Abstract
Lipids and lipoproteins are a diverse group of substances and their interactions with the blood-brain barrier (BBB) is similarly diverse. Some lipoproteins such as high density lipoprotein (HDL), apolipoprotein (apo) A-I, apoJ, some free fatty acids, and triglycerides cross the BBB whereas others such as apoE do not. Some forms of cholesterol can cross the BBB and others do not. Lipids can have effects on BBB preservation and function: HDL may protect the BBB during multiple sclerosis, cholesterol can disrupt the BBB, and triglycerides inhibit the transport of leptin across the BBB and the activation of the hypothalamic leptin receptor. ApoE is associated with many effects on the BBB, with the specific isoform apoE4 having detrimental effects. In summary, the diverse ways in which lipids, lipoproteins, and apolipoproteins interact with the BBB is important in both health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The brain is one of the most lipid-rich organs in the body. Whether those lipids and their related molecules are derived in part or whole from outside the central nervous system (CNS) depends on their ability to cross the blood-brain barrier (BBB). The best studied component of the BBB is the vascular BBB which consists of the capillary bed and the immediately adjacent arterioles and venules. Small, lipid soluble molecules can readily cross the membranes which form the BBB. Lipids, lipoproteins, and apolipoproteins that can cross the BBB do so largely by using saturable transporters. Here, we give an overview as to what is known about these transport systems and their ligands.
Interactions of lipids, lipoproteins, and apolipoproteins include actions not readily appreciated in other tissues. For example, many functions of the BBB, including transporters, transcytosis activity, and even BBB integrity are affected by triglycerides, apolipoproteins, and cholesterol, respectively (Fig. 1). Once in brain, triglycerides and/or their derivative free fatty acids (FFAs) can affect CNS receptor functions. These and other aspects of interactions with the BBB are reviewed below.
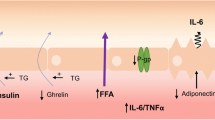
Lipids can have both direct and indirect effects on the BBB. Direct effects include a) BBB transport of lipids. Some lipids, such as free fatty acids (FFA), triglycerides and high-density lipoprotein (HDL), and certain apolipoproteins (apo) such as apoJ and apoA-I (green symbol), can be transported across the BBB (solid lines) while apoE (maroon symbol) and cholesterol in their free form are unable to be transported across the BBB (dotted line). b) However, these serum lipids can also act on receptors at the BBB, which could induce transport as is the case for FFA or elicit intracellular signaling pathways, as occurs for apoE. Some of these interacting receptors are known, like MFSD2A and LRP1, yet there are still others that are likely unknown at this time. c) Lastly, serum lipids including apoE, cholesterol, and FFA can affect BBB permeability. Serum lipids can also have indirect affects at the BBB. d) The most classic example is that triglycerides are known to decrease or inhibit (dotted line) the transport of leptin (blue spheres) across the BBB, while insulin and ghrelin (green and red spheres) transport is increased (solid line). The interactions of lipids at the BBB have not really been separated for luminal versus abluminal impact and remains to be explored. Figure generated at www.biorender.com.
Transport of FFA, Cholesterol, and Triglycerides, across the BBB
Free Fatty Acid (FFA) Transport across the BBB
Fatty acids are a major component of lipids and are an important dietary source of fuel as well as an important structural component for cells. Fatty acids are formed from carbohydrates primarily in the liver and adipose tissue. There are multiple types of fatty acids, classified by saturation, carbon content, or branching. Circulating fatty acids are known as free fatty acids (FFA).
As with many substances, FFA were once assumed to not cross the BBB. Work in the 1970’s to 1990’s as reviewed elsewhere (1) with radioactive ligands showed that a number of FFA, including arachidonate (AA), docosahexaenoate (DHA), and palmitate, were rapidly taken up from blood by brain by saturable transporters and incorporated into brain lipids. Oldendorf reported a transporter for monocarboxylic acids that was optimal for octanoate and did not transport the larger FFA (2). In blood, FFA are about 99.99% bound to plasma proteins, but the free fraction crosses the BBB very rapidly (1). Rapid transport across the BBB of the unbound fraction, a rapid replenishment of the unbound fraction from the very large pool of bound FFA, and a brain-to-blood efflux system for FFA not incorporated into brain tissue combine in a way so that the brain has an unlimited source of FFA on an as needed basis without having to transport or retain large amounts of FFA. This also means that the rate of FFA transport reflects the metabolic demand by the brain (1). For example, AA and DHA are involved in membrane signal transduction and so stimulation with the M1 cholinergic agonist arecoline results in an increased uptake of AA and DHA by regions of the brain where the M1 receptor resides (3).
Later studies have discovered transporters for FFA. Major facilitator superfamily domain-containing protein 2 (Mfsd2a) is a major transporter for DHA and lysophophatidylcholines. Once DHA-lysoPC reaches the BBB, it detaches from albumin and binds to Mfsd2a, which then transfers DHA to the inner membrane leaflet of endothelial cells lining the BBB, allowing it to bypass the tight junctions. Additionally, a class of membrane transporters, fatty acid transport proteins (FATP) 1, 4, and 5as well as fatty acid translocase (CD36) have been located in brain microvascular endothelial cells and shown to play a role in DHA BBB transport (4,5,6). The apolipoprotein E4 (apoE4) receptor, low density lipoprotein receptor (LDLR) has been reported to be another transporter for DHA (7).
Lower levels of brain DHA have been linked with Alzheimer’s disease (8) and apoE4 carriers respond well to increased intake of DHA (9). Decreased DHA BBB transport could contribute to lower CNS DHA levels. Mfsd2a can also regulate the lipid composition of brain endothelial cells, affecting the structure and function of the BBB, playing a critical role in BBB homeostasis (10). Loss of this transporter in mice results in cognitive deficits (11) and BBB permeability (10).
Cholesterol Transport across the BBB
Not only is cholesterol an important component of cell membranes, but it is also a building block of many steroid hormones. In the brain, cholesterol is an important component of myelin sheaths that surround axons of neurons to enhance neurotransmission. The brain is the most cholesterol-rich organ in the body, containing 25% of the body’s cholesterol, yet only a select group of CNS cells (astrocytes and oligodendrocytes) can synthesize cholesterol in the adult brain (12). Cholesterol synthesis in the brain is greatest during development and efficient recycling occurs in adulthood, with a half-life lasting up to 5 years (13).
Cholesterol synthesis in the brain is the primary source for CNS cholesterol. While free cholesterol cannot cross the BBB, cholesterol present on lipoproteins and modified oxysterols may be able to traverse the BBB. In addition to triglycerides, cholesterol is a component of lipoproteins. The scavenger receptor class B type I (SR-BI) aids in the flux of cholesterol between lipoproteins, particularly LDL and HDL, and cell membranes at the BBB (Balazs J Neurochem 2004). Lipoprotein receptors are able to mediate transcytosis of lipoproteins, expanded on below in section 3. It was first shown that lipoproteins can cross the BBB in Drosophila (14).
Cholesterol can also be converted into hydroxycholesterol, both in the brain and the periphery. Hydroxycholesterol in the CNS has been shown to modulate neuron function and plays a controversial role in Alzheimer’s disease (15). When converted in the periphery, hydroxycholesterol can cross the BBB (16). Additionally, hydroxycholesterol generated in the CNS can also be transported across the BBB, from brain to blood by the organic anion transporting polypeptide 2 (OATP2) (17). This process is saturable and specific for hydroxycholesterol as it does not transport cholesterol. Once transported into the brain, hydroxycholesterol can act to regulate brain cholesterol metabolism. The possibility of cholesterol transport at the choroid plexus has been recently reviewed (18). Ultimately, cholesterol transport across the BBB can occur, but CNS synthesis is apparently the main source of cholesterol for brain.
Triglyceride Transport across the BBB
Triglycerides consist of a combination of three fatty acids attached to a glycerol backbone. They are synthesized by esterification in the endoplasmic reticulum of cells in the adipose tissue and liver. They are the main component of adipose tissue and are most known by the saturation status: saturated or unsaturated. Triglycerides are present in blood, shuttling fuel from adipose tissue to muscles or following a meal prior to storage.
As discussed further below, circulating triglycerides inhibit leptin transport across the BBB, while stimulating transport of insulin and ghrelin (19). This suggests that triglycerides, which are elevated in starvation, would inhibit leptin’s anorectic effects and promote ghrelin-related feeding.
Triglycerides were recently shown to cross the murine BBB rapidly after their intravenous administration and accumulate over time (20). This likely explains several effects of circulating triglycerides, including their ability to affect levels of hypothalamic feeding hormones, to impair cognition, and to modulate brain microglial activity (21,22,23). Triglycerides in the murine CSF are low with a level of about 0.65 mg/dl, just 0.6% of blood levels. CNS triglycerides impair activation of both the insulin and leptin receptors within the hypothalamus and block the anorectic effect of CNS leptin (20). Thus, triglycerides in the CNS have regulatory properties on brain and BBB function. Due to the composition of triglycerides and enzymes present within the CNS, transport of triglycerides across the BBB can also be a source for CNS FFA.
.
Effects of Lipids on Hormonal Transport and Other BBB Functions
Lipids are known to affect BBB structure and function. First, while some lipids of various forms are able to cross the BBB, they are also able to regulate transport of other substrates across the BBB. While the exact mechanisms are unknown, it could be postulated that lipids alter the brain endothelial cell lipid bilayer, which inadvertently alters the transport proteins present on the luminal surface. In addition, lipids, likely by a similar mechanism, are also able to affect BBB structure and permeability (Fig. 1). Specific examples of these two regulatory processes are described next.
Triglyceride Effects on Peptide Transport
Not only has triglyceride transport across the BBB been investigated as described above, but the impact of circulating triglycerides on BBB peptide transport, including leptin, insulin, and ghrelin has also been explored (Fig. 1). This regulation of hormonal BBB transport has huge implications within the CNS as they act within the brain to regulate feeding behavior, amongst many other roles, but must first navigate the BBB. Leptin and insulin act within the brain to suppress appetite while ghrelin is orexogenic, stimulating food intake. Therefore, it has been proposed that triglycerides can act on BBB transport to also mediate energy intake. For example, it is thought triglycerides block leptin transport from blood into the hypothalamus to suppress appetite. In addition, during periods of starvation, triglyceride levels increase, thereby inhibiting the effect of leptin on suppressing food intake.
Indeed, intravenous injection of triglycerides, and not FFA, decreases the amount of leptin transport across the BBB (19). Conversely, in obese, starved mice, triglycerides increase the amount of insulin transport across the BBB (24). These differences between leptin and insulin transport highlight the differences in regulation of these two transport systems. Similar to insulin, triglycerides enhanced transport of ghrelin across the BBB (25). Clearly, circulating triglycerides can play a critical role in the regulation of BBB hormone transport.
Additionally, circulating triglycerides can affect hypothalamic levels of particular orexigenic peptides that elicit a feeding response (21). Since serum glucose, insulin, and leptin levels were unaffected, these data suggest triglycerides can act on the BBB to alter CNS signaling independent of serum hormone levels. There are three possibilities of how triglycerides could elicit a CNS effect independent of serum insulin and leptin levels: 1) the triglycerides are metabolized and the metabolites cross the BBB to act within the CNS, 2) the triglycerides interact with the BBB directly to elicit a signaling process within the CNS similar to adiponectin, or 3) the triglycerides are affecting circulating levels of some unknown peptide or protein that can cross the BBB and act within the CNS. Whatever the mechanism, it is clear there are many unanswered questions regarding how peripheral triglycerides interact with the BBB to affect CNS signaling processes.
Lipid Regulation of BBB Transcytosis and BBB Disruption
Brain endothelial cell membranes have a unique composition of lipids that helps contribute to BBB functionality. These cells are inherently known to have low levels of vesicle trafficking. Vesicle trafficking, or transcytosis, when it does occur, is often clathrin-mediated, although caveolae-mediated transcytosis is also known. As mentioned above, Mfsd2a transports lipids, which helps create a unique lipid composition of brain endothelial cells that limits caveolae-mediated transcytosis (26).
However, lipid membrane composition requires a proper balance of dietary lipids, in order to aid in BBB function, without disrupting the structure of the BBB. In pre-clinical studies, a diet rich in cholesterol and saturated fatty acids (SFA) can lead to BBB disruption to plasma proteins and neuroinflammation (27, 28), with some studies reporting alterations to tight junction proteins (29) (Fig. 1). Statins have been shown to prevent this increase in BBB permeability, improving the integrity of the BBB (27, 28). Whether these findings can be extended into humans is currently unknown.
It is thought that the BBB disruption due to excess intake of cholesterol and SFA can impact the transport of amyloid β into the brain, thus having implications in Alzheimer’s disease (30). The cholesterol-lowering agent, probucol, has been shown to prevent the BBB disruption due to cholesterol and SFA, without affecting circulating lipid levels (31). On the other hand, diets rich in unsaturated fats such as DHA, seem to promote BBB integrity (30), protecting individuals from Alzheimer’s disease. Indeed, Mediterranean diets, as well as other diets low in saturated fatty acids, are known to protect against cognitive impairment and reduce the risk for developing Alzheimer’s disease (32).
Apolipoproteins (apo) are also known to regulate BBB permeability. The most widely studied lipoprotein on BBB permeability is apoE. Bell et al. in 2012 found that apoE knock-out mice exhibited an increase in BBB permeability (33). Additionally, apoM knock-out mice have an increase in BBB permeability depending on the type of blood vessel (34). That is, arterioles have disruption, but capillaries and venules are unchanged. Activation of the G protein coupled receptor signaling protein sphingosine-1-phosphate receptor 1 (S1PR1) was able to prevent the increase in permeability due to loss of apoM. More about the interactions of apolipoproteins with the BBB are discussed next.
Lipoprotein Interactions with the BBB
Lipoproteins are complex particles containing a single lipid layer, arranged so that the outer layer contains the hydrophilic membrane, with a hydrophobic inner core. These particles are the primary delivery route for lipids throughout the body. The outer surface contains apolipoproteins, other proteins, phospholipids, and cholesterol which can interact with cell membranes, including brain endothelial cells.
ApoE and Interactions at the BBB
ApoE is primarily produced by the hepatocytes in the periphery and by astrocytes in the CNS. In addition to regulating BBB structure, apoE secreted by non-brain endothelial cells also has effects on BBB function. ApoE in humans exists in three isoforms: apoE2, apoE3, and apoE4. While apoE3 is the primary isoform expressed in humans, apoE4 is associated with an increased risk for Alzheimer’s disease and has detrimental effects on the BBB compared to the other isoforms (35). The best evidence that apoE does not cross the BBB is in patients undergoing liver transplants, whose post-operative isoform of apoE in blood matched that of the donor liver, whereas the apoE isoform in the CSF remained that of the recipient (36).
ApoE4 expression can impact BBB transport. Specific transport systems that have been investigated include DHA, glucose, and diazepam, all of which are decreased due to apoE4. DHA (specifically DHA, 22:6(n-3)) transport is decreased in mice expressing apoE4 (37). ApoE4 carriers respond well cognitively from fish DHA but not from supplemental DHA. This is likely due to the form of DHA. Natural DHA from fish is primarily in the phospholipid form while supplemental DHA is not (mainly FFA), which affects the metabolism in vivo (9). This suggests DHA in the form of DHA-lysoPC is superior to free DHA as it can cross the BBB.
Additionally, there is evidence in humans that apoE genotype can affect triglyceride BBB transport. A 5-h triglyceride infusion in humans, led to increased CSF levels of lipids, specifically increasing diacylglycerols and lysophosphatidylcholines and reduced dihydroceramides (38). Following the triglyceride infusion, CSF levels of three lipids differed due to apoE genotype status: unsaturated FFA linoleic acid (18:2) and palmitoleic acid (16:1) and diacylglycerol 18:1, suggesting apoE4 status can affect appearance of triglyceride components within the CNS.
Lastly, it has been shown at least in astrocytes that apoE4 can impair endocytosis (39). Probing clathrin-mediated internalization specifically, there was a decrease in the internalized ligands epidermal growth factor and transferrin in apoE4 iPSC-derived astrocytes compared to apoE3. These disruptions occur independent of amyloid β, suggesting a common defect in many diseases of which apoE4 increases the risk. Due to the close interactions between astrocytes and brain endothelial cells, dysregulated endocytosis could have detrimental implications at the BBB.
These data suggest that while apoE does not readily cross the BBB, apoE and its particular isoforms can not only affect the structure of the BBB but can also impact the function of the BBB in altering specific serum substrate BBB transport and generic endocytosis processes.
Actions of the LRPs at the BBB
Low-density-lipoprotein receptors (LDLRs) are present at the BBB and not only contribute to lipoprotein metabolism and cell signaling but also play a role in transporting substrates into and out of the brain (Fig. 1). LDLR family members consist of LDLR, low-density lipoprotein receptor-related protein-1 (LRP1), LRP2, and very-low density lipoprotein receptor (VLDLR) to name a few. LRP1 can bind over 40 structurally diverse ligands, including LDL, apoE and amyloid β (40). LRP1 interactions with apoE vary based on isoform expression (33, 41). Additionally, LRP1 aids in amyloid β efflux from the brain.
LDLRs can be cleaved to release soluble levels of the receptors in serum and interstitial fluid. This shedding can not only impact intracellular signaling but also BBB transport, as only membrane bound protein is able to mediate brain endothelial cell internalization. LDLR and LRP1 are shed more when exposed to apoE4 and amyloid β (42). Statins can increase LRP1 levels at the BBB (43), thereby potentially affecting BBB transport of ligands including amyloid β.
LRP1 can compartmentalize into distinct plasma membrane microdomains, existing both in lipid rafts and clathrin-coated pits. This dynamic distribution is one of the mechanisms proposed for the separation of endocytosis versus mediating intracellular signaling by LRP1 (44). The specific signaling mechanisms of this compartmentalization and the impact on BBB substrate transport and intracellular signaling remains to be determined.
HDL and the BBB
HDL is the smallest of the lipoproteins, ranging in diameter from 5 to 17 nm; by comparison, albumin is about 7 nm in diameter. It is also the densest of the lipoproteins owning to its high ratio of proteins to lipids. It is involved in reverse cholesterol transport, that is, the transport of cholesterol from peripheral tissues to the liver. Two of the most common apolipoproteins in HDL are apolipoprotein A-I and apolipoprotein A-II. However, over 200 proteins have been tentatively associated with HDL and 85 have been consistently so identified (45). Most of these proteins are not related to lipid metabolism, but endow HDL with antioxidant and anti-inflammatory properties, as well as effects on endothelial activation, coagulation, and platelet aggregation. Only 10 proteins have been found to be associated with HDL in the CSF. Therefore, HDL in blood and in the CSF have very different characteristics and possibly different functions. HDL is removed from the blood by HDL receptors, such as scavenger receptor B1 (SR-B1).
In vitro studies using monolayer cultures of human primary BECs show that HDL particles are transported across the BBB (46). Transport was self-inhibited, indicating a saturable transport mechanism, and was dynamin dependent. Although dynamin is usually associated with clathrin-dependent or caveolin-dependent transcytosis, HDL used neither of these mechanisms to cross the BEC. Although uptake was SR-B1 dependent, SR-B1-mediated uptake did not involve the adaptor protein PDZK1. Constitutive nitric oxide may have played a role in HDL transport, but stimulation of nitric oxide did not further enhance HDL transport. Nystatin inhibited HDL transport, suggesting that cholesterol was required for HDL transport, although it should be noted that nystatin has other properties, such as acting as an ionophore (47). In brief, HDL crosses BECs using mechanisms similar to, yet distinct, from that of other endothelial cells.
ApoA-I with a molecular weight of 45.4 kDa is one of the most abundant apolipoproteins found in HDL and also crosses the BBB. An ApoA-I polymorphism is associated with an increased risk for early development of Alzheimer’s disease (48). As reviewed by Wellington et al. (45), blood, CSF, and brain levels of apoA-1 and HDL are lower in AD patients in some, but not all, studies. ApoA-1’s ability to bind to lipopolysaccharide is thought to be a major reason why HDL has anti-inflammatory properties. ApoA-1 along with apoE are the most abundant lipoproteins in the CSF, measuring about 0.37 mg/dl and 0.30 mg/dl respectively (49). Of the 9 apolipoproteins present in CSF, apoA-1 is the only one whose message has not been found in the CNS (50). The presence of apoA-1 in CSF with no source of CNS production is one proof for its ability to cross the BBB. Direct proof was provided by Zhou et al., who showed that radioactively labeled apoA-1 crossed the murine BBB. They further showed that, like HDL, apoA-1 transport was not dependent on clathrin and was inhibited by nystatin (51).
HDL may offer the BBB protection in some conditions. Higher serum HDL and apoA-I levels were associated with less BBB damage in patients with multiple sclerosis (52). HDL levels were also associated with decreased immune cell extravasation into the CNS. An 18 amino acid peptidomimetic of apoA1 which crosses the BBB about 1000 times faster than apoA1 increased the brain-to-blood efflux rate of amyloid β1–42 (53).
ApoJ, or clusterin, is another apolipoprotein in abundance in CSF HDL particles. ApoJ has various associations with AD, including from GWAS studies and an ability to aid in the blood-to-brain transfer of amyloid β. ApoJ crosses the BBB (54), but blood levels are much higher than the Km of its transporter and so it is saturated at physiologic levels (55).
Conclusions
Interactions of the BBB with lipids, lipoproteins, and apolipoproteins are diverse. The BBB prevents the entry of some of these into the CNS while serving to transport others. They interact with the BBB and with the CNS, affecting BBB transport systems, BBB integrity, BBB vesicular activity, and CNS receptor activity. These interactions are important to the normal functioning of the BBB and in some cases contribute to disease states. It is likely that additional interactions between the BBB and these classes of molecules will be discovered in the future.
References
Banks WA, Kastin AJ, Rapoport SI. Permeability of the blood-brain barrier to circulating free fatty acids. In: Yehuda S, Mostofsky DI, editors. Handbook of essential fatty acid biology: biochemistry, physiology, and behavioral neurobiology. Totowa, NJ: Human Press; 1997. p. 3–14.
Oldendorf WH. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol. 1973;224:1450–3.
Jones CR, Arai T, Bell JM, Rapoport SI. Preferential in vivo incorporation of [ 3 H]arachidonic acid from blood into rat brain synaptosomal fractions before and after cholinergic stimulation. J Neurochem. 1996.
Low YL, Jin L, Morris ER, Pan Y, Nicolazzo JA. Pioglitazone increases blood-brain barrier expression of fatty acid-binding protein 5 and docosahexaenoic acid trafficking into the brain. Mol Pharm. 2020;17(3):873–84.
Ochiai Y, Uchida Y, Ohtsuki S, Tachikawa M, Aizawa S, Terasaki T. The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport. J Neurochem. 2017;141(3):400–12.
Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117(4):735–46.
Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–85.
Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. 2016;8(2):99.
Patrick RP. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J. 2019;33(2):1554–64.
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–11.
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–6.
Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103(3):405–13.
Wang H, Eckel RH. What are lipoproteins doing in the brain? Trends Endocrinol Metab. 2014;25(1):8–14.
Brankatschk M, Eaton S. Lipoprotein particles cross the blood-brain barrier in Drosophila. J Neurosci. 2010;30(31):10441–7.
Gamba P, Giannelli S, Staurenghi E, Testa G, Sottero B, Biasi F, Poli G, Leonarduzzi G. The controversial role of 24-S-Hydroxycholesterol in Alzheimer's disease. Antioxidants (Basel). 2021;10(5).
Bjorkhem I, Leoni V, Svenningsson P. On the fluxes of side-chain oxidized oxysterols across blood-brain and blood-CSF barriers and origin of these steroids in CSF (review). J Steroid Biochem Mol Biol. 2019;188:86–9.
Ohtsuki S, Ito S, Matsuda A, Hori S, Abe T, Terasaki T. Brain-to-blood elimination of 24S-hydroxycholesterol from rat brain is mediated by organic anion transporting polypeptide 2 (oatp2) at the blood-brain barrier. J Neurochem. 2007;103(4):1430–8.
Pifferi F, Laurent B, Plourde M. Lipid transport and metabolism at the blood-brain Interface: implications in health and disease. Front Physiol. 2021;12:645646.
Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–60.
Banks WA, Farr SA, Salameh TS, Niehoff ML, Rhea EM, Morley JE, HAnson AJ, Hansen KM, Craft S. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int J Obes. 2018;42:391–7.
Chang GQ, Karatayev O, Daydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2006;145:3904–12.
Morley JE, Banks WA. Lipids and cognition. J Alzheimers Dis. 2010;20:737–47.
Toscano R, Millan-Linares MC, Lemus-Conejo A, Claro C, Sanchez-Margalet V, Montserrat-de la Paz S. Postprandial triglyceride-rich lipoproteins promote M1/M2 microglia polarization in a fatty-acid-dependent manner. J Nutr Biochem. 2020;75:108248.
Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149(7):3592–7.
Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29(11):2061–5.
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of Caveolae-mediated transcytosis. Neuron. 2017;94(3):581–94 e585.
Jiang X, Guo M, Su J, Lu B, Ma D, Zhang R, Yang L, Wang Q, Ma Y, Fan Y. Simvastatin blocks blood-brain barrier disruptions induced by elevated cholesterol both in vivo and in vitro. Int J Alzheimers Dis. 2012;2012:109324.
Pallebage-Gamarallage M, Lam V, Takechi R, Galloway S, Clark K, Mamo J. Restoration of dietary-fat induced blood-brain barrier dysfunction by anti-inflammatory lipid-modulating agents. Lipids Health Dis. 2012;11:117.
Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC. Aging-related changes in blood-brain barrier integrity and the effect of dietary fat. Neurodegener Dis. 2013;12(3):125–35.
Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Mamo JC. Dietary fats, cerebrovasculature integrity and Alzheimer’s disease risk. Prog Lipid Res. 2010;49(2):159–70.
Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Dhaliwal SS, Mamo JC. Probucol prevents blood-brain barrier dysfunction in wild-type mice induced by saturated fat or cholesterol feeding. Clin Exp Pharmacol Physiol. 2013;40(1):45–52.
Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimer's & Dementia : The Journal of the Alzheimer's Association. 2015;11(9):1007–14.
Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–6.
Mathiesen Janiurek M, Soylu-Kucharz R, Christoffersen C, Kucharz K, Lauritzen M. Apolipoprotein M-bound sphingosine-1-phosphate regulates blood-brain barrier paracellular permeability and transcytosis. Elife. 2019;8.
Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA. Healthy aging and the blood-brain barrier. Nature Aging. 2021;1:243–54.
Linton MF, Gish R, Hubl ST, Bütler E, Esquivel C, Bry WI, Boyles JK, Wardell MR, Young SG. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88(1):270–81.
Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem N Jr, Calon F, Plourde M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem. 2014;129(3):516–26.
Hanson AJ, Banks WA, Bettcher LF, Pepin R, Raftery D, Craft S. Cerebrospinal fluid lipidomics: effects of an intravenous triglyceride infusion and apoE status. Metabolomics. 2019;16(1):6.
Narayan P, Sienski G, Bonner JM, Lin YT, Seo J, Baru V, Haque A, Milo B, Akay LA, Graziosi A, Freyzon Y, Landgraf D, Hesse WR, Valastyan J, Barrasa MI, Tsai LH, Lindquist S. PICALM rescues endocytic defects caused by the Alzheimer's disease risk factor APOE4. Cell Rep. 2020;33(1):108224.
Storck SE, Pietrzik CU. Endothelial LRP1 - a potential target for the treatment of Alzheimer's disease : theme: drug discovery, development and delivery in Alzheimer's disease guest editor: Davide Brambilla. Pharm Res. 2017;34(12):2637–51.
Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286(20):17536–42.
Bachmeier C, Shackleton B, Ojo J, Paris D, Mullan M, Crawford F. Apolipoprotein E isoform-specific effects on lipoprotein receptor processing. NeuroMolecular Med. 2014;16(4):686–96.
Shinohara M, Sato N, Kurinami H, Takeuchi D, Takeda S, Shimamura M, Yamashita T, Uchiyama Y, Rakugi H, Morishita R. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J Biol Chem. 2010;285(29):22091–102.
Laudati E, Gilder AS, Lam MS, Misasi R, Sorice M, Gonias SL, Mantuano E. The activities of LDL receptor-related Protein-1 (LRP1) compartmentalize into distinct plasma membrane microdomains. Mol Cell Neurosci. 2016;76:42–51.
Robert J, Cheng WH, Hayat A, Ward-Able T, Wellington CL. High-density lipoproteins at the interface between central nervous system and plasma lipoprotein metabolism. Clin Lipidol. 2015;10:69–81.
Fung KY, Wang C, Nyegaard S, Heit B, Fairn GD, Lee WL. SR-BI mediated transcytosis of HDL in brain microvascular endothelial cells is independent of caveolin, clathrin, and PDZK1. Front Physiol. 2017;8:841.
Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 1994;44(5):433–73.
Vollbach H, Heun R, Morris CM, Edwardson JA, McKeith IG, Jessen F, Schulz A, Maier W, Kölsch H. APOA1 polymorphism influences risk for early-onset nonfamiliar AD. Ann Neurol. 2005;58(3):436–41.
Elliott DA, Weickert CS, Garner B. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin Lipidol. 2010;51:555–73.
Stukas S, Robert J, Lee M, Kulic I, Carr M, Tourigny K, Fan J, Namjoshi D, Lemke K, DeValle N, Chan J, Wilson T, Wilkinson A, Chapanian R, Kizhakkedathu JN, Cirrito JR, Oda MN, Wellington CL. Intravenously injected human apolipoprotein A-I rapidly enters the central nervous system via the choroid plexus. J Am Heart Assoc. 2014;3(6):e001156.
Zhou AL, Swaminathan SK, Curran GL, Poduslo JF, Lowe VJ, Li L, Kandimalla KK. Apolipoprotein A-I crosses the blood-brain barrier through Clathrin-independent and cholesterol-mediated endocytosis. J Pharmacol Exp Ther. 2019;369(3):481–8.
Fellows K, Uher T, Browne RW, Weinstock-Guttman B, Horakova D, Posova H, Vaneckova M, Seidl Z, Krasensky J, Tyblova M, Havrdova E, Zivadinov R, Ramanathan M. Protective associations of HDL with blood-brain barrier injury in multiple sclerosis patients. J Lipid Res. 2015;56(10):2010–8.
Swaminathan SK, Zhou AL, Ahlschwede KM, Curran GL, Lowe VJ, Li L, Kandimalla KK. High-density lipoprotein mimetic peptide 4F efficiently crosses the blood-brain barrier and modulates amyloid-β distribution between brain and plasma. J Pharmacol Exp Ther. 2020;375(2):308–16.
Zlokovic BV, Martel CL, Mackic JB, Matsubara E, Wisniewski T, McComb JG, Frangione B, Ghiso J. Brain uptake of circulating apolipoproteins J and E complexed to Alzheimer's amyloid ·. Biochem Biophys Res Commun. 1994;205:1431–7.
Shayo M, McLay RN, Kastin AJ, Banks WA. The putative blood-brain barrier transporter for the ·-amyloid binding protein apolipoprotein J is saturated at physiological concentrations. Life Sci. 1996;60:L115–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sheng Qi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rhea, E.M., Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm Res 38, 1469–1475 (2021). https://doi.org/10.1007/s11095-021-03098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03098-6