Abstract
An evaluation of the gaseous species production by the discharge, i.e., discharge chemical activity, is very important for determining its potential for practical applications. In this work, production of gaseous species by the multi-hollow surface dielectric barrier discharge generated in a perforated ceramic substrate with the air-exposed electrode is investigated under conditions of various discharge powers (1–5 W), air flow rates (0.25–2.4 L/min) and air relative humidities (0–80%). Production of ozone O3, nitrous oxide N2O, nitric oxide NO, nitrogen dioxide NO2, dinitrogen pentoxide N2O5 and nitric acid HNO3 is evaluated in terms of concentration (ppm), production yield (g/kWh) and production rate (mg/h). The work demonstrates a critical impact of both air flow rate and relative humidity on prevailing discharge mode (“O3 mode” vs. “NOx mode”) and, thus, on overall composition and concentration of produced gaseous species. For low discharge power, the discharge operates in the “O3 mode”, when O3, N2O, N2O5 and HNO3 are dominant gaseous products. With the increasing power, the discharge transfers into the “NOx mode”, when N2O and HNO3 along with NO and NO2 are mostly produced. In dry air, transition from “O3 mode” to “NOx mode” is found for the specific input energy of 1000–1100 J/L. With an increase of air relative humidity from 20 to 80%, the transition gradually decreases from approximately 600 to 450 J/L, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nonthermal plasma (NTP), as it is very well known, can create a high-reactive environment by initiating and maintaining various chemical reactions at ambient conditions of temperature and pressure. This feature represents one of the most important benefits of the NTP in contrast to the thermal plasma. The most frequently employed NTP sources for effective plasma chemistry are electrical discharges which have been utilised in numerous applications including surface treatment of materials [1, 2], air flow modification and control [3, 4], air and water pollution control [5, 6], bio-decontamination [7, 8], medical [9, 10] and agricultural applications [11]. Among the discharges, the dielectric barrier discharges (DBDs) have a special position due to their simplicity, scalability, and the availability of reliable, efficient, and affordable power supplies [12]. Out of them the surface dielectric barrier discharges (SDBDs) are getting an increasing attention because of their versatility, small dimensions, and possibility for electrodes encapsulation into the dielectric, what prevents their aging and damage. To optimise utilisation of the discharges in selected applications, their proper chemical characterisation (i.e., their chemical effects) must be known in detail especially under ambient conditions.
Chemical effects of NTP are in general mainly associated with high-energy electrons with energy of the order 10 eV or even higher. The electrons collide with bulk gas molecules (such as N2, O2, H2O) leading to their dissociation and production of short-lived high-reactive species, also known as radicals (e.g., ·O, ·N, ·OH, etc.). Apart from molecular dissociation, ion formation may also occur (e.g., O+, O2+, N+, N2+, etc.). In all reactions, an excess of energy is transferred not only to kinetic energy of reaction products, but also to excitation of atoms and molecules (e.g., N2*, O*, etc.). In addition, the electronic or vibrational excitation of species can also take place in direct reactions with electrons if they do not possess enough energy for dissociation or ionisation [13]. Reactive species can further undergo mutual post-discharge reactions [14] or react with bulk gas molecules and form more complex long-lived secondary species.
Evaluation of production of gaseous long-lived species by different discharges under various conditions, i.e., the discharge gas-phase chemical activity, have been already studied by many authors experimentally [15, 16] and by numerical modelling [17, 18]. In the air-like mixtures, ozone O3 and nitrogen oxides NOx (mainly nitric oxide NO, nitrogen dioxide NO2 and nitrous oxide N2O) represent the most frequent long-lived products of atmospheric pressure air discharges [15]. The production of the gaseous species generally depends on discharge parameters (e.g., applied voltage), and working conditions (e.g., composition of a gas mixture) and their formation can be used as a measure of the discharge chemical activity.
In this manuscript, a relatively novel type of discharge, the multi-hollow SDBD (also known as micro-hollow SDBD [19,20,21,22,23]), was investigated. The discharge was generated in a perforated ceramic substrate with the air-exposed electrode. A unique geometry forces the carrier gas to pass through holes (hollows) of the ceramic substrate inside which the discharge is formed. Although the multi-hollow SDBD has been already studied in relevant applications [20, 24,25,26,27,28,29] and its physical characteristics investigated by several authors [20,21,22, 30], detailed evaluation of gaseous species produced by the discharge have not been examined yet except for ozone O3 production [31,32,33]. Moreover, the NOx and HNO3 production promoted by nonthermal plasma have been identified as promising and emerging technology for N2 fixation under ambient air conditions [34]. In this sense, evaluation of NOx and HNO3 produced by the discharge under various working conditions is also important. Therefore, the main objective of this work was to evaluate the gas-phase chemical activity of the multi-hollow SDBD. This research follows our previous work [35] in which the electrical and optical characteristics of the discharge were investigated. Out of the gaseous species produced by the discharge, the production of ozone O3, nitrogen oxides NOx (N2O, NO, NO2, N2O5) and nitric acid HNO3 were evaluated under conditions of various discharge powers (1–5 W), air flow rates (0.25–2.4 L/min) and air relative humidities (0–80%). The effect of air flow rate was examined under dry air conditions, while the effect of air relative humidity was studied for two air flow rates (0.5 and 1 L/min). In addition, the gas as well as surface temperature of the ceramic substrate were also monitored.
Experimental Setup and Methods
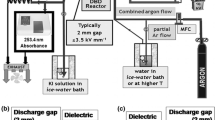
The experimental setup is depicted in Fig. 1. The multi-hollow SDBD was generated in a perforated ceramic square substrate (KD-EB2B10, Kyocera) with the dimensions of 50 × 50 × 1 mm (Fig. 2a). It consisted of 170 holes (hollows) with an inner diameter of 1.5 mm and two electrodes—one was embedded inside the ceramic while the other one (made of Ni/Au alloy) was printed on the ceramic surface (air-exposed electrode). More details about the substrate and its parameters can be found in our previous work [35]. The substrate was powered by AC high voltage (HV) power supply consisting of function generator (GwInstek SFG-1013), signal amplifier (Omnitronic PAP-350) and HV transformer. The HV was connected to the air-exposed electrode while the embedded electrode was grounded. Upon application of the AC HV, the discharge was generated at the edge (circumference) of each single hole (Fig. 2b). The waveform of the applied AC HV was measured by HV probe (Tektronix P6015A) and the discharge current by a current probe (Pearson Electronics 2877) both connected to a digital oscilloscope (Tektronix TBS2104). The power consumption of the discharge was evaluated using the Lissajous figure method with an 82 nF capacitor and a voltage probe (Tektronix P2220). Synthetic air (purity 5.0) supplied from a pressure tank was used as the carrier gas and its flow rate was controlled by mass flow controllers (MFC) (Bronkhorst El-Flow Prestige FG-201CV). The air was alternatively enriched by water vapours by passing it through a water cell. The air relative humidity (RH) was monitored by a capacitive humidity sensor (Arduino). Then the air was led into the reactor chamber from the bottom side of ceramic substrate, it passed through the substrate holes, and it exited the substrate by its top side with the air-exposed electrode and left the reactor chamber. At the outlet of the reactor chamber, a thermocouple was placed to measure the gas temperature after passing the discharge zone. Surface temperature of the ceramic substrate was measured by infrared thermal camera (Workswell WIC2) (Fig. 2c). Gas-phase chemical activity of the discharge was evaluated by means of FTIR spectroscopy (Shimadzu IR-Affinity 1S) with a resolution of 0.5 cm−1 inside a gas cell with an optical path length of 10 cm equipped with CaF2 windows. Main gaseous species (O3, N2O, NO, NO2, N2O5, HNO3) were identified in the FTIR spectra, and their concentrations were evaluated using absorption bands as follows: O3 at 1055 cm−1; N2O at 2236 cm−1; NO at 1900 cm−1; NO2 at 1602 cm−1; N2O5 at 1245 cm−1; HNO3 at 1325 cm−1. The absolute concentrations were determined based on calibrations with commercial gas mixtures (NO, NO2), UV absorption (O3) or by modelling the spectra (N2O, N2O5, HNO3) using a set of absorption lines from HITRAN database (more details can be found in [36]). The approximate detection limits of evaluated species were as follows: 70 ppm for O3; 5 ppm for N2O; 70 ppm for NO; 15 ppm for NO2; 5 ppm for N2O5 and 15 ppm for HNO3. In addition to FTIR measurements, O3 concentration was also evaluated by a homemade analyser based on UV absorption at 254 nm using a mercury lamp and the fiber optic emission spectrometer (Ocean Optics SD2000) in a 12.5 cm gas cell. The presence of NO was also monitored by an electrochemical sensor (Membrapor NO/SF-1000).
In addition to discharge power, the performance of the multi-hollow SDBD was also evaluated with the help of following variables:
-
Specific input energy (SIE) (i.e., energy density or discharge energy per gas volume):
$$\mathrm{SIE }\left(\mathrm{J}/\mathrm{L}\right)= \frac{P}{Q},$$(1)where P and Q represent discharge power and total air flow rate, respectively.
-
Production yield (PY) (i.e., the amount of produced gaseous species per SIE):
$$\mathrm{PY }\left(\mathrm{g}/\mathrm{kWh}\right)= \frac{c}{\mathrm{SIE}}=\frac{Qc}{P},$$(2)where c represents the concentration of gaseous species produced by the discharge. PYs of all evaluated species were calculated for room temperature of 22 °C.
-
Production rate (PR) (i.e., the amount of produced gaseous species per unit of time):
$$\mathrm{PR }\left(\mathrm{mg}/\mathrm{h}\right)= Qc.$$(3)
The experiments were performed with applied voltage amplitude in a range of 3.5–6.5 kV at a frequency of 1 kHz resulting in discharge power of 1–5 W. Gas-phase chemical activity of the multi-hollow SDBD was evaluated under conditions of air flow rates in a range of 0.25–2.4 L/min, air RH in a range of 0–80% and the SIE of 25–1200 J/L. Upon application of applied voltage, the temperature of the ceramic substrate gradually increased. Since a production of gaseous species is generally temperature-dependent, their concentrations were measured once the gas temperature and O3 concentration at the outlet of the reactor chamber were fully stabilised (usually 15–30 min).
Results and Discussion
Figure 3 depicts typical infrared absorption spectra of gaseous species produced by the discharge operated in two distinct modes: “O3 mode” and “NOx mode” for the same SIE of 600 J/L (5 W, 0.5 L/min). As we will present in "The Effect of the Air Relative Humidity" section, the presence of humidity in the air can significantly alter the prevailing discharge mode. In the spectra the following absorption bands of the species are denoted: O3 (1055 and 2125 cm−1), nitrous oxide N2O (1270 and 2236 cm−1), nitrogen dioxide NO2 (1627 cm−1), nitric acid HNO3 (1325 and 1711 cm−1) and dinitrogen pentoxide N2O5 (1245 and 1720 cm−1) (Fig. 3).
The Effect of the Air Flow Rate
The gas residence time in the discharge zone can be controlled by varying of gas flow rate. Variation of gas flow rate has a strong impact on gas heating, residual humidity, and gas pre-ionisation and consequently strongly influences the production of gaseous species [20, 37]. It also affects mixing of produced species and heat exchange in the reaction zone [38]. Therefore, the effect of air flow rate on gas-phase chemical activity of the multi-hollow SDBD was investigated in a range of 0.25–2.4 L/min by using dry air as a carrier gas. The following “Ozone O3 and Nitrous Oxide N2O” section is devoted to the results obtained for O3 and N2O. In the "Dinitrogen Pentoxide N2O5 and Nitric acid HNO3" section, we present results for N2O5 and HNO3 since they were observed under dry air conditions for all tested air flow rates. Finally, the results for NO and NO2 can be found in both sections within the discussion on other species, as they were not detected under all conditions tested, but only under the specific ones.
Ozone O3 and Nitrous Oxide N2O
Ozone O3 represents one of the most common gaseous products formed by atmospheric-pressure air DBDs. Production of O3 is strongly dependent on atomic oxygen species that may be produced by following reactions [18, 39]:
Then, O3 is produced by a well-known three-body reaction:
where M can be nitrogen N2 or oxygen O2 molecule or oxygen atom ·O [40].
Figure 4a shows the O3 concentration as a function of discharge power for various dry air flow rates. Since the SIE (energy density) is a very important parameter determining the chemical activity of the discharge and is not constant for a given discharge power and various air flow rates, the concentration of O3 is also depicted as a function of the SIE (Fig. 4b). The results showed a common feature: an increase of O3 concentration with a decrease of the air flow rate for a given discharge power (except for air flow rate of 0.25 L/min). The same result for O3 production by multi-hollow SDBD was also reported by Nayak et al. [32].
Dependence of O3 concentration on discharge power (or SIE) for a given air flow rate was found nontrivial (Fig. 4). For the air flow rates of 1 and 2.4 L/min the O3 concentration firstly increased and then culminated, whereas for air flow rates of 0.25 and 0.5 L/min, the increase and the culmination were followed by a subsequent decrease of O3 concentration (Fig. 4). The same trend of O3 concentration with an increase of DBD power (i.e., the initial increase, culmination, and the following decrease) was also reported by many authors [41,42,43]. Šimek et al. found a maximum O3 concentration of 750 ppm for the SIE ~ 110 J/L [43], while we observed the maximum concentration ~ 1840 ppm for much higher SIE of 480 J/L (i.e., 2 W and 0.25 L/min). As is well known, the DBD-based reactors can be operated in two distinct modes. The “O3 mode” is typical for low SIEs with O3 dominating among the products. On the other hand, the “NOx mode” prevails for high SIEs with nitric oxide NO and nitrogen dioxide NO2 among the products [44, 45].
Production of NO is given by the following set of reactions known as the Zeldovich mechanism [46, 47]:
NO2 is then formed by subsequent oxidation of NO [48, 49]:
Substantial drop of O3 concentration to zero for very high SIEs when using dry air flow rate of 0.25 L/min (Fig. 4b) is related to the transition of the discharge from “O3 mode” to “NOx mode”. The transition was observed between the SIE of 1000 and 1100 J/L and was characterised by a complete depletion of O3 associated with an increase of NO and NO2 [45, 48]. This effect is also known as “discharge poisoning” [50, 51] and will be discussed in detail later.
In addition to O3, NO and NO2, nitrous oxide N2O represents another frequent gaseous product of atmospheric pressure air discharges. Its production and decomposition are predominantly affected by the first excited state of molecular nitrogen N2(A) via following reactions [52, 53]:
Other reaction pathways leading to N2O formation are reactions of excited nitrogen atom N(2D) with NO and nitrogen radical ·N with NO2 [54, 55]:
On the contrary, decomposition of N2O may happen via reaction with excited oxygen atom O(1D) [53, 54]:
Unlike O3 concentration, the N2O concentration as a function of discharge power (or SIE) and air flow rate was found monotonic when operating the discharge in “O3 mode” (i.e., SIE < 1000 J/L) (Fig. 5). It increased almost linearly with discharge power and SIE, and significantly decreased with air flow rate. Maximum concentration of 121 ppm was reached for 4 W and 0.25 L/min (i.e., the SIE of 960 J/L). The linear increase of N2O concentration with the discharge power was also observed in [41, 51] and is probably linked with an increase of the electron energy, what leads to higher production of reactive nitrogen species important for N2O formation [56]. Tang et al. observed an increase of N2O concentration with an increase of SIE up to 1500 J/L in a coaxial cylindrical DBD reactor [53]. Above this level a saturated concentration of N2O was obtained that is related to an equilibrium between the processes of N2O formation and depletion that are mediated mainly by excited state of molecular nitrogen N2(A) (Eqs. 11, 12) and excited oxygen atom O(1D) (Eqs. 15, 16). Similar trend in N2O monotonic increase up to a specific discharge power and following N2O saturation was also reported in [52]. When using the lowest dry air flow rate (0.25 L/min) and, thus, the highest SIE (> 1000 J/L), a slight decrease of N2O concentration was observed (Fig. 5b). This effect may be associated with a transition of the discharge from “O3 mode” to “NOx mode”. A similar observation was also reported by Kogelschatz and Baessler [51].
Production yields (PYs) of O3 and N2O were also calculated (Fig. 6). In contrast to O3 and N2O concentrations, they decreased monotonically with the discharge power for all tested air flow rates (except for PY of N2O for 2.4 L/min that showed a nontrivial trend). The effect of increase of PY of O3 with an increase of the air flow rate for a given discharge power was also observed by other authors [31, 57]. This may be a result of more efficient cooling of the ceramic substrate. Maximum PYs of O3 and N2O were 77.6 and 1.0 g/kWh for the SIE of 25 and 240 J/L, respectively.
To assess “energy effectiveness” of O3 production by the discharge, the results of our work are compared to those of other authors who used various other types of DBD operated at atmospheric pressure in dry air. Figure 7 presents PY of O3 as a function of the SIE for all these DBD arrangements. All results presented have the same trend – the PY of O3 decreases with an increase of SIE. Homola et al. investigated the multi-hollow SDBD with both electrodes embedded inside the ceramic and obtained much higher PY of O3 (> 150 g/kWh) than we obtained with the same discharge in configuration with the air-exposed electrode (Fig. 7) [31]. Their PR of O3 (~ 90 mg/h for PY of 205.5 g/kWh) was comparable with our results (~ 80 mg/h for PY of 77.6 g/kWh). Maximum PR of O3 we obtained was ~ 220 mg/h for PY of 43.8 g/kWh. The other SDBD reactors are also compared: coplanar SDBD showed higher [42], while the other selected SDBD reactors showed comparable [58] or lower PYs of O3 [43, 56] than our discharge (Fig. 7). Finally, cylindrical coaxial DBD showed also almost identical results than our discharge [57] (Fig. 7). Lower PY of O3 obtained with our multi-hollow SDBD compared to its another configuration (embedded electrodes) as well as coplanar SDBD may be related to arrangement of the ceramic substrate itself. The metal electrode exposed to the flowing air could contribute to the catalytic decomposition of O3, thereby reducing the PY of O3 [59]. On the other hand, higher PY of O3 obtained with our multi-hollow SDBD compared to other SDBD-based reactors could be attributed to the reactor geometry that enables efficient air mixing with generated NTP.
In addition to O3, PY of N2O was also evaluated by Šimek et al. for the SDBD similar to coplanar SDBD and reached ~ 0.8 g/kWh for 250 J/L [43] that can be well compared to our results (~ 1.0 g/kWh for 240 J/L). Besides, maximum PR of N2O in our experiment reached ~ 3.7 mg/h.
Our results clearly showed that the air flow rate plays an essential role in both O3 and N2O production by the discharge. Upon increasing the SIE up to ~ 350–500 J/L, O3 production dominated over O3 decomposition (Fig. 4b). With further increase of the SIE, the O3 concentration started to gradually decline implying an increasing influence of O3 decomposition. Braun et al. proposed that following reactions of O3 with electrons, O and N species are important for O3 decomposition under discharge operation [41]:
Pekárek and Mikeš [58] and Kim et al. [60] reported the direct electron impact reaction (Eq. 17) as a dominant reaction leading to O3 decomposition at a gas temperature below 77 ºC. Kim et al. [60] further stated that at higher gas temperatures, the recombination of O3 with O2 becomes dominant (Eq. 18). Another important O3 loss reaction especially at higher temperatures is the reaction with excited oxygen atom O(1D) (Eq. 19) [61]. An increasing effect of both reactions (Eqs. 18, 19) lies in an exponential dependence of their reaction rates on temperature [62, 63]. However, many authors who observed a decrease of O3 concentration for higher discharge power explained their results referring to the term “O3 thermal decomposition” without further explanation. One can also find that reported gas temperature threshold, when O3 thermal decomposition starts to dominate, varies significantly among the authors. For example, Homola et al. [42] reported a gas temperature of around 80 °C, Osawa and Yoshioka [64] a temperature of 120 °C and Jodzis even temperature above 200 °C. Here, it must be noted that in addition to gas temperature, the temperature in the microdischarge channels should be also considered as it is significantly higher than a gas temperature [61]. In our experiment, we monitored the temperature of both gas and ceramic substrate surface. For the SIE of 600 J/L, the maximum temperatures were almost equal and not too high (~ 70 °C) [35], so we suppose that temperature is not a dominant factor affecting O3 decomposition in our case.
Besides the reactions of direct O3 decomposition presented above, the consumption of ·O atoms that are essential for O3 production must be also taken into account. At higher gas temperatures the ·O atoms can be consumed by reactions with N2 and O2 molecules (denoted as M):
that may also contribute to the overall decline of O3 concentration [40, 56]. Based on the discussion above, we suppose that an observed gradual decrease of O3 concentration from the SIE of 450–500 J/L is given by combination of several factors. Since concentrations of O and N species increase with an increase of the SIE, their reactions with O3 (Eqs. 19, 20) may play important roles. Other influencing factors are increasing temperature and its effect on reaction rates, as well as increased consumption of ·O radicals via reactions with N2 and O2 molecules (Eq. 21).
As we have already mentioned, when the SIE exceeded ~ 1000–1100 J/L, the discharge transition from “O3 mode” to “NOx mode” was accompanied by a significant change in the composition of produced gaseous species. According to Eliasson and Kogelschatz [48], the transition happens when the NOx concentration reaches the level at which ·O atoms react faster with NO and NO2 than with O2 to form O3. As a result, an enhanced recombination of ·O atoms takes place by the following reaction cycle [50, 51]:
In addition, the previously formed O3 is effectively consumed by the reaction cycle involving both NO and NO2 as follows [41, 50]:
As the SIE increases, the vibrationally excited N2 species start to play a dominant role in formation of NO, which is then a major quencher of O3 (the first reaction of the cycle Eq. 23) [44, 65]. Since NO and NO2 concentrations increase with an increase of the SIE and gas temperature [43, 48], the two reaction cycles considerably affect the O3 concentration especially under conditions of high energy densities (SIE > 1000 J/L). Whereas the first cycle (Eq. 22) contributes to consumption of ·O radicals, the second cycle (Eq. 23) to consumption of O3 itself. This confirms the role of the reactions Eqs. 19 and 21 in the observed decline of O3 concentration in dry air under high energy densities (SIE) (Fig. 4b), as the contribution of these reactions is enhanced by a presence of NO and NO2.
The maximum concentrations of NO and NO2 in dry air were 130 and 395 ppm with corresponding PYs of 0.5 and 2.3 g/kWh and PRs of 2.4 and 11 mg/h for 1200 J/L, respectively. It is also important to note that when the discharge was operated in “O3 mode” (i.e., SIE < 1000 J/L) regardless of working conditions, NO and NO2 were not detected in the FTIR spectra.
Dinitrogen Pentoxide N2O5 and Nitric Acid HNO3
Until now, we have discussed the formation of O3, N2O, NO and NO2. However, in a strongly oxidising environment, NO2 can further undergo the oxidation processes promoted by O3 and ·O as follows [18, 51]:
Consequently, N2O5 can be formed via reaction of NO2 with NO3:
Figure 8 show the N2O5 concentration as a function of discharge power and SIE for various dry air flow rates. Here it must be noted that N2O5 production was not observed for the lowest air flow rate of 0.25 L/min. The maximum concentration of N2O5 was 23 ppm (5 W, 1 L/min, 300 J/L). N2O5 production was found to increase almost linearly with an increase of discharge power (SIE) (Fig. 8). Unlike the other evaluated compounds in “O3 mode” (O3, N2O, HNO3), a maximum N2O5 concentration was observed for a moderate air flow rate of 1 L/min (Fig. 8a). N2O5 production by the discharge is generally dependent on several factors including not only discharge power and air flow rate, but also the molar ratio of O3/NO, geometrical parameters, and temperature of the reactor zone [51, 66]. These factors may explain the observed nontrivial trends of N2O5 concentration.
In humid air-like mixtures, nitric acid HNO3 can be also produced by gradual oxidation of NOx mediated by ·H, ·OH and HO2· radicals formed from dissociation of water molecules. The following set of selected reactions can be considered [18, 67]:
Linear trend of HNO3 production as a function of discharge power and air flow rate (Fig. 9) was similar to that of N2O production (Fig. 5). In our dry air conditions, only residual (trace) moisture could serve as a source of the radicals (·H, ·OH and HO2·) that mediate HNO3 production. The same effect of the presence of HNO3 traces in dry air conditions was also observed by Braun et al. [41]. Upon transition of the discharge from “O3 mode” to “NOx mode”, concentration of HNO3 further slightly increased and reached a maximum of 148 ppm (5 W, 0.25 L/min, 1200 J/L) (Fig. 9a).
PYs of N2O5 and HNO3 are presented in the Fig. 10. Similar to PY of O3, the PY of N2O5 followed the same increasing trend with an increase of air flow rate for a given discharge power (Fig. 10a). A maximum of 3.2 g/kWh was reached for 1 W and 2.4 L/min (SIE of 25 J/L). It is worth mentioning that for air flow rates of 0.5 and 1 L/min, the PY of N2O5 showed almost constant values with an increase of discharge power. Unlike other evaluated compounds, the PY of HNO3 during discharge operation in “O3 mode” increased with an increase of discharge power for all tested air flow rates. The maximum of 1.9 g/kWh was found for 5 W and 1 L/min (SIE of 300 J/L) (Fig. 10b). Besides PYs, the PRs of both N2O5 and HNO3 were also evaluated with maxima of ~ 9.9 and ~ 9.5 mg/h, respectively.
Here, it must be noted that in order to explain the presence of N2O5 and HNO3 in the FTIR spectra, formation of NO and NO2 in the “O3 mode” of the discharge must be considered as they are both precursors for N2O5 and HNO3 formation. As we have already noted, NO and NO2 were detected only when the discharge was operated in the “NOx mode”. However, it does not mean they did not form in the “O3 mode”. We suppose that at least traces of NO and NO2 were produced in the discharge and their absence in the FTIR spectra indicates either their concentrations below the detection limits or, more probably, their fast consumption by O3 which is a dominant product of the discharge in the “O3 mode” (Eqs. 10, 24) [43, 56]. The second effect could be even more pronounced as the FTIR spectrometer was placed in some distance from the reactor chamber. Once the air came to the gas cell, NO and NO2 were already consumed very probably by O3. Additionally, to monitor NO the electrochemical sensor was placed just behind the reactor chamber. However, even the sensor did not detect any NO, thus confirming its fast consumption in the discharge in the “O3 mode”. Consequently, NO2 and NO3 may further react and form N2O5 (Eq. 26) or even HNO3 (Eq. 35) if at least a trace humidity is present. Braun et al. reported that when a large surplus of O3 is present especially at low gas temperatures, the equilibrium of Eq. 26 is shifted almost completely towards N2O5 without detection of NO2 and NO3 what corresponds with our results [41]. The same absence of NO and NO2 was also observed by Abdelaziz et al. when using SDBD-based reactor [56]. Hence, we can conclude that the gaseous species produced by the multi-hollow SDBD operated in the “O3 mode” include O3, N2O, N2O5 and HNO3. When the discharge is transitioned to the “NOx mode”, in addition to N2O and HNO3, NO and NO2 are also present. The observed concentrations of O3, N2O and N2O5 in the “O3 mode” agree with the typical feature of this mode reported by Eliasson and Kogelschatz [48]: the O3 concentration was roughly two orders of magnitude higher than the concentrations of N2O and N2O5.
The Effect of the Air Relative Humidity
Humidity of a gas mixture affects the discharge from many aspects. It influences not only the physical processes of discharge formation, propagation, and its characteristics [68, 69], but also it substantially affects and alters the plasma chemistry [67, 70]. To evaluate the effect of air humidity on gas-phase chemical activity of the multi-hollow SDBD, experiments were performed for various air relative humidity (RH) levels of 20, 40, 60 and 80% at atmospheric pressure with a constant room temperature of ~ 22°C, so the absolute humidity of the air (water content) was 3.9, 7.8, 11.7, 15.6 g/m3, respectively. The effect of humidity was studied for a fixed air flow rate of 1 L/min and discharge powers of 1–5 W, so the maximum SIE was 300 J/L. Under these conditions, the discharge was operated only in the “O3 mode”. Decreasing the air flow rate to 0.5 L/min allowed the transition of the discharge to “NOx mode” with the maximum SIE of 600 J/L.
Ozone O3 and Nitrous Oxide N2O
Figure 11a shows the O3 concentration as a function of discharge power for various air RHs. Our results confirmed a well-known fact that production of O3 by the discharges significantly decreases with any impurity in the gas mixture including water H2O, hydrocarbons, etc. [57, 71]. A decrease of O3 concentration was found non-linear. It means that the rate of O3 concentration decrease is greater for low than for high levels of air RH (Fig. 11a). A non-linear decrease of O3 production was also reported by Chen and Wang when investigating DC corona discharge in humid air [70]. The significant decrease of O3 concentration when water vapour is present can be explained by the consumption of ·O radicals triggered by HO2· and ·OH radicals formed from dissociation of H2O and O2 molecules:
or by reaction with water (Eq. 28) [57, 70]. Thus, consumption of ·O radicals accelerates with an increase of air humidity leading to lower O3 production. Decline in formation of ·O radicals can also be caused by a reduction in electron density due to an electronegativity of water molecules [67]. In addition, humidity also accelerates the relaxation rate of the vibration-to-translation of N2 and O2 molecules leading to the decline of O as well as N excited species [67]. Another possible pathway for O3 consumption may be by direct reactions of O3 with ·OH and ·H radicals [70, 72]:
When the reaction of ·H with O3 (Eq. 39) is followed by the reaction of ·OH with ·O (Eq. 37), giving the net reaction of ·O with O3 (Eq. 19), the ·H and ·OH radicals can act as a “catalyst” to reduce O3 concentration (similar to NOx in the reaction cycle Eq. 23) [72].
Figure 11b shows N2O concentration as a function of discharge power for various air RHs. For low discharge powers (< 2 W), the maximum concentration of N2O was obtained in dry air, while for higher discharge powers (> 4 W), it was reached in air with RH of 20% (Fig. 11b). In general, production of nitrogen excited species N2(A) important for N2O production decreases with an increase of air humidity. This is due to very efficient quenching of N2(A) species by water molecules [49]. This explains a decrease of N2O concentration when increasing the air RH from 20 to 80%. However, an increase of N2O concentration with air RH from 0 to 20% for 4 and 5 W may indicate that other reactions leading to N2O formation may also play important roles under these specific conditions. Abdelaziz et al. [67] and Matsui et al. [73] observed an increase of NO and NO2 concentrations upon increasing the air humidity, so the reactions of N2O formation moderated by NO and NO2 (Eqs. 13, 14) may dominate.
In addition to O3 and N2O concentrations, their corresponding PYs were also evaluated (Fig. 12). Similar to O3 concentration, the PY of O3 followed the same trend with an increase of air RH: it significantly decreased for a given discharge power (Fig. 12a). Moreover, in air with RH higher than 40%, the increasing humidity had no effect on PY of O3 for the highest tested discharge powers (4 and 5 W). In addition, the PY of N2O followed a trend similar to the concentration reaching maximum values in dry air (for 1 and 2 W) or in air with RH of 20% (for 4 and 5 W) (Fig. 12b).
Dinitrogen Pentoxide N2O5 and Nitric Acid HNO3
In humid air, N2O5 was not detected in the FTIR spectra regardless of discharge power and air flow rate. Similar observations were also reported by other authors [41, 51]. This can be explained by the complete consumption of N2O5 by reaction with H2O leading to the formation of HNO3 (Eq. 35).
Figure 13a shows the HNO3 concentration as a function of discharge power for various air RHs. It initially increased with an increase of air RH from 0 to 40%, but with its further increase, the concentration of HNO3 declined. The initial increase of HNO3 concentration can be easily explained, as precursors to its production (radicals ·H, ·OH, HO2·) are expected to rise with an increase of humidity [49]. In this sense, the decline of HNO3 concentration at air RH > 40% seems counterintuitive. Similar trend of HNO3 concentration increase followed by a decline was also observed by Matsui et al. [73] with a maximum concentration at air RH of 30%. They suggested that at higher air RH, HNO3 produced by the discharge can be easily absorbed by the water which is previously adsorbed on the inner surface of the connecting tubes due to its high miscibility. If HNO3 is absorbed by the water inside the experimental system before it enters the gas cell, it may cause a decline of HNO3 in the measured spectra [67]. In addition to concentration, the PY of HNO3 followed a similar trend (Fig. 13b). The highest concentration of HNO3 was 142 ppm corresponding to PY of 4.4 g/kWh (air RH 40%, 300 J/L).
Transition of the Discharge from “O3Mode” to “NOx Mode” at Higher Humidities
In a previous section, the effect of air RH on production of gaseous species by the discharge was evaluated for air flow rate of 1 L/min and maximum SIE of 300 J/L, i.e., under “O3 mode” conditions. To investigate the effect of air RH on transition of the discharge from “O3 mode” to “NOx mode”, the SIE must be further increased. Therefore, the air flow rate was decreased to 0.5 L/min obtaining the SIE up to 600 J/L.
The results showed that the air RH has a significant impact on the SIE value for the transition between the two discharge modes. Figure 14 show concentrations of O3, N2O, HNO3 and NO2 as a function of discharge power (or SIE) in air with various relative humidities (20–80%). According to these results it can be concluded that a presence of humidity substantially decreases the SIE value for transition between the two discharge modes. In dry air, the transition from “O3 mode” to “NOx mode” was found between 1000 and 1100 J/L. In air with RH of 20 and 40%, the transition was observed between 500 and 600 J/L (Fig. 14a, b), while in air with RH of 60 and 80%, it was found around 450 J/L (Fig. 14c, d). In humid air under “NOx mode” conditions, NO was not found in the FTIR spectra. Although we did not observe it, it does not mean that it was not produced. In humid air, the radicals formed by water dissociation (·OH, HO2·) can mediate formation of NO through the following reactions [67]:
These reactions can compensate for decreasing production of NO through reactions where ·N, ·O and N2(A) are involved [49]. Moreover, NO can further undergo an oxidation to NO2 or HNO3 promoted by ·O, O3 and HO2· (Eqs. 9, 10, 31, 32). Therefore, we suppose that absence of NO and presence of NO2 and HNO3 in the FTIR spectra in humid air under “NOx mode” conditions may indicate fast consumption of NO to higher oxidation products NO2 and HNO3 probably mediated by ·OH and HO2· radicals. The maximum NO2 concentrations were found in a range of 350–402 ppm for the SIE of 600 J/L (Fig. 14) with corresponding PYs of 4–4.6 g/kWh and PRs of 20–23 mg/h, respectively. Concentration of HNO3 reached maximum of 229 ppm at air RH of 40% for 480 J/L (Fig. 14b) with PY of 4.5 g/kWh and PR of 17.9 mg/h. These results indicate that humid air promoted a production of NO2 and HNO3 compared to their production in dry air for the same air flow rate (0.5 L/min). A similar effect was also observed by Baerdemaeker et al. [74] using multi-hollow SDBD with both electrodes embedded inside the ceramic.
To explain decreasing SIE value for transition between the two discharge modes with an increase of air RH, one must consider the O3/NOx ratio. In the “O3 mode”, the O3/NOx ratio is typically very high (\(\gg \hspace{0.17em}\)1) as O3 dominates the products. On the other hand, in the “NOx mode” the ratio is typically low (< 1) due to very low O3 concentration. Our results showed that the O3 concentration substantially decreased upon increasing the air RH. Therefore, the increase of humidity can significantly decrease the O3/NOx ratio and, thus, may promote the transition between the discharge modes for lower SIE in contrast to dry air.
Conclusions
Evaluation of the gaseous species production by the discharge, i.e., the discharge chemical activity, is one of the key factors for its effective employment for practical applications. In this manuscript, the gas-phase chemical activity of the multi-hollow surface dielectric barrier discharge generated in a perforated ceramic substrate with an air-exposed electrode was presented. The unique discharge geometry allowed the carrier gas to pass through holes (hollows) of the substrate and, thus, enabled efficient production of gaseous species. The production of ozone O3, nitrous oxide N2O, nitric oxide NO, nitrogen dioxide NO2, dinitrogen pentoxide N2O5 and nitric acid HNO3 was evaluated in terms of concentration (ppm), production yield (PY in g/kWh) and production rate (PR in mg/h) under conditions of various discharge powers (1–5 W), air flow rates (0.25–2.4 L/min) and air relative humidities (RH; 0–80%). The effect of air flow rate was examined under dry air conditions, while the effect of air RH was studied for two air flow rates (0.5 and 1 L/min).
The work demonstrated a critical impact of both the air flow rate and the relative humidity on the prevailing discharge mode (“O3 mode” vs. “NOx mode”) and, thus, on production and composition of the gaseous species. Whereas the air flow rate determines the gas residence time in a discharge zone and gas heating, the air relative humidity influences the discharge from physical (processes of discharge formation, propagation, and its characteristics) as well as chemical (production of ·H, ·OH, HO2· radicals) aspects. When the discharge was operated in the “O3 mode”, O3, N2O, N2O5 and HNO3 were observed among the gaseous products, while in the “NOx mode”, N2O, HNO3, NO and NO2 were present.
In dry air, the results showed that the concentrations of O3, N2O and HNO3 increase with a decrease of the air flow rate for a given discharge power, except for the lowest tested air flow rate (0.25 L/min) due to the discharge mode transition. On the contrary, the concentration of N2O5 was found the highest for the moderate air flow rate of 1 L/min (23 ppm with corresponding PY of 1.2 g/kWh for 300 J/L). Moreover, concentrations of N2O, HNO3 and N2O5 monotonously increased with an increase of discharge power in “O3 mode”, while concentration of O3 showed a nontrivial trend for 0.25 and 0.5 L/min. Upon increasing the discharge power or specific input energy (SIE), the O3 concentration firstly increased, culminated, and then gradually declined, or eventually dropped down to zero (for 0.25 L/min). A complete depletion of O3 was related to the discharge transition from “O3 mode” to “NOx mode” associated with an increase of NO and NO2 with maximum concentrations of 130 and 395 ppm with corresponding PYs of 0.5 and 2.3 g/kWh for 1200 J/L, respectively. The maximum O3 concentration was 1840 ppm with corresponding PY of 27 g/kWh for 480 J/L, whereas the maximum PY of O3 (78 g/kWh) was reached at concentration of 272 ppm for 25 J/L. For a comparison, the maximum N2O concentration was 121 ppm with corresponding PY of 0.8 g/kWh for 960 J/L.
In humid air, the O3 concentration substantially decreased with an increase of air RH for a given discharge power and a rate of reduction was larger for low than for high levels of air RH. The N2O concentration showed more complicated trend: for low discharge powers (< 2 W), the maximum concentration was obtained in dry air, while for higher discharge powers (> 4 W), it was reached in air with RH of 20%. Further, the maximum HNO3 concentration (142 ppm) was observed at air RH of 40% with corresponding PY of 4.4 g/kWh for 300 J/L. On the other hand, N2O5 was not detected at all under humid air conditions.
Overall, the results showed that a presence of air humidity substantially decreases the SIE (energy density) value for discharge transition from “O3 mode” to “NOx mode”. In dry air, a transition was found between 1000 and 1100 J/L, while with an increase of air RH from 20 to 80%, it gradually decreased from approximately 600 to 450 J/L, respectively. We suppose that upon increasing the humidity a decrease of O3/NOx ratio may be responsible for the observed effect.
The work demonstrated that the multi-hollow surface dielectric barrier discharge is capable of production of various gaseous species in a wide range of concentrations that can be controlled by proper discharge operating conditions. Therefore, we suppose the discharge may eventually find many possible applications, particularly in plasma pollution control and biomedicine.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Cvelbar U, Walsh JL, Černák M et al (2019) White paper on the future of plasma science and technology in plastics and textiles. Plasma Process Polym 16:1–37. https://doi.org/10.1002/ppap.201700228
Šimek M, Černák M, Kylián O et al (2019) White paper on the future of plasma science for optics and glass. Plasma Process Polym 16:1–23. https://doi.org/10.1002/ppap.201700250
Corke TC, Enloe CL, Wilkinson SP (2010) Dielectric barrier discharge plasma actuators for flow control. Annu Rev Fluid Mech 42:505–529. https://doi.org/10.1146/annurev-fluid-121108-145550
Boeuf JP, Pitchford LC (2005) Electrohydrodynamic force and aerodynamic flow acceleration in surface dielectric barrier discharge. J Appl Phys 97:103307. https://doi.org/10.1063/1.1901841
Hackam R, Akiyama H (2000) Air pollution control by electrical discharges. IEEE Trans Dielectr Electr Insul 7:654–683. https://doi.org/10.1109/94.879361
Magureanu M, Bradu C, Parvulescu VI (2018) Plasma processes for the treatment of water contaminated with harmful organic compounds. J Phys D Appl Phys 51:313002. https://doi.org/10.1088/1361-6463/aacd9c
Machala Z, Hensel K, Akishev Y (2012) Plasma for bio-decontamination, medicine and food security. NATO Science for Peace and Security Series A—Chemistry and Biology, Springer, Dordrecht
Moreau M, Orange N, Feuilloley MGJ (2008) Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv 26:610–617. https://doi.org/10.1016/j.biotechadv.2008.08.001
Weltmann KD, Von Woedtke T (2017) Plasma medicine—current state of research and medical application. Plasma Phys Control Fusion 59:14031. https://doi.org/10.1088/0741-3335/59/1/014031
Fridman G, Friedman G, Gutsol A et al (2008) Applied plasma medicine. Plasma Process Polym 5:503–533. https://doi.org/10.1002/ppap.200700154
Puač N, Gherardi M, Shiratani M (2018) Plasma agriculture: a rapidly emerging field. Plasma Process Polym 15:1–5. https://doi.org/10.1002/ppap.201700174
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46. https://doi.org/10.1023/A:1022470901385
Fridman A (2008) Plasma Chemistry. Cambridge University Press
Ono R (2016) Optical diagnostics of reactive species in atmospheric-pressure nonthermal plasma. J Phys D Appl Phys 49:83001. https://doi.org/10.1088/0022-3727/49/8/083001
Liu K, Zheng Z, Liu S, Hu Y (2019) Study on the physical and chemical characteristics of DBD: the effect of N2/O2 mixture ratio on the product regulation. Plasma Chem Plasma Process 39:1255–1274. https://doi.org/10.1007/s11090-019-09998-1
Pavlovich MJ, Clark DS, Graves DB (2014) Quantification of air plasma chemistry for surface disinfection. Plasma Sources Sci Technol 23:065036. https://doi.org/10.1088/0963-0252/23/6/065036
Eliasson B, Egli W, Kogelschatz U (1994) Modelling of dielectric barrier discharge chemistry. Pure Appl Chem 66:1275–1286. https://doi.org/10.1351/pac199466061275
Peyrous R (1990) The effect of relative humidity on ozone production by corona discharge in oxygen or air—a numerical simulation—Part II: Air. Ozone Sci Eng 12:41–64. https://doi.org/10.1080/01919519008552454
Homola T, Krumpolec R, Zemánek M et al (2017) An array of micro-hollow surface dielectric barrier discharges for large-area atmospheric-pressure surface treatments. Plasma Chem Plasma Process 37:1149–1163. https://doi.org/10.1007/s11090-017-9792-z
Nayak G, Du Y, Brandenburg R, Bruggeman PJ (2017) Effect of air flow on the micro-discharge dynamics in an array of integrated coaxial microhollow dielectric barrier discharges. Plasma Sources Sci Technol 26:035001. https://doi.org/10.1371/journal.pone.0080705
Sakai O, Kishimoto Y, Tachibana K (2005) Integrated coaxial-hollow micro dielectric-barrier-discharges for a large-area plasma source operating at around atmospheric pressure. J Phys D Appl Phys 38:431–441. https://doi.org/10.1088/0022-3727/38/3/012
Tachibana K, Nakamura T, Motomura H (2016) Monolithic structure of integrated coaxial microhollow dielectric barrier discharges: Characterization for environmental and biomedical applications. Jpn J Appl Phys 55:07LB01. https://doi.org/10.7567/JJAP.55.07LB01
Cimerman R, Hensel K (2021) Generation of honeycomb discharge assisted by micro-hollow surface dielectric barrier discharge. Int J Plasma Environ Sci Technol 15:e01003. https://doi.org/10.34343/ijpest.2021.15.e01003
Krumpolec R, Richter V, Zemánek M, Homola T (2019) Multi-hollow surface dielectric barrier discharge for plasma treatment of patterned silicon surfaces. Surf Interfaces 16:181–187. https://doi.org/10.1016/j.surfin.2019.01.014
Nayak G, Aboubakr HA, Goyal SM, Bruggeman PJ (2017) Reactive species responsible for the inactivation of feline calicivirus by a two-dimensional array of integrated coaxial microhollow dielectric barrier discharges in air. Plasma Process Polym 15:1–12. https://doi.org/10.1002/ppap.201700119
Aboubakr HA, Nisar M, Nayak G et al (2020) Bactericidal efficacy of a two-dimensional array of integrated, coaxial, microhollow, dielectric barrier discharge plasma against Salmonella enterica Serovar Heidelberg. Foodborne Pathog Dis 17:157–165. https://doi.org/10.1089/fpd.2019.2698
Kelar Tučeková Z, Vacek L, Krumpolec R et al (2021) Multi-hollow surface dielectric barrier discharge for bacterial biofilm decontamination. Molecules 26:910. https://doi.org/10.3390/molecules26040910
Gebremical GG, Admassu S, Berhanu T et al (2019) Optimization and influence of multi-hollow surface dielectric barrier discharge plasma operating conditions on the physical quality of peanut. Eur Phys J D 73:1–16. https://doi.org/10.1140/epjd/e2019-90616-0
Gebremical GG, Emire SA, Berhanu T (2019) Effects of multihollow surface dielectric barrier discharge plasma on chemical and antioxidant properties of peanut. J Food Qual 2019:3702649. https://doi.org/10.1155/2019/3702649
Shimizu K, Ishii T, Blajan M (2010) Emission spectroscopy of pulsed power microplasma for atmospheric pollution control. IEEE Trans Ind Appl 46:1125–1131. https://doi.org/10.1109/TIA.2010.2044968
Homola T, Prukner V, Hoffer P, Šimek M (2020) Multi-hollow surface dielectric barrier discharge: an ozone generator with flexible performance and supreme efficiency. Plasma Sources Sci Technol 29:095014. https://doi.org/10.1088/1361-6595/aba987
Nayak G, Sousa JS, Bruggeman PJ (2017) Singlet delta oxygen production in a 2D micro-discharge array in air: effect of gas residence time and discharge power. J Phys D Appl Phys 50:105205. https://doi.org/10.1088/1361-6463/aa5764
Voráč J, Britun N, Nikiforov A et al (2019) Photofragmentation laser-induced fluorescence of ozone: an in situ tool for precise mapping of ozone concentration in non-thermal plasmas. Appl Phys Express 12:106001. https://doi.org/10.7567/1882-0786/ab3d15
Cherkasov N, Ibhadon AO, Fitzpatrick P (2015) A review of the existing and alternative methods for greener nitrogen fixation. Chem Eng Process 90:24–33. https://doi.org/10.1016/j.cep.2015.02.004
Cimerman R, Maťaš E, Sárený M, Hensel K (2022) Electrical and optical characterization of multi-hollow surface dielectric barrier discharge in configuration with the air-exposed electrode. Phys Plasmas 29:113510. https://doi.org/10.1063/5.0101496
Janda M, Hensel K, Tóth P et al (2021) The role of HNO2 in the generation of plasma-activated water by air transient spark discharge. Appl Sci 11:7053. https://doi.org/10.3390/app11157053
Höft H, Becker MM, Kettlitz M (2016) Impact of gas flow rate on breakdown of filamentary dielectric barrier discharges. Phys Plasmas 23:033504. https://doi.org/10.1063/1.4943278
Jodzis S, Smoliński T, Sówka P (2011) Application of a concentric actuator for ozone synthesis under surface discharge conditions. IEEE Trans Plasma Sci 39:1055–1060. https://doi.org/10.1109/TPS.2011.2105285
Chen J, Davidson JH (2002) Ozone production in the positive DC corona discharge: model and comparison to experiments. Plasma Chem Plasma Process 22:495–522. https://doi.org/10.1023/A:1021315412208
Zhang Y, Liang X, Xu M, Wei L (2018) Energy conversion and temperature dependence in ozone generator fed by synthetic air. Ozone Sci Eng 40:361–371. https://doi.org/10.1080/01919512.2018.1431520
Braun D, Kuchler U, Pietsch G (1988) Behaviour of NOx in air-fed ozonizers. Pure Appl Chem 60:741–746. https://doi.org/10.1351/pac198860050741
Homola T, Pongrác B, Zemánek M, Šimek M (2019) Efficiency of ozone production in coplanar dielectric barrier discharge. Plasma Chem Plasma Process 39:1227–1242. https://doi.org/10.1007/s11090-019-09993-6
Šimek M, Pekárek S, Prukner V (2012) Ozone production using a power modulated surface dielectric barrier discharge in dry synthetic air. Plasma Chem Plasma Process 32:743–754. https://doi.org/10.1007/s11090-012-9382-z
Shimizu T, Sakiyama Y, Graves DB et al (2012) The dynamics of ozone generation and mode transition in air surface micro-discharge plasma at atmospheric pressure. New J Phys 14:103028. https://doi.org/10.1088/1367-2630/14/10/103028
Kogelschatz U, Eliasson B, Hirth M (1988) Ozone generation from oxygen and air: discharge physics and reaction mechanisms. Ozone Sci Eng 10:367–377. https://doi.org/10.1080/01919518808552391
Machala Z, Tarabová B, Sersenová D et al (2019) Chemical and antibacterial effects of plasma activated water: correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J Phys D Appl Phys 52:034002. https://doi.org/10.1088/1361-6463/aae807
Pawłat J, Terebun P, Kwiatkowski M et al (2019) Evaluation of oxidative species in gaseous and liquid phase generated by mini-gliding Arc discharge. Plasma Chem Plasma Process 39:627–642. https://doi.org/10.1007/s11090-019-09974-9
Eliasson B, Kogelschatz U (1991) Modeling and applications of silent discharge plasmas. IEEE Trans Plasma Sci 19:309–323. https://doi.org/10.1109/27.106829
van Gaens W, Bogaerts A (2014) Reaction pathways of biomedically active species in an Ar plasma jet. Plasma Sources Sci Technol 23:035015. https://doi.org/10.1088/0963-0252/23/3/035015
Pekárek S (2011) Effect of catalysts on dc corona discharge poisoning. Eur Phys J D 61:657–662. https://doi.org/10.1140/epjd/e2010-10246-4
Kogelschatz U, Baessler P (1987) Determination of nitrous oxide and dinitrogen pentoxide concentrations in the output of air-fed ozone generators of high power density. Ozone Sci Eng 9:195–206. https://doi.org/10.1080/01919518708552335
Fan X, Kang S, Li J, Zhu T (2018) Formation of nitrogen oxides (N2O, NO, and NO2) in typical plasma and plasma-catalytic processes for air pollution control. Water Air Soil Pollut 229:351. https://doi.org/10.1007/s11270-018-4011-y
Tang X, Wang J, Yi H et al (2017) N2O formation characteristics in dielectric barrier discharge reactor for environmental application: effect of operating parameters. Energy Fuels 31:13901–13908. https://doi.org/10.1021/acs.energyfuels.7b02428
Kučerová K, Machala Z, Hensel K (2020) Transient spark discharge generated in various N2/O2 gas mixtures: reactive species in the gas and water and their antibacterial effects. Plasma Chem Plasma Process 40:749–773. https://doi.org/10.1007/s11090-020-10082-2
Teodoru S, Kusano Y, Bogaerts A (2012) The effect of O2 in a humid O2/N2/NOx gas mixture on NOx and N2O remediation by an atmospheric pressure dielectric barrier discharge. Plasma Processes Polym 9:652–689. https://doi.org/10.1002/ppap.201100187
Abdelaziz AA, Ishijima T, Seto T et al (2016) Characterization of surface dielectric barrier discharge influenced by intermediate frequency for ozone production. Plasma Sources Sci Technol 25:035012. https://doi.org/10.1088/0963-0252/25/3/035012
Zhang X, Lee BJ, Im HG, Cha MS (2016) Ozone production with dielectric barrier discharge: effects of power source and humidity. IEEE Trans Plasma Sci 44:2288–2296. https://doi.org/10.1109/TPS.2016.2601246
Pekárek S, Mikeš J (2014) Temperature-and airflow-related effects of ozone production by surface dielectric barrier discharge in air. Eur Phys J D 68:1–8. https://doi.org/10.1140/epjd/e2014-50393-x
Batakliev T, Georgiev V, Anachkov M et al (2014) Ozone decomposition. Interdiscip Toxicol 7:47–59. https://doi.org/10.2478/intox-2014-0008
Kim HY, Kang SK, Kwon HC et al (2013) Gas temperature effect on reactive species generation from the atmospheric pressure air plasma. Plasma Process Polym 10:686–697. https://doi.org/10.1002/ppap.201200163
Jodzis S (2011) Application of technical kinetics for macroscopic analysis of ozone synthesis process. Ind Eng Chem Res 50:6053–6060. https://doi.org/10.1021/ie102603z
Benson SW, Axworthy AE (1957) Mechanism of the gas phase, thermal decomposition of ozone. J Chem Phys 26:1727–1733. https://doi.org/10.1063/1.1743610
Itoh H, Taguchi M, Suzuki S (2020) Thermal decomposition of ozone at high temperature leading to ozone zero phenomena. J Phys D Appl Phys 53:185206. https://doi.org/10.1088/1361-6463/ab71a9
Osawa N, Yoshioka Y (2015) Ozone and by-products generation characteristics by novel air-fed ozone generator which combines homogeneous discharge and filamentary discharge. In: 22th international symposium on plasma chemistry. Antwerp, Belgium, pp 5–9
Pavlovich MJ, Chang HW, Sakiyama Y et al (2013) Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J Phys D Appl Phys 46:145202. https://doi.org/10.1088/0022-3727/46/14/145202
Wang H, Zhuang Z, Sun Ch et al (2016) Numerical evaluation of the effectiveness of NO2 and N2O5 generation during the NO ozonation process. J Environ Sci (China) 41:51–58. https://doi.org/10.1016/j.jes.2015.05.015
Abdelaziz AA, Ishijima T, Osawa N, Seto T (2019) Quantitative analysis of ozone and nitrogen oxides produced by a low power miniaturized surface dielectric barrier discharge: effect of oxygen content and humidity level. Plasma Chem Plasma Process 39:165–185. https://doi.org/10.1007/s11090-018-9942-y
Falkenstein Z, Coogan JJ (1997) Microdischarge behaviour in the silent discharge of nitrogen-oxygen and water-air mixtures. J Phys D Appl Phys 30:817–825. https://doi.org/10.1088/0022-3727/30/5/015
Ryzko H (1965) Drift velocity of electrons and ions in dry and humid air and in water vapour. Proc Phys Soc 85:1283–1295. https://doi.org/10.1088/0370-1328/85/6/327
Chen J, Wang P (2005) Effect of relative humidity on electron distribution and ozone production by DC coronas in air. IEEE Trans Plasma Sci 33:808–812. https://doi.org/10.1109/TPS.2005.844530
Lopez JL, Vezzu G, Freilich A, Paolini B (2013) Effects of hydrocarbon contamination on ozone generation with dielectric barrier discharges. Eur Phys J D 67:1–8. https://doi.org/10.1140/epjd/e2013-40126-2
Ono R, Oda T (2003) Dynamics of ozone and OH radicals generated by pulsed corona discharge in humid-air flow reactor measured by laser spectroscopy. J Appl Phys 93:5876–5882. https://doi.org/10.1063/1.1567796
Matsui K, Ikenaga N, Sakudo N (2015) Effects of humidity on sterilization of Geobacillus stearothermophilus spores with plasma-excited neutral gas. Jpn J Appl Phys 54:06GD02. https://doi.org/10.7567/JJAP.54.06GD02
de Baerdemaeker K, Nikiforov A, de Geyter N, Devlieghere F (2022) Cold plasma for bacterial decontamination: Impact of food matrix composition and RH of the input gas. In: 9th Central European symposium on plasma chemistry (CESPC-9) joint with COST action CA19110 Plasma Applications for Smart and Sustainable Agriculture (PlAgri). Faculty of Mathematics, Physics and Informatics, Comenius University in Bratislava, p 98
Acknowledgements
This work was supported by Slovak Research and Development Agency Grants APVV-20-0566 and APVV-17-0382, and Slovak Grant Agency VEGA 1/0822/21.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. As stated in the Acknowledgement, this work was supported by Slovak Research and Development Agency grants APVV-20–0566 and APVV-17–0382, and Slovak Grant Agency VEGA 1/0822/21.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Experiments, analyses, data collection and processing were performed by R.C. The first draft of the manuscript was written by R.C. Revision of the draft of the manuscript was performed by K.H. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
This declaration is “not applicable”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cimerman, R., Hensel, K. Multi-hollow Surface Dielectric Barrier Discharge: Production of Gaseous Species Under Various Air Flow Rates and Relative Humidities. Plasma Chem Plasma Process 43, 1411–1433 (2023). https://doi.org/10.1007/s11090-023-10381-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10381-4