Abstract
Introduction
High-fat diets increase risk for Alzheimer’s disease, but individuals with the risk gene APOE ε4 (E4) paradoxically have improved memory soon after high fat feeding. Little is known about how dietary lipids affect CNS lipids, especially in older adults.
Objectives
We analyzed the lipidomic signature of cerebrospinal fluid (CSF) in older adults who underwent both a saline and TG infusion. We further analyzed these data by E4 carrier status.
Methods
Older adults (n = 21, age 67.7 ± 8.6) underwent a 5-h TG and saline infusion on different days in random crossover design; lumbar CSF was collected at the end of the infusion. Lipids were extracted using dichloromethane/methanol and 13 classes of lipids analyzed using the Lipidyzer platform consisting of an AB Sciex 5500 MS/MS QTraps system equipped with a SelexION for differential mobility spectrometry (DMS). Multiple reaction monitoring was used to target and quantify 1070 lipids in positive and negative ionization modes with and without DMS.
Results
The TG infusion increased total lipids in the CSF, including the appearance of more lipids at the detection limit in the TG samples compared to saline (Chi square p < 0.0001). The infusion increased the total level of diacylglycerols and lysophosphatidylcholines and reduced dihydroceramides. Of the possible 1070 lipids detectable, we found 348 after saline and 365 after TG infusion. Analysis using MetaboAnalyst revealed 11 specific lipids that changed; five of these lipids decreased after TG infusion, and four of them differed by E4 status, but none differed by cognitive diagnosis or sex.
Conclusion
These results in older adults show that blood lipids affect lipid profiles in CSF and such profiles are modified by APOE status. This suggests that how the CNS handles lipids may be important in the AD phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intake of the “Western diet” characterized by high levels of saturated fat is a risk factor for Alzheimer’s disease (AD) (Barnes and Yaffe 2011; Gustaw-Rothenberg 2009; Kivipelto et al. 2008; Solfrizzi et al. 2011). Conversely, high intake of polyunsaturated fatty acids (PUFA) found in olive oil and fish is protective for AD (Gardener et al. 2012; Martinez-Lapiscina et al. 2013; Tsivgoulis et al. 2013). Another risk factor for AD is carriage of the epsilon 4 allele (E4) of the APOE lipid carrier protein (Farrer et al. 1997). Interestingly, the connection between a high saturated fat diet and AD risk is attenuated in E4 carriers, (Barberger-Gateau et al. 2011; Huang et al. 2005) and we found that E4 carriers improved in terms of cognitive and plasma AD biomarkers after acute high fat feeding, compared to E4 non-carriers (Hanson et al. 2015). In a responder analysis of clinical trials involving PUFA supplementation, only E4 non-carriers showed a cognitive benefit (Quinn et al. 2010). Therefore, there is a need to better understand responses to high lipid states in this group at higher risk for AD.
The lipid content of the brain is significant, and neurons rely on a constant lipid supply for several important functions including turnover of membranes and myelin, (Braverman and Moser 2012) cellular signaling, (Kasser et al. 1986; Moulle et al. 2012) and neurotransmitter metabolism (Ebert et al. 2003). Although the brain makes its own cholesterol, it relies on peripheral delivery of TG and free fatty acids (FFA), which are taken up by the brain via passive diffusion and facilitated transport (Kasser et al. 1986; Moulle et al. 2012). Lipidomics analyses from CSF and brain extracts from dementia patients have revealed significant differences in fatty acids, glycerophospholipids, sphingolipids, and cholesterol, and in one study, these differences correlated with cognitive measures (Astarita et al. 2011; Lam et al. 2014; Wood 2012). However, little is known about how these differences might be modulated by acute lipid intake, or by APOE genotype. Here in this study we analyzed the lipid content of the CSF of older adults in a crossover design after a triglyceride (TG) and saline infusion to better understand lipid transport in older adults at risk for developing AD.
2 Methods
2.1 Subjects
The Institutional Review Boards of the University of Washington and the Veterans Affairs Puget Sound Health Care System approved the study. All potential subjects underwent a physical examination, laboratory testing, and APOE genotyping using standard PCR (Hixson and Vernier 1990). Inclusion criteria were age ≥ 50 and in good physical health, and sample was enriched for those with mild cognitive impairment or Alzheimer’s disease, as per NINCDS/ADRDA criteria. Exclusion criteria included serious systemic illnesses, abnormal lipid metabolism, diabetes, and medications including anticoagulants, statins, and sedatives.
2.2 Infusion
All study subjects were blinded to the intervention and arrived after a 12 h fast. Bilateral 20-gauge IVs were placed, and blood was drawn at time 0 and 5 h. The participants received a 5-h infusion of normal saline (0.9% 15 cc/h) or 20% triglyceride emulsion at 45 cc/h (Liposyn II for the first few participants, then Intralipid for the remainder) on different days in random order. The infusion consisted of emulsified fat particles of approximately 0.4 micron in diameter, similar to naturally occurring chylomicrons, and contained 10% safflower oil, 10% soybean oil, and egg yolk phosphatides. Contents of the infusion are listed in Table 5.
2.3 LP procedure
Three hours after the TG infusion was stopped, the L4–L5 space was accessed with a 24-gauge Sprott spinal needle (Sprott; Pajunk), and 30 mL of CSF was withdrawn into sterile syringes. Samples were divided into aliquots in prechilled polyethylene tubes, immediately frozen with dry ice, and stored at − 70 °C until assays were performed.
2.4 Lipidomics
Materials: LC–MS grade methanol, dichloromethane, and ammonium acetate were purchased from Fisher Scientific (Pittsburgh, PA) and HPLC grade 1-propanol was purchased from Sigma-Aldrich (Saint Louis, MO). Milli-Q water was obtained from an in-house Ultrapure Water System by EMD Millipore (Billerica, MA). The Lipidyzer isotope labeled internal standards mixture consisting of 54 isotopes from 13 lipid classes was purchased from Sciex (Framingham, MA).
2.5 Sample preparation
Frozen CSF samples were thawed at room temperature (25 °C) for 30 min and vortexed; 250 μL of CSF was transferred to a borosilicate glass culture tube (16 × 100 mm). Next, 250 μL of water, 1 mL of methanol, and 450 μL of dichloromethane were added to all samples. The mixture was vortexed for 5 s and 25 μL of the isotope labeled internal standards mixture were added to the tube. Samples were left to incubate at room temperature for 30 min. Next, another 500 μL of water and 450 μL of dichloromethane were added to the tube, followed by gentle vortexing for 5 s, and centrifugation at 2500×g at 15 °C for 10 min. The bottom organic layer was transferred to a new tube and 900 μL of dichloromethane was added to the original tube for a second extraction. The combined extracts were concentrated under nitrogen and reconstituted in 250 μL of the running solution (10 mM ammonium acetate in 50:50 methanol:dichloromethane).
2.6 Mass spectrometry
Quantitative lipidomics was performed with the Sciex Lipidyzer platform consisting of Shimadzu Nexera X2 LC-30AD pumps, a Shimadzu Nexera X2 SIL-30AC autosampler, and a Sciex QTRAP® 5500 mass spectrometer equipped with SelexION® for differential mobility spectrometry (DMS). 1-propanol was used as the chemical modifier for the DMS. Samples were introduced to the mass spectrometer by flow injection analysis at 8 uL/min. Each sample was injected twice, once with the DMS on (PC/PE/LPC/LPE/SM), and once with the DMS off (CE/CER/DAG/DCER/FFA/HCER/LCER/TAG). The lipid molecular species were measured using multiple reaction monitoring (MRM) and positive/negative polarity switching. Positive ion mode detected lipid classes SM/DAG/CE/CER/DCER/HCER/DCER/TAG and negative ion mode detected lipid classes LPE/LPC/PC/PE/FFA. A total of 1070 lipids and fatty acids were targeted in the analysis. Data were acquired and processed using Analyst 1.6.3 and Lipidomics Workflow Manager 1.0.5.0.
2.7 Statistical analysis
Data were analyzed using MetaboAnalyst 4.0 (Canada) for data processing, normalization and analysis, and where indicated, t tests and Chi square tests in Excel and repeated measures ANOVA using SAS University (2018 edition). We determined whether each species had been previously characterized in the Human Metabolome Database (Version 4.0) which includes 450 species previously characterized in the CSF (Wishart et al. 2018).
3 Results
3.1 Demographics and plasma data
Table 1 shows the demographics of the population, including age, BMI and fasting lipids drawn on a separate day from the study visits. None of the baseline demographics differed by E4 status. Nine of the 21 participants had cognitive impairment (three with AD, six with MCI). Plasma TG levels pre- and post-infusion were available for 19 of the 21 participants, which significantly increased after infusion (repeated measures ANOVA p < 0.0001 for time × condition); findings did not differ by E4 status (Supplementary Table 1).
3.2 Lipid profiles
Our method can detect up to 1070 individual lipid species in the biological sample of interest. We detected a total of 373 unique lipids in the CSF (348 lipids after saline and 365 lipids after TG) with an average CV of 5.2% for lab QC technical replicates. For quality control, an instrument QC of pooled human serum was run every ten samples (at the beginning, middle, and end of each batch). Samples were run in two batches, and the average CV for batch 1 was 3.75 and for batch 2 was 3.98.
To further assess whether there were more detectable lipids after the TG, we counted the number of blank versus filled cells for each metabolite and compared the counts using Chi square analysis; we counted 48% filled cells for saline versus 52% filled for TG (p < 0.0001). To understand overall trends, we summed all species within 13 lipid classes and compared the average concentration after each treatment (Table 2). The lipid class LPE was omitted from the table as only one individual had a single measurable LPE after the TG infusion. Both the diacylglycerol (DAG) and lysophosphatidylcholine (LPC) class increased after the TG infusion and the dihydroceramide (DCER) class decreased (p values < 0.05). We next determined whether the class variables of sex, cognitive diagnosis, or E4 status influenced these lipid concentrations. Neither sex nor cognitive diagnosis influenced the concentrations or the change by treatment (data not shown); however, E4 carriers had higher FFA in the CSF (F statistic 5.46, p = 0.031) and post hoc analyses revealed it was driven by higher FFA in both conditions (saline: E4− 8.467 ± 1.222, E4+ 9.787 ± 2.04, p = 0.0772, TG: E4− 8.547 ± 0.834, E4+ 9.523 ± 1.462, p = 0.0646).
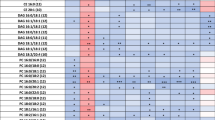
To aid in analysis of lipid species in MetaboAnalyst, lipids with the same FA chain were combined and concentrations of species were then summed. We counted 136 lipids overall and 105 lipids which were detectable in at least 50% of participants’ CSF after the TG infusion (Supplementary Table 2). For the remainder of analyses, we deleted metabolites which had < 50% detectable data in the TG infusion. We used MetaboAnalyst to further analyze the lipidomic data, and blank values were estimated by a small value. Figure 1 shows the principal component analysis (PCA) plot of the data (free fatty acids combined). Table 3 shows the results of MetaboAnalyst paired t test by condition, using an FDR cut-off of 0.1 (equivalent to a p value at 0.01 or below for a paired t-test), resulting in 11 lipid species (concentrations given in nmol/g). We also indicated if species were cataloged in the Human Metabolome Database (HMDB) including in CSF (Wishart et al. 2018). Four of the 11 species had previously been characterized in human fluid but not in CSF (marked with *), and the two DAGs were not listed in the database likely due to the focus of HMDB on small molecule metabolites.
We then analyzed all 11 species by repeated measures ANOVA to determine whether findings differed by the categorical variables of E4 status, cognitive diagnosis or sex. For 4 of the 11 lipids, E4 status appeared to influence CSF lipid levels, with lower baseline lipids in the E4 non-carrier group which then increased to E4 carrier levels after the TG infusion (Table 4). These analyses were unchanged when controlled for age or BMI, and none of these lipids differed by sex or by cognitive diagnosis.
3.3 Summary of findings
Table 5 shows the components of the lipid infusion compared to the CSF findings. For the unsaturated fatty acids, one increased in CSF as FFA (18:2), one increased as a DAG (18:1), and one decreased (FFA 18:3). For the two saturated fatty acids, neither significantly changed in CSF between the treatments. However, the top hit which was detectable in only one subject after saline but in 11 subjects after TG was FFA 16:1 which is most likely palmitoleic acid but could also be sapienic acid as our methods can only detect the number of carbon atoms and double bonds, not the location of the double bonds. Given that sapienic acid was not identified in any human fluid per HMDB, and that stearic acid can convert to palmitoleic acid by fatty acid desaturase—an enzyme known to be in the brain (Thon et al. 2017)—this species is most likely palmitoleic acid. The appearance of the LPC in CSF likely reflects the purified egg phosphatides which, according to the package insert, contains a mixture of phosphatidylcholine and phosphatidylethanolamine.
4 Discussion
Here we present a CSF lipid profile in older adults in a fasting state as well as in response to a triglyceride infusion. We detected 373 distinct lipids and showed that a TG infusion produced statistically significant changes in lipid species number and composition. These changes included increases and decreases in several lipid species, and lipid composition did differ by APOE genotype.
4.1 Baseline lipid composition of CSF
Overall our results are comparable to other CSF lipidomics studies, but with some variations. In one analysis of 45 patients using an untargeted approach, 550 unique lipids were identified within five major lipid families (free fatty acids, sphingolipids, glycerophospholipids, glycerolipids, and sterol lipids) and 304 of these distinct lipid species were identified in both positive and negative ion modes (Seyer et al. 2016). Another group that characterized the lipidomic signature of CSF in amyotrophic lateral sclerosis (ALS) patients detected approximately 200 lipids partitioned into 11 classes, and identified a unique signature that discriminated ALS from controls that included ceramides, glycosylceramides, sphingomyelin, and triglycerides (Blasco et al. 2017). These findings are in line with our identification of 373 unique lipids in CSF in an older population.
We identified 26 unique FFA in human CSF which were present in all samples, and others have found similar numbers of FFA: one group reported 20 (Fonteh et al. 2014), another group reported 21 (Jumpertz et al. 2012). The three highest FFA levels in our study (16:0, 18:0 and 18:1) were the same in these two reports. Interestingly, the Blasco group did not identify any FFA in their CSF samples with their approach, possibly reflecting different MS extraction or analyses methods (Blasco et al. 2017).
Cholesterol esters (CEs) were the second highest component, and we detected 20 of the 26 measurable CEs in the CSF. Cholesterol esters have been linked to amyloid and tau pathology, and one group using Lipidyzer technology was able to show in induced pluripotent stem cell (iPSC) astrocytes and neurons that specific cholesterol esters independently regulated both tau and amyloid breakdown products. Furthermore, these pathways could be modified by drugs that target cholesterol synthesis (van der Kant et al. 2019).
Phospholipids encompass several subclasses including PC, PE, SM and LPC, and in some studies comprise up to 50% of total CSF lipid composition (Kosicek et al. 2012). In our study, phospholipids comprised ~ 30% of the total lipid concentration. One group showed that when CSF is separated into nanometer particles and supernatant fluid, different fractions contain different lipid patterns; particularly concerning glycerophospholipids. These types of studies reveal the importance of standardization of fluid collection and processing (Fonteh et al. 2013). In summary our data appears to fit into the general knowledge of what is found in CSF although different methodologies, different class definitions of lipids, and different populations must be taken into account when comparing different studies.
4.2 Response to a TG infusion
The brain is comprised of many types of lipids and requires a constant lipid supply for neurotransmitter building blocks and myelin turnover, through de novo synthesis or transport (Hamilton and Brunaldi 2007). Much of what we know about brain lipid synthesis and transport is limited to animal models, and the paradigm is that cholesterol and larger lipid molecules such as TG do not cross the BBB under normal conditions and therefore must be synthesized in the brain. However, we recently showed that the TG triolein (three oleic acids, 18:1) readily crosses the BBB in mice (Banks et al. 2017). In this study, we found that a TG infusion did not increase FFA or TG as a class but did increase DAGs, and the top hit in the change analysis was DAG that contained palmitoleic acid (FFA 16:1). This finding raises interesting questions as to how the brain is either transporting or immediately processing post-prandial lipids. This may be important given that some DAGs may serve as second messengers or sources for neurotransmitters, (Garcia del Cano et al. 2014) and processing of DAGs by diacylglycerol kinases is important in many neuronal functions including long-term potentiation (Sakane et al. 2018).
Free fatty acids cross the BBB in several experimental models; however, the kinetics depend on the specific FFA. A study of labeled FFA showed that linoleate (18:2) clearance from the plasma is greater than that of palmitate (16:0) or oleate (18:1) (Nelson et al. 2013). Available FFA can then enter the brain by diffusion or protein-mediated transport, and transport rate also depends on plasma albumin binding and the rate of activity of lipoprotein lipase located on the cerebral microvessel endothelium (Chen et al. 2008; Edmond 2001; Jumpertz et al. 2012). The brain itself can also synthesize FFA from acetyl-CoA which requires only transport of glucose, and given that most FFA in CSF do not correlate with plasma levels may suggests that synthesis is the main source of FFA in the brain (Hamilton and Brunaldi 2007; Jumpertz et al. 2012). Using radiolabeled FFA, Edmond et al. showed that palmitic, stearic, and oleic acids do not cross the BBB in developing rat brains, whereas linoleic acid did (Edmond 2001). However, others have demonstrated that palmitate does enter the brain in rodents and primate models (Alberghina et al. 1993; Arai et al. 1995; Williams et al. 1997). These groups also demonstrated that the brain preferentially incorporated fatty acids into glycerophospholipids in a rapid fashion. Here we demonstrated that the two saturated FFA components (FFA 18:0 and 16:0) did not increase in the CSF after the infusion. However, the lipid with the most significant change was DAG 16:1 which could be evidence of stearic acid (16:0) crossing then immediately converting to palmitoleic acid and packaging into DAG, or it is also possible that this particular DAG is able to cross the BBB. Our findings support the idea that linoleic acid (18:2) can cross the BBB but interestingly the unsaturated FFA linolenic acid (18:3) was decreased compared to saline, despite being a component of the infusion.
Glycerophospholipids are integral components of cell membranes which turn over frequently in brain (Frisardi et al. 2011). Lysophospholipids appear transiently during glycerophospholipid metabolism, and in vitro can alter membrane permeability and activate microglia (Frisardi et al. 2011). Lipidomics analysis of brain extracts in patients with AD revealed that glycerophospholipid metabolism was altered in AD brains compared to controls (Paglia et al. 2016). Seyer et al. detected more LPCs than LPEs in their CSF samples, and also detected the non-lyso forms of PCs and PEs (Seyer et al. 2016). In human CSF, no differences in total PC were detected between AD patients and controls, but LPC levels were lower, leading the authors to speculate that the metabolite LPC is a more sensitive parameter for changes in PC turnover (Mulder et al. 2003). One component of our TG infusion was egg yolk phosphatides which are reported by the manufacturers to be a 50/50 mixture of phosphatidylcholine (PC) and phosphatidylethanolamine (PE), presumably the non-lyso version of these compounds. In this study, the non-lyso forms of PC and PE were detected in the CSF but did not change between conditions, LPCs were identified and increased after TG infusion, and LPEs were not detected despite being a component of the infusion.
Ceramides are comprised of a FFA chain attached to a sphingosine by an amide linkage, and are bioactive lipids implicated in the regulation of numerous neuronal processes including protein traffic, neuronal plasticity and neurotransmitter release, among many others (Haughey et al. 2013). Ceramides are increased in both the brain and spinal fluid of AD patients, and in vitro these lipids promote the amyloidogenic pathway (Touboul and Gaudin 2014). They are also implicated in other neurodegenerative disease including ALS, (Seyer et al. 2016) MS, (Vidaurre et al. 2014) and cognitive impairment due to HIV (Bandaru et al. 2013). In our study, we noted a reduction in dihydroceramides in the CSF of TG infusion, compared to saline, whereas the other classes of ceramides remained unchanged. Of note, ceramide metabolism is complicated with several classes of ceramides detected in CSF (Haughey et al. 2013); understanding how interventions change various ceramide concentrations may help us understand how to use this class of lipids as a biomarker for AD trials. Rats fed a high fat diet promoted cholesterol accumulation in the hippocampus as well as elevated galactosyl ceramide and SM (Stranahan et al. 2011). We noted a significant reduction in SM containing the FFA 24:0 after TG infusion. CSF SM levels were shown to be altered in individuals with prodromal AD compared to controls, suggesting that alterations in the SM metabolism may contribute to AD pathology (Kosicek et al. 2012).
Overall our findings support previous literature that FFA brain transport is dependent on the specific FFA and suggest that diet interventions acutely change the composition of several classes of lipids such as DAGS and ceramides. We also show here that lysoforms of PC (LPC) may be dynamic markers of glycerophospholipid metabolism as previously suggested (Mulder et al. 2003). Finally, many lipid species decreased in response to the TG infusion, suggesting CSF redistribution or metabolism after a high TG state.
4.3 APOE genotype and lipidomics
Those who carry the E4 allele have higher plasma TG levels in some but not all studies and the apoE protein in vitro can modulate lipoprotein lipase and hepatic lipase activity (Dallongeville et al. 1992). We did not find a difference in peripheral TG by E4 but small numbers of subjects, exclusion of lipid disorders and those on statins, and high variability of peripheral TG may limit the generalizability of these findings. In the brain, apoE is the predominant lipoprotein and as such plays an important role in brain lipid transport and metabolism (Grimm et al. 2017). Carriage of the E4 allele is a potent risk factor for developing AD, and we and others have shown that the E4 isoform of apoE is less able to hold on to lipid (less lipidated) which affects its ability, at least in vitro, to bind and clear amyloid peptides, and other important functions (Fan et al. 2009; Hanson et al. 2013). How this protein isoform affects brain lipid transport and function is an active area of study. In the PUFA trials, E4 carriers did not show cognitive improvements to supplementation, and in this group, levels of plasma PUFA did not increase to the extent seen in non-carriers; CSF lipids were not measured (Plourde et al. 2009). A study in soldiers showed that those with traumatic brain injury or post-traumatic stress exhibited lower plasma phospholipids—particularly those containing monounsaturated FA. In addition, they showed that E4 carriers had higher plasma phospholipid levels than non-carriers, indicating that APOE genotype should be accounted for when using lipidomics as a disease biomarker (Emmerich et al. 2016).
Few studies have analyzed CSF lipids by E4 status. A lipidomic analysis from brain tissue revealed that AD brains had lower stearic acid (18:0) and arachidonic acid (20:4), whereas oleic acid (18:1) and palmitic acid (16:0) were increased, no effects were noted based on APOE status (Touboul and Gaudin 2014). In mice with the human E4 gene knocked in, a group found APOE4-specific differential expression of several important metabolite groups, but interestingly found that several FFA were lower in E4 mice than E3 (Nuriel et al. 2017). APOE genotype affected the hippocampal metabolome in E4+ compared to E3+ mice fed a high fat diet, and this included metabolites involved in sphingolipid and glycerophospholipid metabolism (Johnson et al. 2017). In our analysis of CSF by E4, we noted that E4 carriers tended to have higher FFA in the fasting condition and a TG infusion in this group tended to make the E4 non-carrier group look more like the E4 carrier group. Overall these findings suggest that lipid differences in CSF may be able to be modulated by diet.
4.4 Limitations
The findings of this study are limited by a small sample size, which particularly limits our ability to look for subgroup effects by sex, diagnosis, or APOE genotype. Early in the study we had to change to a different manufacturer for the lipid infusion; however, the two infusions had the same components per the manufacturer. It is unknown whether dietary lipid would give the same results as a TG infusion, and we are not able to draw direct conclusions about CSF lipid transport given that the conditions were on different days.
5 Conclusions
We demonstrate here that lipidomic analysis of human CSF identifies a comprehensive list of lipid species in various lipid classes. Our CSF lipid composition is similar to other reports but with some important differences especially involving FFA, stressing the importance of method standardization for clinical studies. We also demonstrate that CSF lipidomics can be used to detect acute differences in treatment conditions such as feeding or drug studies. If standardized, these types of crossover studies may help us make inferences as to what types of lipids (or building blocks) cross the BBB, as radiolabeled studies or repeated CSF sampling are more difficult to do in humans. Finally, differences in lipid profiles of E4 carriers who are at higher risk of developing AD may be able to be modulated by diet or lipid supplementation.
References
Alberghina, M., Lupo, G., Anfuso, C. D., & Moro, F. (1993). Palmitate transport through the blood-retina and blood-brain barrier of rat visual system during aging. Neuroscience Letters, 150, 17–20.
Arai, T., Wakabayashi, S., Channing, M. A., Dunn, B. B., Der, M. G., Bell, J. M., et al. (1995). Incorporation of [1-carbon-11]palmitate in monkey brain using PET. Journal of Nuclear Medicine, 36, 2261–2267.
Astarita, G., Jung, K. M., Vasilevko, V., Dipatrizio, N. V., Martin, S. K., Cribbs, D. H., et al. (2011). Elevated stearoyl-CoA desaturase in brains of patients with Alzheimer’s disease. PLoS ONE, 6, e24777.
Bandaru, V. V., Mielke, M. M., Sacktor, N., McArthur, J. C., Grant, I., Letendre, S., et al. (2013). A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology, 81, 1492–1499.
Banks, W. A., Farr, S. A., Salameh, T. S., Niehoff, M. L., Rhea, E. M., Morley, J. E., et al. (2017). Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. International Journal of Obesity (London), 42(3), 391–397.
Barberger-Gateau, P., Samieri, C., Feart, C., & Plourde, M. (2011). Dietary omega 3 polyunsaturated fatty acids and Alzheimer’s disease: interaction with apolipoprotein E genotype. Current Alzheimer Research, 8, 479–491.
Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology, 10, 819–828.
Blasco, H., Veyrat-Durebex, C., Bocca, C., Patin, F., Vourc’h, P., Kouassi Nzoughet, J., et al. (2017). Lipidomics reveals cerebrospinal-fluid signatures of ALS. Scientific Reports, 7, 17652.
Braverman, N. E., & Moser, A. B. (2012). Functions of plasmalogen lipids in health and disease. Biochimica et Biophysica Acta, 1822, 1442–1452.
Chen, C. T., Green, J. T., Orr, S. K., & Bazinet, R. P. (2008). Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukotrienes and Essential Fatty Acids, 79, 85–91.
Dallongeville, J., Lussier-Cacan, S., & Davignon, J. (1992). Modulation of plasma triglyceride levels by apoE phenotype: A meta-analysis. Journal of Lipid Research, 33, 447–454.
Ebert, D., Haller, R. G., & Walton, M. E. (2003). Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. Journal of Neuroscience, 23, 5928–5935.
Edmond, J. (2001). Essential polyunsaturated fatty acids and the barrier to the brain: The components of a model for transport. Journal of Molecular Neuroscience, 16, 181–193.
Emmerich, T., Abdullah, L., Crynen, G., Dretsch, M., Evans, J., Ait-Ghezala, G., et al. (2016). Plasma lipidomic profiling in a military population of mild traumatic brain injury and post-traumatic stress disorder with apolipoprotein E varepsilon4-Dependent Effect. Journal of Neurotrauma, 33, 1331–1348.
Fan, J., Donkin, J., & Wellington, C. (2009). Greasing the wheels of Abeta clearance in Alzheimer’s disease: The role of lipids and apolipoprotein E. BioFactors, 35, 239–248.
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA, 278, 1349–1356.
Fonteh, A. N., Chiang, J., Cipolla, M., Hale, J., Diallo, F., Chirino, A., et al. (2013). Alterations in cerebrospinal fluid glycerophospholipids and phospholipase A2 activity in Alzheimer’s disease. Journal of Lipid Research, 54, 2884–2897.
Fonteh, A. N., Cipolla, M., Chiang, J., Arakaki, X., & Harrington, M. G. (2014). Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PLoS ONE, 9, e100519.
Frisardi, V., Panza, F., Seripa, D., Farooqui, T., & Farooqui, A. A. (2011). Glycerophospholipids and glycerophospholipid-derived lipid mediators: A complex meshwork in Alzheimer’s disease pathology. Progress in Lipid Research, 50, 313–330.
Garcia del Cano, G., Montana, M., Aretxabala, X., Gonzalez-Burguera, I., Lopez de Jesus, M., Barrondo, S., et al. (2014). Nuclear phospholipase C-beta1 and diacylglycerol LIPASE-alpha in brain cortical neurons. Advances Biological Regulation, 54, 12–23.
Gardener, S., Gu, Y., Rainey-Smith, S. R., Keogh, J. B., Clifton, P. M., Mathieson, S. L., et al. (2012). Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Translational Psychiatry, 2, e164.
Grimm, M. O. W., Michaelson, D. M., & Hartmann, T. (2017). Omega-3 fatty acids, lipids, and apoE lipidation in Alzheimer’s disease: A rationale for multi-nutrient dementia prevention. Journal of Lipid Research, 58, 2083–2101.
Gustaw-Rothenberg, K. (2009). Dietary patterns associated with Alzheimer’s disease: Population based study. International Journal of Environmental Research and Public Health, 6, 1335–1340.
Hamilton, J. A., & Brunaldi, K. (2007). A model for fatty acid transport into the brain. Journal of Molecular Neuroscience, 33, 12–17.
Hanson, A. J., Bayer, J. L., Baker, L. D., Cholerton, B., VanFossen, B., Trittschuh, E., et al. (2015). Differential effects of meal challenges on cognition, metabolism, and biomarkers for apolipoprotein E varepsilon4 carriers and adults with mild cognitive impairment. Journal of Alzheimer’s Disease, 48, 205–218.
Hanson, A. J., Bayer-Carter, J. L., Green, P. S., Montine, T. J., Wilkinson, C. W., Baker, L. D., et al. (2013). Effect of apolipoprotein E genotype and diet on apolipoprotein E lipidation and amyloid peptides: Randomized clinical trial. JAMA Neurology, 70(8), 1–9.
Haughey, N. J., Zhu, X., & Bandaru, V. V. (2013). A biological perspective of CSF lipids as surrogate markers for cognitive status in HIV. Journal of Neuroimmune Pharmacology, 8, 1136–1146.
Hixson, J. E., & Vernier, D. T. (1990). Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of Lipid Research, 31, 545–548.
Huang, T. L., Zandi, P. P., Tucker, K. L., Fitzpatrick, A. L., Kuller, L. H., Fried, L. P., et al. (2005). Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology, 65, 1409–1414.
Johnson, L. A., Torres, E. R., Impey, S., Stevens, J. F., & Raber, J. (2017). Apolipoprotein E4 and insulin resistance interact to impair cognition and alter the epigenome and metabolome. Scientific Reports, 7, 43701.
Jumpertz, R., Guijarro, A., Pratley, R. E., Mason, C. C., Piomelli, D., & Krakoff, J. (2012). Associations of fatty acids in cerebrospinal fluid with peripheral glucose concentrations and energy metabolism. PLoS ONE, 7, e41503.
Kasser, T. R., Deutch, A., & Martin, R. J. (1986). Uptake and utilization of metabolites in specific brain sites relative to feeding status. Physiology & Behavior, 36, 1161–1165.
Kivipelto, M., Rovio, S., Ngandu, T., Kareholt, I., Eskelinen, M., Winblad, B., et al. (2008). Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: A population-based study. Journal of Cellular and Molecular Medicine, 12, 2762–2771.
Kosicek, M., Zetterberg, H., Andreasen, N., Peter-Katalinic, J., & Hecimovic, S. (2012). Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer’s disease. Neuroscience Letters, 516, 302–305.
Lam, S. M., Wang, Y., Duan, X., Wenk, M. R., Kalaria, R. N., Chen, C. P., et al. (2014). Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiology of Aging, 35, 2369–2381.
Martinez-Lapiscina, E. H., Clavero, P., Toledo, E., Estruch, R., Salas-Salvado, J., San Julian, B., et al. (2013). Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. Journal of Neurology, Neurosurgery and Psychiatry, 84, 1318–1325.
Moulle, V. S., Cansell, C., Luquet, S., & Cruciani-Guglielmacci, C. (2012). The multiple roles of fatty acid handling proteins in brain. Frontiers Physiology, 3, 385.
Mulder, C., Wahlund, L. O., Teerlink, T., Blomberg, M., Veerhuis, R., van Kamp, G. J., et al. (2003). Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer’s disease. Journal of Neural Transmission, 110, 949–955.
Nelson, R. H., Mundi, M. S., Vlazny, D. T., Smailovic, A., Muthusamy, K., Almandoz, J. P., et al. (2013). Kinetics of saturated, monounsaturated, and polyunsaturated fatty acids in humans. Diabetes, 62, 783–788.
Nuriel, T., Angulo, S. L., Khan, U., Ashok, A., Chen, Q., Figueroa, H. Y., et al. (2017). Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nature Communications, 8, 1464.
Paglia, G., Stocchero, M., Cacciatore, S., Lai, S., Angel, P., Alam, M. T., et al. (2016). Unbiased metabolomic investigation of Alzheimer’s disease brain points to dysregulation of mitochondrial aspartate metabolism. Journal of Proteome Research, 15, 608–618.
Plourde, M., Vohl, M. C., Vandal, M., Couture, P., Lemieux, S., & Cunnane, S. C. (2009). Plasma n-3 fatty acid response to an n-3 fatty acid supplement is modulated by apoE epsilon4 but not by the common PPAR-alpha L162V polymorphism in men. British Journal of Nutrition, 102, 1121–1124.
Quinn, J. F., Raman, R., Thomas, R. G., Yurko-Mauro, K., Nelson, E. B., Van Dyck, C., et al. (2010). Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA, 304, 1903–1911.
Sakane, F., Mizuno, S., Takahashi, D., & Sakai, H. (2018). Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Advances in Biological Regulation, 67, 101–108.
Seyer, A., Boudah, S., Broudin, S., Junot, C., & Colsch, B. (2016). Annotation of the human cerebrospinal fluid lipidome using high resolution mass spectrometry and a dedicated data processing workflow. Metabolomics, 12, 91.
Solfrizzi, V., Panza, F., Frisardi, V., Seripa, D., Logroscino, G., Imbimbo, B. P., et al. (2011). Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Review of Neurotherapeutics, 11, 677–708.
Stranahan, A. M., Cutler, R. G., Button, C., Telljohann, R., & Mattson, M. P. (2011). Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. Journal of Neurochemistry, 118, 611–615.
Thon, M., Hosoi, T., Chea, C., & Ozawa, K. (2017). Loss of stearoyl-CoA desaturase-1 activity induced leptin resistance in neuronal cells. Biological and Pharmaceutical Bulletin, 40, 1161–1164.
Touboul, D., & Gaudin, M. (2014). Lipidomics of Alzheimer’s disease. Bioanalysis, 6, 541–561.
Tsivgoulis, G., Judd, S., Letter, A. J., Alexandrov, A. V., Howard, G., Nahab, F., et al. (2013). Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology, 80, 1684–1692.
van der Kant, R., Langness, V. F., Herrera, C. M., Williams, D. A., Fong, L. K., Leestemaker, Y., et al. (2019). Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-beta in iPSC-derived Alzheimer’s Disease neurons. Cell Stem Cell, 24(363–375), e9.
Vidaurre, O. G., Haines, J. D., Katz Sand, I., Adula, K. P., Huynh, J. L., McGraw, C. A., et al. (2014). Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain, 137, 2271–2286.
Williams, W. M., Chang, M. C., Hayakawa, T., Grange, E., & Rapoport, S. I. (1997). In vivo incorporation from plasma of radiolabeled palmitate and arachidonate into rat brain microvessels. Microvascular Research, 53, 163–166.
Wishart, D. S., Feunang, Y. D., Marcu, A., Guo, A. C., Liang, K., Vazquez-Fresno, R., et al. (2018). HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Research, 46, D608–D617.
Wood, P. L. (2012). Lipidomics of Alzheimer’s disease: Current status. Alzheimers Research Therapy, 4, 5.
Acknowledgements
Alzheimer’s disease research center at University of Washington, and the Northwest Metabolomics Research Center.
Funding
This study was funded by 1K23AG047978-01A1 and 1S10OD021562-01 from the National Institutes of Health, the Veterans Affairs Medical Center, and the Chair of Medicine scholars award from the University of Washington School of Medicine.
Author information
Authors and Affiliations
Contributions
AJH conceptualized the study, undertook statistical analysis and interpreted results and wrote/revised manuscript; WAB conceptualized the study, assisted with result interpretation and revised manuscript; LFP and RP collected data, assisted with result interpretation and revised manuscript. DR conceptualized the study, assisted with result interpretation and revised manuscript. SC collected data and was the PI of the parent clinical study, and revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of the University of Washington and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hanson, A.J., Banks, W.A., Bettcher, L.F. et al. Cerebrospinal fluid lipidomics: effects of an intravenous triglyceride infusion and apoE status. Metabolomics 16, 6 (2020). https://doi.org/10.1007/s11306-019-1627-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1627-x