correlation
- IVPT:
-
In vitro permeation test
- Jmax :
-
Maximum flux
- logP:
-
Logarithm of octanol–water partition coefficient
- %PE:
-
Percent Prediction Error
- PK:
-
Pharmacokinetics
- TDS:
-
Transdermal delivery system
- TEWL:
-
Transepidermal water loss
- UIR:
-
Unit impulse response
References
Shin SH, Thomas S, Raney SG, Ghosh P, Hammell DC, El-Kamary SS, Chen WH, Billington MM, Hazem HE, Stinchcomb AL. In vitro – in vivo correlations for nicotine transdermal delivery systems evaluated by both in vitro skin permeation (IVPT) and in vivo serum pharmacokinetics under the influence of transient heat application. J Control Release. 2018;270:76–88.
Petersen KK, Rousing ML, Jensen C, Arendt-Nielsen L, Gazerani P. Effect of local controlled heat on transdermal delivery of nicotine. Int J Physiol Pathophysiol Pharmacol. 2011;3(3):236–42.
Grissinger M. Fentanyl transdermal patches: more protection needed for patients and their families. P T. 2009;34(7):343–90.
Ashburn MA, Ogden LL, Zhang J, Love G, Basta SV. The pharmacokinetics of transdermal fentanyl delivered with and without controlled heat. J Pain. 2003;4(6):291–7.
Barkve TF, Langseth-Manrique K, Bredesen JE, Gjesdal K. Increased uptake of transdermal glyceryl trinitrate during physical exercise and during high ambient temperature. Am Heart J. 1986;112(3):537–41.
Klemsdal TO, Gjesdal K, Bredesen JE. Heating and cooling of the nitroglycerin patch application area modify the plasma level of nitroglycerin. Eur J Clin Pharmacol. 1992;43(6):625–8.
Shomaker TS, Zhang J, Ashburn MA. Assessing the impact of heat on the systemic delivery of fentanyl through the transdermal fentanyl delivery system. Pain Med. 2000;1(3):225–30.
Shomaker TS, Zhang J, Ashburn MA. A pilot study assessing the impact of heat on the transdermal delivery of testosterone. J Clin Pharmacol. 2001;41(6):677–82.
Marriott TB, Charney MR, Stanworth S. Effects of application durations and heat on the pharmacokinetic properties of drug delivered by a lidocaine/tetracaine patch: a randomized, open-label, controlled study in healthy volunteers. Clin Ther. 2012;34(10):2174–83.
Hao J, Ghosh P, Li SK, Newman B, Kasting GB, Raney SG. Heat effects on drug delivery across human skin. Expert Opin Drug Deliv. 2016;13(5):755–68.
Ling W. Buprenorphine implant for opioid addiction. Pain Manag. 2012s;2(4):345–50.
Pharmacology - Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction - NCBI Bookshelf. 2018. pp. 1–13.
Butrans® Patch [package insert]. Purdue Pharma, Parsipanny, NJ; 2009. (http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022122s010lbl.pdf). Accessed 01 Jun 2022
Clinical Pharmacology and Biopharmaceutics Review(s) for Butrans®. (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/021306Orig1s000ClinPharmR.pdf) Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Application number 21–306, September 2011.
Thomas S, Shin SH, Hammell DC, Hazem HE, Stinchcomb AL. Effect of controlled heat application on topical diclofenac formulations evaluated by in vitro permeation tests (IVPT) using porcine and human skin. Pharm Res. 2020;37:49.
Prodduturi S, Sadrieh N, Wokovich AM, Doub WH, Westenberger BJ, Buhse L. Transdermal delivery of fentanyl from matrix and reservoir systems: Effect of heat and compromised skin. J Pharm Sci. 2010;99(5):2357–66.
Shin SH, Ghosh P, Newman B, Hammell DC, Raney SG, Hassan HE, Stinchcomb AL. On the road to development of an in vitro permeation test (IVPT) model to compare heat effects on transdermal delivery systems: Exploratory studies with nicotine and fentanyl. Pharm Res. 2017;34:1817–30.
Chattaraj SC, Kanfer I. Release of acyclovir from semi-solid dosage forms: a semi-automated procedure using a simple plexiglass flow-through cell. Int J Pharm. 1995;125:215–22.
Akomeah F, Nazir T, Martin GP, Brown MB. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci. 2004;21:337–45.
Yang Y, Manda P, Pavurala N, Khan MA, Krishnaiah YSR. Development and validation of in vitro–in vivo correlation (IVIVC) for estradiol transdermal drug delivery systems. J Control Release. 2015;210(C):58–66.
Lehman PA, Raney SG, Franz TJ. Percutaneous absorption in man: in vitro-in vivo correlation. Skin Pharmacol Physiol. 2011;24:224–30.
Shin SH, Yu M, Hammell DC, Ghosh P, Raney SG, Hassan HE, Stinchcomb AL. Evaluation of in vitro/in vivo correlations for three fentanyl transdermal delivery systems using in vitro skin permeation testing and human pharmacokinetic studies under the influence of transient heat application. J Control Release. 2022;342:134–47.
Bond JR, Barry BW. Limitations of hairless mouse skin as a model for in vitro permeation studies through human skin: hydration damage. J Invest Dermatol. 1988;90(4):486–9.
Rigg PC, Barry BW. Shed snakeskin and hairless mouse skin as model membranes for human skin during permeation studies. J Invest Dermatol. 1990;94(2):235–40.
Ghosh P, Milewski M, Paudel K. In vitro/in vivo correlations in transdermal product development. Ther Deliv. 2015;6(9):1117–24.
Rougier A, Dupuis D, Lotte C, Roguet R, Schaefer H. In vivo correlation between stratum corneum reservoir function and percutaneous absorption. J Invest Dermatol. 1983;81:275–8.
Lawson EE Angbogu, AN, Williams AC Barry BW Edwards HG. Thermally induced molecular disorder in human SC lipids compared with a model phospholipid. Spectrochim Acta A Mol Biomol Spectrosc. 1998;54A(3):543–58.
FDA Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070239.pdf) U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), September 1997.
Huestis MA, Cone EJ, Pirnay SO, Umbricht A, Preston KL. Intravenous buprenorphine and norbuprenorphine pharmacokinetics in humans. Drug Alcohol Depend. 2013;131(3):258–62.
Acknowledgements
Biospecimens were provided by the NCI funded CHTN. Other investigators may have received specimens from the same tissue specimens. The CHTN is comprised of six academic institutions who collect and distribute remnant human biospecimens from routine surgical and autopsy procedures to investigators for basic and applied science to advance biomedical research.
Funding
This publication was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [FAIN] (Research Award U01FD004955) totaling $2,699,996 with 100 percent funded by FDA/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The Authors declare no financial conflict of interest for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, S., Hammell, D.C., Hassan, H.E. et al. In Vitro—In Vivo Correlation of Buprenorphine Transdermal Systems Under Normal and Elevated Skin Temperature. Pharm Res 40, 1249–1258 (2023). https://doi.org/10.1007/s11095-023-03487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03487-z