Abstract
This comprehensive review explores decade-long research conducted on the applications of Cold Atmospheric Plasma (CAP) and Plasma Activated Liquids (PAL), including Plasma Activated Water (PAW), in the fields of Medicine and Dentistry. CAPs, operating at atmospheric pressure, offer unique advantages over conventional medical devices, generating Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) that interact with substances, enabling diverse applications. The review examined CAP and PAL efficacy against infectious diseases using in vitro, ex vivo, in vivo, and direct application methods. Significant strides were observed in wound healing, cancer treatment, and dental care. However, ensuring patient safety through rigorous plasma source standards remains crucial. The study underscores CAPs and PALs’ potential to transform medical and dental therapies, urging further research and development in these groundbreaking technologies. These findings highlight the transformative impact of CAPs and PALs, offering promising avenues for innovative medical and dental treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the fields of medicine and dentistry, a diverse range of therapeutic tools has been harnessed to address various health conditions and challenges. From traditional pharmaceutical interventions to cutting-edge surgical techniques, the quest for effective and innovative therapies continues to drive research and development in these crucial domains.
Among the recent advancements, Cold Atmospheric Plasmas (CAPs) have emerged as a promising technology with the potential to revolutionize various applications in academia and industry. What sets CAPs apart from conventional medical devices is their unique ability to operate at atmospheric pressure, distinguish them from other technologies requiring different pressure levels for therapeutic interventions.
The distinguishing feature of operating at atmospheric pressure empowers CAPs to generate a diverse array of active agents, each holding immense potential for applications across a multitude of medical and dental scenarios. Figure 1 showcases CAP generation with diverse sources, such as RONS, UV radiation, and electric fields, promising applications in direct and indirect treatments. Among the noteworthy active agents produced by CAPs are Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), collectively referred to as RONS. These reactive species have garnered significant attention due to their remarkable ability to interact with various substances and induce chemical reactions, thereby playing a pivotal role in numerous fields. In the biomedical sector, RONS have demonstrated valuable antimicrobial properties, making them particularly advantageous for sterilization and disinfection processes. Their potency against harmful microorganisms has shown promise in effectively combating infections and safeguarding patients in clinical settings [1, 2]. Moreover, CAPs have the capacity to generate ultraviolet (UV) photons, characterized by their high energy levels. These UV photons hold tremendous significance in a myriad of applications. For instance, they are instrumental in sterilization processes where their ability to initiate photochemical reactions proves invaluable. By targeting the DNA of microorganisms, UV photons can lead to their inactivation, ensuring a hygienic environment and enhancing patient safety [3]. Furthermore, CAPs possess the unique capability to generate charged particles, offering vast potential in numerous applications. In healthcare, these charged particles find utility in ionizing radiation treatments, while in technology, they contribute to the creation of microelectronic devices, among other uses. The cancer therapy is one area that stands to benefit significantly from CAPs, as the charged particles produced by these plasmas can cause DNA damage in cancer cells, ultimately leading to their death [4].

(adapted from [5])
A general scheme illustrating different sources of atmospheric cold plasma generation, capable of producing reactive agents such as reactive oxygen and nitrogen species (RONS), UV radiation, electric fields, among others, for direct and indirect treatment methodologies, with high potential for application in medical sciences.
Over the past decade, CAPs have drawn considerable interest in the scientific community due to their distinctive properties and diverse potential applications in the fields of engineering, materials science, environment, medicine, etc. In the field of medicine, CAP have made a significant impact, providing innovative therapeutic applications such as wound healing, cancer treatment, and medical equipment sterilization [5]. Furthermore, the interaction of CAPs with living tissues has unveiled a multitude of simultaneous biological effects, encompassing antimicrobial, anti-inflammatory, tissue repair, and antineoplastic properties [2, 6]. In dentistry, CAPs have also demonstrated their transformative potential, contributing notably to dental implant sterilization, tooth whitening, and the treatment of various oral diseases [7], further underlining their promise in revolutionizing dental treatments, and enhancing patient outcomes.
Another exciting area of research within the field of CAPs is plasma-activated liquids (PAL), with plasma-activated water (PAW) being the most extensively studied. PAW refers to water that has been exposed to CAPs, resulting in the generation of reactive species and other active agents within the water [7]. This PAW has shown promising potential in various applications, such as disinfection, agriculture, food safety, and wastewater treatment [8,9,10]. Additionally, recent studies have introduced the concept of indirect plasma treatment, wherein PAL is utilized as a treatment agent in various applications, further expanding the potential of CAP technology [9,10,11].
Understanding CAPs’ biological effects, especially in treating infectious diseases, involves a comprehensive investigation employing diverse methodologies (in vitro, ex vivo, and in vivo). This approach provides valuable insights into the potential benefits and limitations of CAPs as therapeutic tools. Furthermore, different methods of CAP application, such as direct tissue contact and the use of PALs, including PAW, have been examined. The goal is to optimize the therapeutic potential of CAPs and PALs and develop tailored treatment strategies for specific medical and dental applications.
The development of plasma sources also comprises a significant aspect of the activities of several research groups in the CAP field. For a plasma source to be suitable for a given medical application, the resulting plasma must adhere to certain safety standards, particularly if the plasma plume is intended to come into contact with human tissues. The gas temperature of the plasma plume and the electrical current that leaks to the patient must not exceed 40 °C and 100 µA-AC, respectively, to avoid potential damage to living tissues [12, 13]. Evaluations of UV light emission and production of harmful gases by the plasma plume are also crucial, as these parameters not only affect the patient but also the operator, and thus determine the plasma exposure time and operating time of the plasma source [14]. Therefore, one of the main focuses in the development of plasma sources has been on creating devices that comply with these safety standards.
This comprehensive review investigates the diverse applications of CAPs in medicine and dentistry, spanning a decade-long research study. It offers an in-depth exploration of CAPs, including their generated chemistry and subsequent biological effects. A particular emphasis is placed on PALs and, more specifically, PAW. Furthermore, we delve into the evolution of CAP devices and their applications in medical and dental fields, revealing groundbreaking advancements in plasma technology that are revolutionizing medical and dental therapies. Safety standards for plasma sources are extensively discussed, along with insights from our work in developing compliant devices. The examination of CAPs and PALs applications provides a comprehensive understanding of their current status and future prospects in the medical and dental realms. As we explore potential applications and advancements, we also highlight the necessity for further research to fully unleash the transformative capabilities of PALs, potentially heralding a new era of medical and dental therapies.
Evolution of CAP Devices and Applications in Medical and Dental Fields
Currently, various sources of atmospheric plasma excitation are available, including high-frequency sources (in the order of kHz), radio-frequency sources (MHz), microwave sources (GHz), and the recent piezoelectric sources. A fascinating historical perspective reveals the initial application of high-frequency irradiation for various diseases, dating back to the early decades of the last century. Frederick Strong, an American physician and electrical engineer, pioneered the use of high-frequency currents for therapeutic purposes [15]. In his 1908 book “High Frequency Currents”, Strong claimed to invent the vacuum electrode in 1897, later used widely in Europe around 1900. Apparatuses unintentionally produced spark effluvia via glass electrodes, representing CAP. Strong connected patients to a Tesla coil with a metal hand electrode, protected by a glass tube to prevent sparks. He replaced it with a Geissler vacuum tube, inventing the Vacuum Electrode [15, 16]. Lowering gas pressure inside the tube generated X-rays and UV light. Strong found diverse physiological and medical effects, like promoting circulation and germicidal action [15]. Recent research shows the vacuum electrode (sometimes called the ‘violet ray’ or ‘violet wand’ [15]) generating antimicrobial plasma, suggesting CAP treatments as a rediscovery. Contemporary investigations echoed Strong’s work, offering potential for further research and therapeutic efficacy rediscovery.
One recent paper compared antimicrobial effects of the historical vacuum electrode device with recent CAP devices [16]. Results align with observations in Crook’s 1909 book on antimicrobial effects of brush discharges [15, 16]. In another study, Daschlein et al. also arrived at the conclusion that the vacuum electrode exhibited no significant difference in antimicrobial activity when compared to modern CAP sources [17]. They emphasized that this advantageous effect is not solely due to the device’s psychosomatic or mystic light show aspects. The vacuum electrode was sold worldwide from the early 20th century until the 1950s and remains accessible today. While its medical value was likely overstated and misused, undeniable similarities to modern plasma devices exist.
In the medical field, surgical procedures have undergone a transformative revolution with the introduction of cutting-edge electrosurgical devices and endoscopic instruments, including plasma scalpels and argon plasma coagulators. Traditionally, these devices relied on high-temperature plasma, characterized by elevated gas temperatures. However, recent years have witnessed significant strides in the development of distinct CAP plasma sources, offering unique effects compared to their high-temperature counterparts [18]. This remarkable advancement has paved the way for specialized methods, particularly in dermatology and dentistry, focusing on treating solid surfaces.
Among the modern plasma devices, several stand out as parallel to historical devices used in local high-frequency current treatments. The dielectric barrier discharge (DBD) plasmas, gliding arc plasma jet, and surfatron microwave-driven plasma are noteworthy examples. These contemporary devices effectively utilize plasma in contact with cells, tissues, or organisms, showcasing similarities with historical devices like the vacuum electrode and other DBD-like configurations. This parallelism illustrates a continuous evolution of research and development in the field of plasma medicine.
Figure 2 provides an insightful timeline, depicting representative plasma devices that have been instrumental in medical and dental treatments from the beginning of the last century up to the present day. Moreover, it is worth noting that recent reviews have extensively explored a wide array of modern non-thermal plasma medical devices, offering comprehensive insights into their applications and potential advancements [18,19,20,21].
In the late 2000s, multiple Low-Temperature Plasma (LTP) sources received approval for both cosmetic and medical purposes. Notably, in 2008, the US FDA approved the Rhytec Portrait® (plasma jet) for use in dermatology. Additionally, several other plasma devices, including the Bovie J-Plasma®, the Canady Helios Cold Plasma and Hybrid Plasma™ Scalpel, are currently in use in the US for various medical applications [22].
In Germany, some CAP devices received “CE certification” for clinical testing in 2013. Notable examples include the kINPen MED (by Neoplas Tools GmbH in Greifswald, Germany), which utilizes an RF-powered (1 MHz) Ar jet [20], and the MicroPlasSter (by ADTEC in Hunslow, UK), operating as a microwave-powered (2.45 GHz) Ar plasma torch [21]. These devices are designed to treat tissues without significant thermal heating. Another device, the PlasmaDerm (by CINOGY GmbH in Duderstadt, Germany), operates with a DBD in open air, functioning in a non-thermal manner [21].
In 2009, the piezoelectric direct discharge (PDD), a new atmospheric pressure gaseous discharge producing cold plasma, was commercially released [23]. It utilizes a piezoelectric cold plasma generator (PCPG) with a resonant piezoelectric transformer (RPT) to generate > 10 kV output voltage at low input voltage (< 25 V). PDD ignites micro-discharges directly at the ceramic surface, distinguishing it from other discharges. Since 2009 the company Relyon Plasma GmbH commercialize PDD based piezo-brushes [23].
Over the last decade, Cold Atmospheric Plasmas (CAPs) have experienced a remarkable surge of interest within the scientific community, primarily due to their unique properties and diverse potential applications in medical sciences. This increasing trend is vividly illustrated by the substantial number of scientific papers published annually, as demonstrated in Fig. 3. The figure provides a breakdown of research areas in “Cold atmospheric plasma”, including subdivisions related to medicine, as per the Scopus database.
(a) Division of research areas in “Cold atmospheric plasmas” with the subdivisions of areas comprising medicine according to the Scopus database. (b) Number of publications as a function of year for the keywords: “Cold plasma + medicine”; “Cold plasma + dentistry” (inside Figure (b), data for “Plasma activated water + Medicine” and “Plasma activated water + Dentistry” are also presented).
Furthermore, Fig. 3(b) displays the number of publications over time for specific keywords like “Cold plasma + medicine” and “Cold plasma + dentistry”. Notably, the data also encompasses information on “Plasma activated water + Medicine” and “Plasma activated water + Dentistry”, particularly emphasizing the growth of research in these areas, especially within the medical field. However, it is important to note that in dentistry, the number of works is still relatively modest.
The information conveyed by Fig. 3 underscores the significant interest and investment in CAP research, especially its potential in medical applications. The emerging prominence of PAW research further demonstrates its relevance and promising role in medical science. Although dentistry shows comparatively fewer works, the overall trajectory indicates a compelling and dynamic landscape for the future of CAP and PAW applications in various clinical domains.
Table 1 provides a comprehensive overview of the diverse applications of CAPs in the field of medicine. The listed review papers highlight the significant research efforts into CAP’s potential across critical areas such as wound healing, cancer treatment, dermatology, virus inactivation, and pathogen inactivation. These reviews emphasize the efficacy of CAP-generated reactive species in addressing a wide range of medical challenges, ranging from cancer attenuation to the inactivation of viruses and pathogens. Particularly noteworthy is CAP’s unique ability to generate Reactive Oxygen and Nitrogen Species (RONS), presenting a valuable resource in exploring new approaches for water treatment and other relevant fields.
In the context of virus inactivation, CAP has demonstrated a noteworthy contribution in addressing the SARS-CoV-2 pandemic, with several studies focusing on its potential for virus inactivation and destruction [32, 43, 48]. The generation of reactive species through physical and cold atmospheric plasma techniques has shown promising results in combating the virus and contributing to the efforts against COVID-19. CAP’s ability to effectively inactivate viruses, including SARS-CoV-2, opens new possibilities in the field of medical science and presents a compelling area for further research and application.
Table 2 sheds light on the transformative potential of Cold Atmospheric Plasma (CAP) in the field of dentistry, encompassing a wide range of dental applications. The reviews emphasize CAP’s role in dental implant sterilization, tooth whitening, and the treatment of oral diseases. Notably, CAP’s antimicrobial properties emerge as a pivotal factor in effectively combating dental pathogens and supporting overall oral health. Its contributions to dental implantology and various dental procedures exemplify CAP’s significance in advancing dental treatments and promoting oral well-being.
The review articles highlight the areas of dentistry where CAP has made significant strides, including endodontics [58, 62, 63], cariology [58, 59], oral oncology [58], oral candidiasis [57, 58], and periodontal disease [61]. CAP’s therapeutic perspective in treating periodontal disease underscores its potential in addressing challenging dental conditions. Additionally, CAP’s applications extend to resin-dentin bonding, adhesive bonding efficiency, and dentin wetting, showing promise in enhancing dental materials and techniques [54,55,56].
Overall, the data of Table 2 emphasizes the wide-reaching impact of CAP in dentistry, reflecting its potential to revolutionize dental practices and enhance patient care. As CAP continues to show promising results and gain momentum in dental research, it opens up new horizons for innovative and advanced dental treatments across various fields within dentistry.
Overview of Cold Atmospheric Plasmas
As seen in the previous section, significant efforts have been devoted to the development and utilization of various distinct CAP systems. These systems, such as DBD-based plasmas, gliding arc discharges, and surface-wave discharges, have garnered significant attention and recognition within the scientific community. This is primarily attributed to their wide range of applications, including liquid activation, surface treatment, sterilization, and water treatment [64,65,66,67,68,69,70].
Each of these plasma generation techniques is characterized by unique plasma configurations and mechanisms that are tailored to facilitate the generation of highly reactive species. These reactive species, upon interaction with liquids, can initiate substantial chemical and biological transformations. Such transformations are pivotal in the fields where these plasma systems are implemented, leading to practical advancements and innovative solutions [71,72,73].
In the following sections, we explore the key features and characteristics of the three plasma generation techniques that have been extensively utilized and studied. To provide a comprehensive overview, we refer to Table 3, which presents the main characteristics of the cold atmospheric plasma devices most commonly used in the literature.
Dielectric Barrier Discharge (DBD)
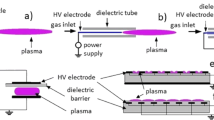
DBD stands as a versatile and flexible plasma generation technique, characterized by its distinct construction. This technique typically consists of two parallel electrodes, with a dielectric barrier inserted between them, thus defining its name. The dielectric barrier plays a crucial role in preventing the transition from a glow discharge into an arc discharge, thereby facilitating a stable and uniform plasma field [84].
The inherent versatility of DBD is largely attributed to its ability to be tailored to a myriad of setups. These setups encompass but are not limited to, planar, cylindrical, and coaxial configurations (Fig. 4). Each configuration manifests its own unique set of properties, deriving from the geometry and arrangement of the electrodes and dielectric [84].
Some types of DBD reactors: (A) Planar with two dielectrics in contact with both electrodes; (B) Planar with one dielectric without contact with both electrodes; (C) Planar with one dielectric in contact with the high-voltage electrode; (D) Coaxial with one dielectric in contact with the high-voltage inner electrode; and (E) Tubular DBD jet reactor with ring grounded electrode
In a planar configuration, the electrodes are arranged flatly, in parallel to each other. This setup is particularly useful for surface treatment applications, where a large, flat surface needs to be uniformly treated. The cylindrical configuration, on the other hand, involves one electrode being wrapped around another, often with the dielectric material in between. This configuration can be especially effective for concentrated plasma applications, where a stronger, more focused plasma field is needed [85].
The coaxial configuration situates the electrodes on the same axis but with one inside the other. This design allows for a more focused and intense plasma field along the axis, making it ideal for applications such as plasma drilling or cutting. Lastly, the sliding configuration, a more complex and dynamic setup, can adjust the plasma field by moving one electrode relative to the other. This can allow for more precise control over the plasma field and is particularly suited to applications that require a varying plasma effect [86, 87].
A particularly important and frequently utilized application of DBD is the plasma jet. DBD plasma jets are typically used for surface treatment applications, biomedical applications, and even in environmental remediation. The plasma jet generates a directed stream of plasma that can be precisely controlled, allowing for localized treatment with high specificity [88].
The unique configuration of DBD equips it with the capability to generate a multitude of reactive species, both in gas and liquid phases. When we delve into the gas phase, DBD facilitates the production of several species, each of which serves specific roles in a variety of applications [89]. For instance, DBD assists in the formation of ozone (O3), a strong oxidant known for its disinfecting and deodorizing properties, making it particularly useful in air and water purification processes. Atomic oxygen (O), another species generated by DBD, is an integral part of many chemical reactions due to its high reactivity, and it is especially significant in combustion processes. Singlet oxygen (O2*), another reactive species produced in the gas phase by DBD, holds notable potential in applications involving disinfection and sterilization [77,78,79, 89]. This is due to its higher reactivity compared to ground-state oxygen, making it more efficient in destroying bacteria and other harmful organisms [79]. Hydroxyl radicals (OH*), also generated by DBD, are powerful oxidants that contribute to numerous reactions and play a crucial role in many environmental and atmospheric processes. Lastly, nitrogen dioxide (NO2), another product of DBD’s gas phase reactions, is an important reactive gas in various industrial and environmental contexts, including air pollution monitoring and control.
Switching to the liquid phase, DBD primarily contributes to the formation of hydrogen peroxide (H2O2). This is achieved through reactions involving hydroxyl radicals (OH*) and atomic oxygen (O) [77, 89]. Hydrogen peroxide is a valuable compound in many applications, such as disinfection, sterilization, and wastewater treatment, to name a few. However, it is crucial to note that the production rate of H2O2 can be influenced by specific experimental conditions and the presence of additional species within the system [76, 77, 90]. This highlights the importance of careful control and understanding of the system to achieve the desired results.
Furthermore, DBD exhibits a unique capability of generating nitrite (NO2−) and nitrate (NO3−), through the activation of reactive species such as hydroxyl radicals (OH*) and atomic oxygen (O) [91]. These are important compounds in a range of biological and environmental contexts, including nitrogen cycling in ecosystems, water quality monitoring, and food preservation [77, 78, 85, 91,92,93].
Gliding Arc
In the medical and dental fields, gliding arc plasma has emerged as a promising and cutting-edge technology with diverse applications. Y. D. Korolev conducted a comprehensive review exploring low-current discharges in a gas flow at atmospheric pressure, highlighting the study of glow discharges in coaxial plasmatrons and gliding arcs [94]. These gliding arcs are known for generating plasma jets containing active chemical species with extensive applications in surface modification, biology, and medicine. Notably, plasma discharges in nitrogen-oxygen mixtures have been utilized to produce nitric oxide (NO), which serves as a valuable vessel dilator in inhalation therapy.
Figure 5 illustrates the structure of a rotating gliding arc reactor. The Gliding Arc (GA) is a plasma system that lies between non-thermal and thermal plasmas, offering unique characteristics and potential applications. It has the capability to simultaneously produce high plasma densities, a high degree of non-equilibrium, higher electron temperatures, lower gas temperatures, and the ability to stimulate specific chemical processes without quenching [94]. This dynamic feature enhances the interaction of the GA plasma with liquids and surfaces, making it a versatile tool with broad applications [72]. In the healthcare realm, especially in medicine and dentistry, the GA technique’s capacity to generate highly energetic and chemically reactive plasma is particularly promising. This leads to the production of various reactive species, including hydroxyl radicals (OH*), nitrogen radicals (N*), atomic oxygen (O), ozone (O3), and nitrogen oxides (NOx) [79, 80]. The high electron density and energy of the GA plasma stimulate intense chemical reactions, enabling the production of these species in significant quantities, expanding its applicability in medicine and dentistry [95, 96].
In dentistry, plasma-liquid technology, such as the generation of plasma-activated water (PAW) through the GA, is gaining significant attention [80]. PAW, rich with active constituents, offers potential clinical advantages in treating various oral diseases [80]. The versatility and potential of plasma liquid technology, found in applications ranging from agriculture and the food industry to healthcare, make it an exciting approach in dentistry. The use of PAW in dentistry is supported by the potential of direct and indirect application of non-thermal atmospheric pressure plasmas, including the GA technique [99,100,101]. The GA technique capabilities open new possibilities for effective and innovative dental treatments, promising advancements in oral healthcare and disease treatment.
Surface-Wave Discharge
The Surface-wave discharge technique, often referred to as the surfatron, is a plasma methodology featuring a plasma source encased within a microwave cavity (Fig. 6). This cavity hosts a plasma-quartz tube, the interior of which is equipped with a slit. The slit is a crucial component of the surfatron, as it allows for the effective coupling of electromagnetic energy to the plasma via surface waves. These waves subsequently propagate along the quartz tube, thereby enabling the creation of plasma in regions distal from the surfatron while maintaining the self-consistent propagation of the surface wave. The production of the plasma column persists until the power supply is insufficient for its sustenance.
The surfatron has been expertly adapted for usage at elevated frequencies, specifically 2.45 GHz, catering to situations that require high microwave power levels over 500 W, or necessitate large tube diameters, measuring 15 mm or more.
As a versatile tool, the surfatron can generate a broad spectrum of reactive species. These include, but are not limited to, hydroxyl radicals (OH*), oxygen radicals (O*), ozone (O3), nitrite (NO2−), nitrate (NO3−), and hydrogen peroxide (H2O2) [20]. Notwithstanding, the precise production levels of these reactive species can fluctuate, subject to a variety of factors. Influencing variables include the choice of gas, the composition of the liquid, the power applied, and the specific experimental conditions. Thus, meticulous optimization of these parameters is paramount to maximize the production of specific reactive species and to ensure the overall efficacy of the process [102].
In the context of medical and dental applications, the surfatron is making strides as a potential tool for advanced treatment protocols. Previous research indicates the promise of plasma technologies in fields such as dentistry, where PAW is being explored for various oral diseases [14, 99,100,101, 103,104,105,106,107,108].
Critical Safety Standards of Plasma Sources
Plasma sources, a critical component in a variety of applications, particularly in the medical and industrial sectors, are required to adhere to a comprehensive set of safety standards [14]. These standards, which encompass a wide array of physical and chemical properties, play a pivotal role in ensuring the safe and effective operation of plasma sources. The adherence to these standards not only ensures the safety of the end-users but also enhances the overall performance and reliability of these plasma sources.
One of the primary standards that plasma sources must comply with is the DIN SPEC 91315:2014-06 (General requirements for plasma sources in medicine) [109]. This standard, recognized globally, provides a detailed framework for the general requirements that plasma sources in the medical field must meet. It mandates a series of rigorous experiments designed to assess the potential health hazards associated with atmospheric pressure plasmas [14]. Key properties, including gas temperature, thermal output, heat flow to the treated surface, patient leakage current, UV radiation, and ozone emission, are carefully evaluated to ensure the plasma source operates within safe parameters for a given “plasma dosage.“ Depending on the exposure or treatment intensity, influenced by plasma device, treatment, and target parameters, plasma can have cell stimulatory or inhibitory effects or induce sub-lethal cellular damage (Fig. 7). This principle aligns with the concepts of “oxidative eustress,“ promoting physiological responses, and “oxidative distress,“ causing pathophysiological signaling, proposed in redox biology [14, 24].
The safety of plasma exposure is of utmost importance, given the potential risks associated with cell toxicity, immunogenicity, sensitization, genotoxicity, and potential carcinogenicity. This concern carries significant weight in various sectors, including medicine, healthcare, food, agriculture, and other fields utilizing plasma technology. Prioritizing safety and comprehending the potential side effects of plasma exposure are critical in unlocking its full potential and expanding its applications in the biomedical domain [24].

(adapted from [24])
The balance between cell stimulatory and cell inhibitory effects is dependent on the plasma exposure/treatment intensity, which is determined by device and treatment parameters, as well as characteristics of the target cells and their environment. Genotoxic effects can occur when DNA damage exceeds or escapes the capacity of cellular repair mechanisms at sub-lethal plasma treatments.
Another crucial standard that plasma sources must adhere to is the IEC DIN EN 60,601 [110]. This standard, developed by the International Electrotechnical Commission (IEC), guarantees the safe operation of the plasma jet as a piece of medical-electrical equipment. It outlines four types of leakage currents: ground leakage current, housing leakage current, patient leakage current, and patient auxiliary current. Each of these leakage currents is carefully measured and controlled to ensure the safety of the patients and the operators. Of these, the patient leakage current under standard conditions is particularly significant in the context of plasma sources [14]. This is because any excessive leakage current can pose a risk to the patient, making its control paramount.
The gas temperature and heat flow of the plasma source are also vital safety considerations that must not be overlooked [14]. Cold plasmas, known for their lower gas temperatures, are commonly employed in medical applications due to their safety and effectiveness. The heat flow of the atmospheric pressure plasma jet (APJ) is ascertained by a detailed examination of the transient temperature curve during the heating-up phase. This analysis helps ensure that the heat flow remains within safe operating limits, thereby preventing any potential thermal damage to the treated tissues or surfaces.
UV emission is another safety factor that requires meticulous monitoring and control [14]. Plasma sources, especially those incorporating nitrogen as a shielding gas, can emit dominant nitrogen bands in the UVB and UVA regions. Exposure limits and threshold values for UV radiation are contingent on various factors, including the velocity and movement of the plasma source over the skin and the skin’s condition. Therefore, careful assessment and control of UV emission are necessary to prevent any potential harm to the patients or operators.
Despite the implementation of nitrogen shielding, a notable concentration of ozone can be detected in the vicinity of the plasma source. This ozone emission, while a natural byproduct of the operation of plasma sources, is a critical safety consideration that must be factored into the final design of a medical device. Excessive ozone concentration can be harmful, and therefore, appropriate measures must be taken to control its emission.
In sum, the safety standards for plasma sources are comprehensive and multifaceted, encompassing a wide range of physical and chemical properties. These standards ensure that the plasma sources operate safely and effectively, thereby enhancing their reliability and performance. Nevertheless, compliance with these standards is not the end of the road. Additional investigations and certifications, such as ISO 13,485 and risk assessment in accordance with DIN EN ISO 14,971, are required to fulfill the stipulations of the Medical Device Directive (MDD) for CE marking [14]. These additional certifications provide an extra layer of assurance, further reinforcing the commitment to safety and quality in the operation of plasma sources. By adhering to these rigorous safety standards and certifications, plasma sources can be confidently employed in biomedical applications, from disinfection and wound healing to blood coagulation and cancer treatment, with the assurance of safety and efficacy.
Evolution of the Plasma Sources Used to Produce He-APPJs
A plasma source can be separated in two basic parts: the power supply and the plasma device. The first provides the energy required to ignite a discharge with the second one. The studies in plasma medicine from our research group started using a parallel plate dielectric barrier discharge (DBD) device in which the discharge was driven by a low-frequency (60 Hz) high-voltage (HV) power supply [111]. In the later years, the research started to be developed using He-APPJs. Then, the plasma source was based on a commercial power supply composed of an AC generator from GBS Elektronik GmbH (model Minipuls4), an arbitrary function generator from RIGOL (model DG1012) and a DC voltage generator. With that power supply it was possible to compare the use of sinusoidal voltage in continuous wave mode or in burst mode. The last can be seen as a pulse-like waveform. An example of that is shown in Fig. 8, where Tr is the repetition period of the burst and Δt is the time interval in which the plasma discharge occurs [112]. The oscillation frequency inside each burst is of the order of 30 kHz.
Burst waveform [112]
One of the requirements for the application of APPJs in dentistry is that the plasma plume must be produced by a handable device in a way that it fits in the oral cavity of a patient. This problem was solved by our research group after the development of a device that is able to produce a remote plasma plume at the end tip of long, thin and flexible plastic tubes [113]. The complete plasma device consists of a dielectric barrier discharge (DBD) reactor, composed by a metal pin electrode encapsulated into a closed-end quartz tube, which, in turn, is placed inside a dielectric chamber. A 1-meter long flexible plastic tube is connected to the reactor exit. Inside this tube is placed a thin copper wire, which ends 2 mm before the outlet tip of the plastic tube.
The basic functioning of this plasma source is as follows: a continuous gas flow is injected inside the reactor and flows out through the long tube. When the high-voltage is applied to the pin electrode, a primary plasma discharge is formed inside the reactor chamber. Once this primary DBD discharge is ignited, it polarizes the wire inside the long tube. Eventually, the electric field induced on the wire tip is sufficient to generate a small (remote) discharge, the plasma jet, at the distal end of the plastic tube. The tip of the conducting wire does not touch the glass tube or any electrified part of the DBD device, so the wire acts as a floating electrode. This plasma production method is also referred to as jet transfer technique. The generation of remote plasma jets was also studied by other research groups. More recently, this technique has been employed in a device aimed for endoscopic applications [114, 115].
By using the plasma device with a long tube and the HV in burst mode, it was possible to generate He-APPJ with a good safety degree. In addition, the physical properties of the plasma jet, mainly production of reactive species and discharge power, proved to be suitable for medical applications [116]. Thus, the studies focused on plasma medicine and dentistry that followed were based on this configuration [117,118,119,120,121,122,123,124]. Most importantly, with such configuration working as expected and proving antimicrobial efficacy it was possible to establish an effective and safe protocol for in vivo treatment of oral candidiasis using He-APPJs [119].
In recent years, the research group started new studies aimed to reduce the dimensions of the plasma source. For this purpose, the first step was to replace the above-mentioned commercial power supply by a home-made one, which is portable and has considerably smaller dimensions. The main physical features of the initial plasma source were maintained (use of a long tube and the jet transfer technique). The waveforms produced by each power supply are quite different, but both produce pulse-like high-voltage signals. The homemade power supply generates a damped-sine waveform, as can be seen in Fig. 9 [125, 126].
Damped-sine waveform (reproduced from [126])
All those differences between the electrical characteristics of the power supplies result in significant differences in some properties of the plasma jets produced by each plasma source, being that the production of reactive species and the discharge power are the main ones. However, an important parameter, the gas temperature (Tgas) in the plasma plume, remained at the same order and below 40 °C when operating in the free jet mode [112, 126]. When using the portable device with the plasma plume impinging on a conducting surface, Tgas is also lower than 40 °C [126]. An interesting phenomenon pointed out in the work by Nascimento et al. [126] was that one of the main heating sources of a He-APPJ can be the helium gas itself and the Tgas values tend to increase as the distance from the plasma outlet is incremented.
Besides the evolution in the development of the plasma source using the jet transfer technique with a thin wire inside the long tube, our research group developed an alternative configuration for the long tube part of the plasma device. Such configuration consists in a concentric arrangement of a long tube composed by a metallic mesh, with 90% closed area, over a polytetrafluoroethylene (PTFE) tube with both placed inside a Nylon 6 tube [126, 127]. Initial tests showed that with this setup the effective electric current passing through the plasma can be up to three times smaller than in the configuration that uses the thin wire [128]. This is basically due to the additional dielectric barrier, the PTFE tube, between the conductor and the plasma jet. A reduction of such electrical current is one of the necessary steps to increase device safety in plasma medicine applications.
Most of the studies with the mesh assembly were carried out using argon as the working gas. However, basic tests with such configuration producing He-APPJs were also performed to assess its antimicrobial efficacy. Satisfactory results were achieved with the formation of inhibition zones in Escherichia coli colonies in in vitro assays. A plasma source employing the mesh configuration is still under development and will eventually replace the one assembled with the wire.
Exploring the Potentialities of Non-Thermal Atmospheric Pressure Plasma Jets
Cold atmospheric plasmas (CAPs) have emerged as a promising technology with remarkable potential in the field of medicine and dentistry due to its antimicrobial, anti-inflammatory, wound healing, and anti-cancer potentialities which encompass a wide range of clinical applications.
CAPs have demonstrated efficacy against a range of microbial species, including bacteria, fungi and virus making them valuable tools for combating various infections. In particular, CAPs have shown inhibitory effects on both planktonic cells and biofilms, which are highly organized microbial communities known for their increased resistance to antimicrobial agents. This resistance poses a significant challenge in medical and dental settings. Therefore, the ability of CAPs to target and reduce the viability of biofilms offers new possibilities for overcoming this obstacle.
Extensive studies have been conducted on the effectiveness of Helium CAP and Helium non-thermal atmospheric pressure plasma jets (He-APPJs) in combating microbial infections. These investigations have revealed the inhibitory effects of CAPs on a variety of microbial species, such as Staphylococcus aureus, E. coli, Enterococcus faecalis, Pseudomonas aeruginosa, Candida albicans, and methicillin-resistant Staphylococcus aureus (MRSA) [129].
Kostov et al. reported the inhibitory effect of Helium CAP generated inside Petri dishes on bacterial suspensions, observing a significant reduction in viable cells of both S. aureus and E. coli, with evidence of cell wall damage detected by scanning electron micrograph [130]. Subsequently, our focus shifted to Helium non-thermal atmospheric pressure plasma jets (He-APPJs), which have the advantage of generating cold plasma plumes not confined by electrodes, making them suitable for biomedical applications. Nishime et al. findings indicated that He-APPJ exhibited antimicrobial effects on the Gram-positive bacterium E. faecalis (ATCC 29,212), the Gram-negative bacterium P. aeruginosa (ATCC 15,442), and the fungus C. albicans (SC 5314) [131]. Notably, E. faecalis was found to be the most susceptible species, while C. albicans demonstrated higher resistance.
CAP’s antifungal effect against medically relevant species has been observed, positioning it as a potential therapeutic alternative for recalcitrant fungal infections. Fungal infections present a significant challenge in medicine due to increasing antifungal resistance and limited therapeutic options. CAP has shown the ability to reduce Trichophyton rubrum growth, germination, and adherence to the nail. Fungal suspensions exposed to the plasma jet for 10 and 15 min were unable to infect nail specimens in an ex vivo infection model [132].
Wang et al. [133]. studied the production of N2O5 using dielectric barrier discharge (DBD) and gliding arc reactor. They found that the presence of N2O5 in the effluent gas led to efficient sterilization effects against bacteria. Moreau et al. [134] provided a comprehensive overview of plasma biodecontamination, emphasizing GA discharge’s ability to generate an abundance of reactive species. Gliding arc techniques showed high efficiency against various bacterial species, making it a promising option for decontamination processes.
In addition to antifungal and antibacterial studies on plasma, plasma-mediated virus inactivation is a recent tool of study on plasma medicine that has shown interesting results. Different kind of viruses such as respiratory and sexually transmitted viruses have been evaluated [32]. CAP treatment inactivated respiratory syncytial virus, the main responsible for lower respiratory tract infections in infants, on a glass surface after 5 min of exposure [135]. Chen et al. investigated CAP effect on SARS-CoV-2 distributed on the surface of metal, plastic, cardboard, and composite leather used for various kind of balls. CAP showed good potential inactivating SARS-CoV-2 on all the evaluated surfaces [136]. These findings point out that CAP could be an interesting source of respiratory virus decontamination, mainly in hospital environments where virus can spread easily. In other study focused HIV virus, macrophages were previously exposed to CAP for three times in a total of 45 s of exposure. After, these cells were infected with HIV and the authors observed that CAP treatment reduced the viral reverse transcriptase activity and impaired the steps necessary for successful of virus infection, with no cytotoxic effects to the cells [137]. These findings hold great promise for the development of future innovative therapeutic approaches for viruses’ treatment.
Considering the broad antimicrobial spectrum, CAPs prove to be particularly interesting for the treatment of infectious diseases. Importantly, our studies have revealed that CAPs exhibit inhibitory effects not only on planktonic cells but also on biofilms, which are highly organized microbial communities. Biofilm formation is one of the most relevant virulence factors of pathogenic microorganisms. Moreover, biofilms have been shown to be more resistant to external factors, such as antimicrobials, when compared to planktonic cells [138].
The utilization of CAPs in various applications, including wound healing has been a key focus of several research groups. The migration and proliferation of keratinocytes and fibroblasts as well as the formation of the new blood vessels (angiogenesis) is necessary to complete wound healing. In this scenario is important the decontamination of the wound as the bacterial colonization with biofilm formation can impair the proper tissue repair [139]. In the context of wound healing, CAPs have demonstrated their potential to reduce the overall viability of biofilms associated with chronic wounds, including those caused by MRSA, P. aeruginosa, and E. faecalis. The protocol was effective against these microorganisms after 5 min of exposure, with low cytotoxicity and genotoxicity to a mouse fibroblastic cell line (3T3) [140]. Additionally, our ongoing in vivo studies on mice and rabbits have shown promising results in terms of tissue repair after exposure to CAPs for 5 min.
Similarly, other studies focused on CAP applications for wound healing have demonstrated satisfactory outcomes in acute and chronic medical conditions that require wound healing. The exposition of acute ulcers to CAP lead to reduced traumatic wound and its associated inflammation, progressive wound repair in genital wart and decreased urticarial and pain of burn wound. For chronic ulcers, the exposition to CAP has also brought positive outcomes such as reduced microbial burden, faster collagen synthesis, angiogenesis, re-epithelialization and finally progressive repair [41].
In vivo studies focused on skin inflammatory diseases have demonstrated an anti-inflammatory potential of CAP, which is crucial for the tissue repair. Positive findings were observed after CAP treatment of allergic contact dermatitis resulting in reduction of inflammation and accelerated healing and for atopic dermatitis, where CAP promoted a decrease of eosinophil and mast cell infiltration, in addition to an inhibition of T helper 2 (Th2) cell differentiation [141, 142].
Vasilets reviewed the potential therapeutic applications of nitric oxide-containing plasma gas. The antimicrobial properties of NO and other species, along with the promotion of tissue regeneration, make it valuable for medical purposes across diverse fields [143]. Nguyen et al. demonstrated the application of GA plasma for the treatment of a failed finger perforator flap [144]. The treatment stimulated tissue repair, exhibited antimicrobial effects, and improved blood circulation, promising advancements in regenerative medicine.
Concern the investigations with surface-wave plasmas, E. Benova et al. have discovered operational regimes that allow the application of a microwave plasma torch for treating biological systems without causing thermal damage. Their investigations have shown significant bactericidal [145] and virucidal [146] effects within remarkably short treatment times (5–30 s), much faster than the typical 2–5 min needed with MicroPlaSter [147]. Furthermore, preliminary experiments on wound treatment in mice [148] have inspired further research in this area. Notably, the production of crucial RONS species for bio-medical plasma effects occurs even when pure argon is used as the working gas, due to the plasma torch’s interaction with ambient air [149]. These findings underscore the potential of microwave plasma torches for diverse bio-medical applications, offering rapid and effective treatments without significant thermal damage.
In the field of dentistry, CAPs offer simultaneous antimicrobial, anti-inflammatory, and tissue-repairing effects, making them highly valuable for the treatment of multiple oral diseases (Fig. 10).
CAP properties on decontamination of caries lesions in minimally invasive techniques have been reported. Dental caries is a multifactorial disease influenced by interactions between the tooth structure and the microbial biofilm formed on the tooth surface. These interactions are modulated by factors such as sugars, salivary properties, and genetic factors [150]. A systematic review that evaluated the effect of CAP on dental pathogens observed that CAP is a promising tool against the bacteria associated with dental caries, such as S. mutans, L. casei and L. fermentum. However, the authors pointed out that most of the studies have evaluated mono-species biofilms [151]. Considering that multi-species biofilms are more representative of a real clinical situation and more difficult to be completely eliminated, future studies should focus on CAP treatment applied to different kind of multi-specie biofilms.
Previously, our group showed that Argon CAP produced by kINPen09™ demonstrated inhibitory effects on multispecies cariogenic biofilms composed of Streptococcus mutans, Streptococcus sanguinis, and Streptococcus gordonii on hydroxyapatite discs [152]. Ongoing studies on the effect of Helium CAP on these biofilms are also showing promising results.
Microorganisms and their products can reach the root canal, promoting the evolution of dental caries to endodontic/periapical disease. E faecalis is a bacterium that can penetrate the radial dentin tubules up to 1000 micrometers. Besides that, E faecalis is highly associated with endodontic reinfection. Effectiveness of CAP in eliminating this pathogen has been observed [151]. Its antimicrobial activity against ex vivo root canals biofilms from extracted teeth has also been reported, although in this study the CAP treatment was not so effective as the use of 6% NaOCl [153].
Candidiasis is a fungal infection commonly caused by the opportunistic pathogen C. abicans, which can proliferate and cause disease in the presence of local and/or systemic predisposing factors. In immunocompromised patients the superficial lesions may progress to life-threatening disseminated infections. The inhibitory effect of Helium-CAP has been reported against C. albicans (SC 5314 and ATCC 18,804) as well as against five clinical isolates from denture stomatitis lesions [154]. Helium-CAP has demonstrated the ability to modulate C. albicans virulence traits, significantly reducing the filamentation rate by nearly 40 times, as well as decreasing adherence to epithelial cells and biofilm formation [155]. Importantly, the CAP protocol showed low cytotoxicity to Vero cells [156]. In an in vivo study on the treatment of experimentally induced oral candidiasis with Helium-CAP, simultaneous anti-inflammatory and inhibitory effects on fungal morphogenesis were observed, with a marked reduction in tissue invasion [156].
CAP effectiveness against C.albicans was also reported by Ebrahimi-Shaghaghi et al., who also analyzed some mechanisms of action related to this potential. CAP showed inhibitory effects on planktonic and biofilm forms of C. albicans [157]. It was observed a reduction of a critical sterol of fungal membrane (ergosterol) in a time dependent manner from 0 to 210 s. The expression of the heat shock protein 90 kDa (HSP90) which is important in the pathogenesis of C. albicans was inhibited in the strains ATCC 10,231 and PFCC 9362 after 210 s of exposure. Additionally, CAP exposition induced intracellular ROS, which may induce membrane damage and fungus cell death [157].
Periodontal disease is an inflammatory oral condition that affects the supporting structures of the teeth composed by the gingiva, periodontal ligament and bone. It is a common cause of tooth loss and may contribute to systemic inflammation, leading to worsening of other pathological conditions. The dysbiosis of oral microbiota and dental plaque formation are crucial for the development of the disease. The bacteria from the dental plaque interacts with the immune defenses of the host, leading to inflammation which is responsible for tissue loss and progression of the disease [158].
In a previous study, inhibitory effects of Helium CAP on Porphyromonas gingivalis / Streptococcus gordonii and P. gingivalis HW24D-1 mature biofilms were reported, along with low cytotoxicity to human gingival fibroblasts [159]. Moreover, it exibited minimal genotoxicity to keratinocytes (OBA-9) [152]. In vivo testing using a model of experimental periodontitis induced in c57bl/6 mice demonstrated that a 5-minute CAP exposure tended to improve periodontal tissue recovery, as evaluated by mineral tissue improvement and Type I collagen percentage [159]. These findings suggest the potential of CAP in promoting periodontal tissue healing.
CAP potentialities on periodontal disease were previously explored in other studies [160]. The authors concluded that the efficacy is firstly related to the breakdown of bacterial DNA, RNA and proteins via ROS/RNS-mediated oxidative stress. Additionally, CAP can attenuate the inflammatory response and help in the soft and hard tissue remodeling. The anti-inflammatory property is related to the downregulation of pro-inflammatory cytokines such as TNFα, IL-6 and IL1-β and upregulation of the anti-inflammatory cytokine IL-10 in human periodontal ligament cells (1hPDLCs). Less number of inflammatory cells has been observed in in vivo CAP-treated periodontal disease, which demonstrate this anti-inflammatory potential. The soft tissue remodeling probably occurs via upregulation of cell proliferation and extracellular matrix factors in human gingival fibroblasts (hHGFs), human gingival keratinocytes (hHGKs) and hPDLCs. Moreover, CAP can also be useful to hard tissue regeneration by osteoblastic differentiation of hPDLCs [160].
The anti-inflammatory potential of CAP has also been explored in the context of oral lichen planus disease, which is an inflammatory disease with an associated autoimmune component that can reach the oral mucosa. The treatment of ex-vivo tissues promoted downregulation of many pro-inflammatory cytokines and reduced number of T-helper (CD4+) and cytotoxic T cells (CD8+) in CAP treated tissues [161]. These promising results still need further in vivo validation, but open perspectives for application of CAP in the field of stomatology focused on inflammatory and immune-mediated diseases.
Another CAP ability that has been explored in medical and dentistry fields is the anti-cancer property. Cancer cells have higher amount of aquaporins (water channels) while they have lower amount of cholesterol compared to normal cells, which make them more susceptible to reactive oxygen and nitrogen species [162]. Some mechanisms of action for plasma efficacy on cancer cells are the impairment of cell division and migration ability, gene expression modulation and apoptosis, induction of immune system, enhance of drug effectiveness and direct tumor cell death by ROS. Many in vitro studies have investigated cancer cell lines from different lineages treated with CAP in combination with the most used medications for cancer treatment. Interesting findings as synergistic anti-cancer effect with increase in the cytotoxic potential of the drug have been observed after CAP treatment [163].
Head and neck squamous cell carcinoma (SCC) represent more than 90% of oral cancers [164]. A previous clinical study with advanced cases of SCC showed that CAP may reduce the pain, the typical fetid odor related to the lesions and may decrease the microbial load with some partial remission of the tumor or wound healing of infected ulcerations [165]. In addition, in vitro studies have demonstrated that CAP may induce EGFR dysfunction in EGFR-overexpressing oral SCC cells through reactive species and may increase the number of dead cancer cells [166]. In this scenario, CAP seems to be a promising tool for (1) ameliorating the side effects of advanced oral cancers and (2) playing an interesting role as an adjunct treatment.
CAP has also been investigated in context of dental implants. A previous study indicated that CAP treatment has not interfered in the elemental composition of different dental implant materials. Additionally, higher proliferation of human gingival fibroblast (HGF-1) and osteoblast-like cells (MG-63) were observed after CAP treatment of the cells for 120 s [167]. CAP treatment of contaminated implant surfaces has demonstrated inactivation or reduction on viability and quantity of biofilms in vitro studies. Moreover, a highly uniform presence of osteogenic tissue and a closer interaction with CAP-treated implant surfaces with improvement of the osseointegration has also been reported in vivo studies [54]. Considering that infection is one of the causes of dental implant failure and the desirable improvement of osseointegration [168], these positive outcomes indicate that CAP could be used in dental implant practice. Its application could be in the biomaterial surface or directly in the treated area at the time of the implantation.
Tooth bleaching is another tool of CAP in dentistry field, which differently from the other conditions previously discussed is this section, is not related to CAP antimicrobial, anti-inflammatory, tissue-repairing or anti-cancer effects. Despite, the generation of ROS is also responsible for tooth bleaching. High-efficiency tooth bleaching has been reached using low concentration of hydrogen peroxide associated with CAP for 10 min [169] or only with CAP treatment after 3 exposures of 60 s [54]. In the last case, CAP was applied in different days simultaneously with root canal disinfection, which is not possible with other bleaching procedures [54]. These findings open perspectives for CAP acting as bleaching agent. The possibility of replacing conventional light sources is desirable as they may present controversy bleaching efficacy and induce high temperatures that can be harmful to surround tissues [54, 169].
Taken together, the potentialities of non-thermal atmospheric pressure plasma in medical and dentistry fields are multiple and dependent on the applied substrate i.e., mammalian cells, cancer cells, different kind of microorganisms, infected ulcers, tooth surface or biomaterial surfaces, such as dental implants. Optimized conditions for each clinical setting should be used to achieve the desired goal for each condition. The adaptation of the plasma device for intra-oral application was a crucial experimental step of our group. A small plasma jet was launched from the end of a long flexible plastic tube, allowing for precise targeting of the plasma jet within the oral cavity [170].
Exploring the Potential of Plasma-Activated Liquids (PAL)
In a recent review by Noala et al. extensive research on Plasma-Activated Liquids (PAL), with a focus on Plasma-Activated Water (PAW), within dentistry is presented [7]. PAL has proven highly advantageous in clinical settings, revolutionizing the treatment of challenging conditions that conventional plasmas struggle to address. Notably, these innovative liquids show promising results in dental applications, such as surgical wound washing, root canal irrigation, and treatment in difficult-to-reach areas within the body.
Various plasma-based devices, including GA plasma jets, surface or coaxial Dielectric Barrier Discharges (DBDs), and surfatron plasma jets, have been utilized to generate PAL from different sources like deionized water, distilled water, tap water, and saline water. For example, the GA plasma jet is used to produce PAL, investigating its antimicrobial properties through direct liquid application [171] and nebulized PAW against bacteria like S. aureus and E. coli [172]. Nebulized PAW has consistent physicochemical properties even during transportation through tubes, making it effective for controlling microbiota in respiratory tract appliances [172].
In another study, the GA device was used to activate different liquids with varying properties, such as deionized water, distilled water, and tap water. The resulting PAL showed significant inhibitory effects on E. coli, and their safety for medical and dental applications was confirmed through minimal cytotoxicity to normal oral keratinocytes (NOK) [173, 174].
The effectiveness of PAW in disinfecting various dental materials, including endodontic devices, silicone, diamond dental drills, metals, titanium alloy surfaces, and titanium disc surfaces, has been demonstrated [7]. The use of PAW as an alternative disinfection method presents advantages over traditional techniques, and its antimicrobial effects are attributed to the main reactive species and low pH acquired during plasma activation [175]. PAW’s long-lasting antimicrobial effect when stored at low temperatures further supports its potential for dental sterilization [176]. Studies also indicate that PAW is effective in decontaminating dental unit waterline system tubes and shows promise in the decontamination of other dental materials [177, 178]. These findings highlight PAW’s potential as a new sterilization method in dentistry, with opportunities for further exploration of other materials and polymicrobial biofilms relevant to clinical settings [179].
Treatment of oral infectious diseases using PAW is a promising field of research, with potential applications in various oral conditions, such as caries, periodontitis, and candidiasis [7]. In the case of dental caries, PAW has shown efficacy against cariogenic bacteria like Streptococcus mutans and Lactobacillus spp., which are responsible for tooth demineralization [180]. PAW activated with a non-thermal plasma of argon and oxygen reduced the viability of S. mutans in both planktonic and biofilm forms [181]. PAW also demonstrated a reduction in S. mutans, Porphyromonas gingivalis, and Actinomyces viscosus within minutes of treatment [180]. Moreover, studies suggest PAW’s potential role in enhancing bond strength in adhesive restorations, which could prevent microleakage and secondary caries [59].
Regarding oral candidiasis, PAW has shown antimicrobial effects against C. albicans in planktonic and biofilm forms [119]. Investigations into the effects of PAW on C. albicans revealed a reduction in viability, and the main components responsible for its antifungal activity were suggested to be hydroxyl radicals and NO3− [182]. However, one study reported no effect of plasma-activated tap water on C. albicans [171]. In endodontic infections, PAW has been investigated for its potential as an antimicrobial irrigator in root canals [182]. Studies demonstrated a reduction in Enterococcus faecalis viability in biofilms after short exposures to PAW [183]. PAW also exhibited antimicrobial effects against E. coli and S. aureus, which are occasionally found in root canals [184]. In periodontal disease treatment, PAW showed a progressive inhibition of Porphyromonas gingivalis in both planktonic and biofilm forms [122]. Considering the restricted access of subgingival biofilm, PAW’s ability to reach such areas makes it an interesting treatment option [180].
PAW has been extensively studied and found to contain various reactive oxygen and nitrogen species (RONS) that offer promising applications in the antimicrobial field [173]. Research has demonstrated its efficacy in treating surfaces contaminated by bacterial biofilms, including those associated with dental devices [174]. Furthermore, PAW has shown potential in reducing bacterial colonies associated with oral infectious diseases [89]. Further in vitro and in vivo investigations are needed to standardize the best parameters for each solution and microorganism to harness the full potential of PAW in treating oral infectious diseases [7].
The non-thermal plasma treatment using PAW has shown promising anti-inflammatory properties and wound healing capabilities, making it a potential alternative for the treatment of infectious diseases in dentistry [7].
PAW has demonstrated selectivity in its antimicrobial action, causing no damage to mammalian cells [185]. Long-term exposure of PAW on vital mouse teeth did not result in significant changes in tooth structure or toxicity to surrounding tissues [186]. Furthermore, PAW has been shown to decrease inflammation in various studies, including oral candidiasis treatment in mice and as an adjuvant therapy for periodontitis in rats [119]. It was observed that PAW could suppress pro-inflammatory genes and cytokines, thus potentially acting against both acute and chronic inflammation [187].
PAW has also demonstrated favorable effects on wound healing, promoting cell proliferation and migration in human keratinocytes [188]. Although direct studies on the effect of PAW or PAL on oral wound healing are lacking, observations in rats and mice suggest a tendency to improve periodontal tissue loss after CAP treatment [189].
The potential applications of PAW in oral surgery and implantology are promising. CAP treatment has shown positive effects on osteoblast-like cells, suggesting potential use in oral surgery for wound closure and healing [190]. Moreover, PAW could enhance dental implant surfaces, promoting better osseointegration [59].
The simplicity of PAW’s use as a mouthwash or irrigation agent, along with its antimicrobial, anti-inflammatory, and wound healing properties, makes it a potential adjuvant tool in dentistry for conditions requiring tissue repair [7].
Indirect CAP treatment using PAW has demonstrated promising anti-cancer properties, with cancer cells being more sensitive to the harmful effects of reactive oxygen and nitrogen species than healthy cells [32]. The higher expression of water channels (aquaporins) in cancer cells allows for easier penetration of reactive species, leading to various types of cell death, such as apoptosis, necrosis, or senescence [32].
Using PAW in cancer therapy is promising due to its ease of use and ability to treat large or deep tumors [32, 191, 192]. The treatment is faster, more comfortable for patients, and can inhibit tumor growth and metastasis [191,192,193]. In oral squamous cell lines, PAL has shown anti-cancer properties, with reductions in cell viability [194].
The potential applications of PAW in oral cancer therapy are significant. PAW could be used before surgery by washing or injecting into deep neoplasms and after surgery to reduce microbial load and promote wound healing, potentially preventing recurrences [7]. PAW could also be used in oral premalignant lesions, offering a non-invasive approach alongside other therapies or as a standalone treatment, depending on individual patient factors [7].
Conclusion
In conclusion, the exploration of CAP and its various applications, including PAL, has opened exciting possibilities in the fields of medicine and dentistry. CAPs have proven to be highly effective in combating a wide range of microbial species, including bacteria, fungi, and viruses. Notably, CAPs have shown inhibitory effects on biofilms, which are notoriously resistant to conventional antimicrobial agents, presenting a significant challenge in clinical settings. Moreover, CAPs demonstrate remarkable potential in wound healing, tissue repair, and inflammatory conditions. The generation of RONS in PAL, such as PAW, further enhances their antimicrobial properties. PAW has shown promise in treating contaminated surfaces, decontaminating dental devices, and addressing oral infectious diseases. The versatility of CAPs lies in their ability to be applied through various plasma sources, such as gliding arc, DBDs, and surfatron plasma jets, each offering specific advantages in the activation process of PAL. Importantly, safety studies have demonstrated minimal cytotoxicity to healthy cells, making these innovations potentially safe for use in diverse medical and dental applications. The ongoing research and development in this field hold great potential for revolutionizing clinical approaches, providing effective and safe treatments for infectious diseases, wound healing, and oral conditions. The antimicrobial, anti-inflammatory, and tissue-regenerating properties of CAPs make them valuable tools for improving patient care and enhancing treatment outcomes. As the understanding of plasma science and its potential applications continues to expand, we can look forward to witnessing even more innovative and transformative uses of CAPs in the medical and dental fields.
Data Availability
Not applicable.
References
Hoffmann C, Berganza C, Zhang J (2013) Cold Atmospheric plasma: methods of production and application in dentistry and oncology. Med Gas Res 3(1):21. https://doi.org/10.1186/2045-9912-3-21PMID: 24083477; PMCID: PMC4016545
Borges AC, Kostov KG, Pessoa RS, de Abreu GMA, Lima GdMG, Figueira LW, Koga-Ito CY (2021) Applications of Cold Atmospheric pressure plasma in Dentistry. Appl Sci 11:1975. https://doi.org/10.3390/app11051975
Braný D, Dvorská D, Halašová E, Škovierová H (2020) Cold Atmospheric plasma: a powerful Tool for Modern Medicine. Int J Mol Sci 21(8):2932. https://doi.org/10.3390/ijms21082932PMID: 32331263; PMCID: PMC7215620
Y, Dayun JH, Sherman, and Michael Keidar (2017) Cold Atmospheric plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 8(9):15977–15995. https://doi.org/10.18632/oncotarget.13304
Chen Z, Chen G, Obenchain R, Zhang R, Bai F, Fang T, Wang H, Lu Y, Wirz RE, Gu Z (2022) Cold atmospheric plasma delivery for biomedical applications. Mater Today 54:153–188. https://doi.org/10.1016/j.mattod.2022.03.001
Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J, Zimmermann JL (2009) Plasma medicine: an introductory review. New J Phys 11:115012
Milhan NVM, Chiappim W, Sampaio AdG, Vegian MRdC, Pessoa RS, Koga-Ito CY (2022) Applications of plasma-activated water in Dentistry: a review. Int J Mol Sci 23:4131. https://doi.org/10.3390/ijms23084131
R, Thirumdas A, Kothakota U, Annapure K, Siliveru R, Blundell R, Gatt VP, Valdramidis (2018) Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci Technol 77:21–31. https://doi.org/10.1016/j.tifs.2018.05.007
Laroussi M, Plasma Medicine (2018) A brief introduction. Plasma 1:47–60. https://doi.org/10.3390/plasma1010005
NH, Neuenfeldt LP, Silva RS, Pessoa LO, Rocha (2023) Cold plasma technology for controlling toxigenic fungi and mycotoxins in food. Curr Opin Food Sci 101045. https://doi.org/10.1016/j.cofs.2023.101045
Melo TFd, Rocha LC, Silva RP, Pessoa RS, Negreiros AMP, Sales Júnior R, Tavares MB, Alves Junior C (2022) Plasma–Saline Water Interaction: A Systematic Review Materials 15:4854. https://doi.org/10.3390/ma15144854
Mann MS, Tiede T, Gavenis K, Daeschlein G, Bussiahn R, Weltmann K-D, Emmert S, von Woedtke T, Almed R (2016) Introduction to DIN-specification 91315 based on the characterization of the plasma jet kINPen® MED. Clin Plasma Med 4:35–45
Gerling T, Helmke A, Weltmann K-D (2018) In: Metelmann H-R et al (eds) Comprehensive clinical plasma medicine: Cold Physical plasma for medical application, 1st edn. Springer International Publishing
A, Lehmann F, Pietag Th, Arnold (2017) Human health risk evaluation of a microwave-driven atmospheric plasma jet as medical device. Clin Plasma Med 7–8. https://doi.org/10.1016/j.cpme.2017.06.001
G (2018) Lessons from Tesla for plasma medicine. IEEE Trans Radiation Plasma Med Sci 2:594–607. https://doi.org/10.1109/TRPMS.2018.2866373
Napp J, Daeschlein G, Napp M, von Podewils S, Gümbel D, Spitzmueller R, Fornaciari P, Hinz P, Jünger M (2015) On the history of plasma treatment and comparison of microbiostatic efficacy of a historical high-frequency plasma device with two modern devices. GMS Hygiene and Infection Control 10. https://doi.org/10.3205/dgkh000251
Daeschlein G, Napp M, von Podewils S, Scholz S, Arnold A, Emmert S, Haase H, Napp J, Spitzmueller R, Gümbel D, Jünger M (2015) Antimicrobial efficacy of a historical high-frequency plasma apparatus in comparison with 2 Modern, Cold Atmospheric pressure plasma Devices. Surg Innov 22:394–400. https://doi.org/10.1177/1553350615573584
Ishikawa K, Takeda K, Yoshimura S, Kondo T, Tanaka H, Toyokuni S, Nakamura K, Kajiyama H (2023) Masaaki Mizuno and Masaru Hori. “Generation and measurement of low-temperature plasma for cancer therapy: a historical review. Free Radic Res 57:239–270. https://doi.org/10.1080/10715762.2023.2230351
Isbary G, Shimizu T, Li Yang-fang, Stolz W, Thomas HM, Morfill GE, Zimmermann JL (2013) Cold atmospheric plasma devices for medical issues. Expert Rev Med Dev 10:367–377. https://doi.org/10.1586/erd.13.4
Reuter S, von Woedtke T, Weltmann K (2018) The kINPen—a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D 51. https://doi.org/10.1088/1361-6463/aab3ad
K (2015) Sigrid and Silke Arndt. “Plasma medicine in dermatology: Mechanisms of action and clinical applications” Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete 66 11 : 819 – 28. https://doi.org/10.1007/s00105-015-3686-x
Laroussi M (2018) “Plasma Medicine: A Brief Introduction.” 1, 5; https://doi.org/10.3390/plasma1010005
Korzec, Darius Dr, Hoppenthaler F, Nettesheim S (2020) “Piezoelectric Direct Discharge: Devices and Applications.” Plasma : n. pag. https://doi.org/10.3390/plasma4010001
Haertel B, von Woedtke T, Weltmann K, Lindequist U (2014) Non-Thermal Atmospheric-Pressure plasma possible application in Wound Healing, vol 22. Biomolecules & Therapeutics, pp 477–490
Boehm D, Bourke P (2018) Safety implications of plasma-induced effects in living cells – a review of in vitro and in vivo findings. Biol Chem 400:3–17
Bernhardt T, Semmler ML, Schäfer M, Bekeschus S, Emmert S, Boeckmann L (2019) Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Medicine and Cellular Longevity, 2019
Mitra S, Nguyen LN, Akter M, Park G, Choi EH, Kaushik NK (2019) Impact of ROS generated by Chemical, Physical, and plasma techniques on Cancer Attenuation. Cancers, p 11
Dai, Xiaofeng Z, Zhang J, Zhang, Kostya Ken Ostrikov (2020) Dosing: the key to precision plasma oncology. Plasma Processes Polym 17:1900178
L, Dawei Y, Zhang M, Xu H, Chen X, Lu, Kostya Ken Ostrikov (2020) Cold atmospheric pressure plasmas in dermatology: sources, reactive agents, and therapeutic effects. Plasma Processes Polym 17:1900218
Boeckmann L, Schäfer M, Bernhardt T, Semmler ML, Jung O, Ojak G, Fischer T, Peters K, Nebe B, Müller-Hilke B, Seebauer C, Bekeschus S, Emmert S (2020) Cold Atmospheric pressure plasma in Wound Healing and Cancer Treatment. Applied Sciences
Braný D, Dvorská D, Halasová E, Skovierová H (2020) Cold Atmospheric plasma: a powerful Tool for Modern Medicine. Int J Mol Sci, 21
Semmler ML, Bekeschus S, Schäfer M, Bernhardt T, Fischer T, Witzke K, Seebauer C, Rebl H, Grambow E, Vollmar B, Nebe JB, Metelmann HR, Woedtke TV, Emmert S, Boeckmann L (2020) Molecular Mechanisms of the efficacy of Cold Atmospheric pressure plasma (CAP) in Cancer Treatment. Cancers (Basel) 12(2):269. https://doi.org/10.3390/cancers12020269
Filipić A, Gutierrez-Aguirre I, Primc G, Mozetič M, Dobnik D (2020) Cold plasma, a New Hope in the field of virus inactivation. Trends Biotechnol 38(11):1278–1291. https://doi.org/10.1016/j.tibtech.2020.04.003
Busco G, Éric, Robert N, Chettouh-Hammas JM, Pouvesle, Grillon C “The emerging potential of cold atmospheric plasma in skin biology.” Free radical biology & medicine (2020): n. pag. https://doi.org/10.1016/j.freeradbiomed.2020.10.004
Malyavko A, Wang DYanQ, Klein AL, Patel K, Sherman JH, Keidar M (2020) Cold atmospheric plasma cancer treatment, direct versus indirect approaches. Mater Adv. https://doi.org/10.1039/d0ma00329h. n. pag
Domonkos M, Tichá P, Trejbal J, Demo P (2021) Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Applied Sciences, 11, 4809
Garner AL, Mehlhorn TA (2021) A review of Cold Atmospheric pressure Plasmas for Trauma and Acute Care. Frontiers of Physics
Scholtz V, Vaňková E, Kašparová P, Premanath R, Karunasagar I, Julák J (2021) Non-thermal plasma treatment of ESKAPE Pathogens: a review. Front Microbiol, 12
M-S (2021) Miguel, Juan Tornín, María Pau Ginebra and Cristina Canal. “Cold Atmospheric plasma: a New Strategy based primarily on oxidative stress for Osteosarcoma Therapy. J Clin Med 10. https://doi.org/10.3390/jcm10040893. n. pag
Chen Z, Chen G, Obenchain R, Zhang R, Bai F, Fang T, Wang H, Lu Y, Wirz RE, Gu Z (2022) Cold atmospheric plasma delivery for biomedical applications. Materials Today
Deepak GD, Atul (2022) Biomedical Applications of Cold plasma. Journal Of Clinical And Diagnostic Research
Bhattacharjee B, Bezbaruah R, Rynjah D, Newar A, Sengupta S, Pegu P, Dey N, Bora S, Barman D (2023) Cold Atmospheric plasma: a Noteworthy Approach in Medical Science. Sciences of Pharmacy
Chupradit S, Widjaja G, Radhi Majeed B, Kuznetsova M, Ansari MJ, Suksatan W, Turki Jalil A, Esfahani G, B (2023) Recent advances in cold atmospheric plasma (CAP) for breast cancer therapy. Cell Biol Int 47:327–340. https://doi.org/10.1002/cbin.11939
Chen Z, Bai F, Jonas SJ, Wirz RE (2022) Cold atmospheric plasma for addressing the COVID-19 pandemic. Plasma Process Polym 19:e2200012. https://doi.org/10.1002/ppap.202200012
Murillo D, Huergo C, Gallego B, Rodríguez R, Tornín J (2023) Exploring the Use of Cold Atmospheric plasma to Overcome Drug Resistance in Cancer, vol 11. Biomedicines
Singh S, Chandra R, Tripathi SK, Rahman H, Tripathi PK, Jain A, Gupta P (2014) The bright future of dentistry with cold plasma - review. IOSR J Dent Med Sci 13:06–13
Zhang, Jingqi F, Li K, Lu W, Zhang, Ma J (2023) Recent advances in cold atmospheric plasma for tumor therapy. Process Biochem. https://doi.org/10.1016/j.procbio.2023.06.009. n. pag
Kaushik N, Mitra S, Baek EJ, Nguyen LN, Bhartiya P, Kim June-hyun (2022) Eun ha Choi and Nagendra Kumar Kaushik. “The inactivation and destruction of viruses by reactive oxygen species generated through physical and cold atmospheric plasma techniques: current status and perspectives. J Adv Res 43:59–71. https://doi.org/10.1016/j.jare.2022.03.002
Dai X, Zhu K (2023) Cold atmospheric plasma: novel opportunities for tumor microenvironment targeting. Cancer Med 12:7189–7206. https://doi.org/10.1002/cam4.5491
Singh S, Chandra R, Tripathi SK, Rahman H, Tripathi PK, Jain A, Pulkit Gupta (2014) The bright future of dentistry with cold plasma - review. IOSR J Dent Med Sci 13:06–13. https://doi.org/10.9790/0853-131040613
Arora, Nikhil, Nk S, Arora P (2013) Cold Atmospheric plasma (CAP) in Dentistry. Dentistry 3000(4):1–6
K, Ch S, Sarada PP, Reddy Ch S, Reddy M, S., Dsv N (2014) Plasma torch toothbrush a new insight in fear free dentistry. J Clin Diagn research: JCDR 8(6):ZE07–10
Li H, Zhang X, Zhu X, Zheng M, Liu S, Qi X, Wang K, Chen J, Xi X, Tan J, Ostrikov KK (2017) Translational plasma stomatology: applications of cold atmospheric plasmas in dentistry and their extension
Hui WL, Perrotti V, Iaculli F, Piattelli A, Quaranta A (2020) The emerging role of Cold Atmospheric plasma in Implantology: a review of the literature. Nanomaterials, 10
Awad MM, Alhalabi F, Alshehri A, Aljeaidi ZA, Alrahlah A (2021) Mutlu Özcan and Hamdi Hosni Hamama. “Effect of Non-Thermal Atmospheric plasma on Micro-Tensile Bond Strength at Adhesive/Dentin interface: a systematic review. Materials 14. https://doi.org/10.3390/ma14041026. n. pag
S, Jovana N, Pficer JK, Miličić B (2021) Nevena Puač and Vesna Miletic. “Effects of non-thermal atmospheric plasma on dentin wetting and adhesive bonding efficiency: systematic review and meta-analysis. J Dent 103765. https://doi.org/10.1016/j.jdent.2021.103765
Jungbauer G, Moser D, Müller S, Pfister W, Sculean A, Eick S (2021) The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics, 10
Borges AC, Kostov KG, Pessoa RS, de Abreu GM, Lima GD, Figueira LW, Koga-Ito CY (2021) Applications of Cold Atmospheric Pressure Plasma in Dentistry. Applied Sciences
Lata S, Chakravorty S, Mitra T, Pradhan PK, Mohanty SK, Patel P, Jha E, Panda PK, Verma SK, Suar M (2021) Aurora borealis in dentistry: the applications of cold plasma in biomedicine. Mater Today Bio, 13
Suresh M, Hemalatha VT, Sundar NM, Nisha AM (2022) Applications of Cold Atmospheric pressure plasma in Dentistry- A Review. Journal of Pharmaceutical Research International
Liu Z, Du X, Xu L, Shi Q, Tang X, Cao Y, Song K (2023 Feb) The therapeutic perspective of cold atmospheric plasma in periodontal disease. Oral Dis 24. https://doi.org/10.1111/odi.14547
Z, Wen Xiu-li, Wang, Huang X (2022) Cold atmospheric pressure plasmas applications in dentistry. Plasma Processes Polym. https://doi.org/10.1002/ppap.202200024. n. pag
M, Bessa A (2023) Mariana Raquel da Cruz Vegian, Lady Daiane Pereira Leite, Diego Morais da Silva, Noala Vicensoto Moreira Milhan, Konstantin Georgiev Kostov and Cristiane Yumi Koga-Ito. “Non-Thermal Atmospheric pressure plasma application in endodontics. Biomedicines 11. https://doi.org/10.3390/biomedicines11051401. n. pag
G, Kamgang-Youbi JM, Herry T, Meylheuc JL, Brisset MN, Bellon-Fontaine A, Doubla M, Naïtali (2009) Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett Appl Microbiol 48:13–18. https://doi.org/10.1111/j.1472-765X.2008.02476.x
G, Kamgang-Youbi JM, Herry MN, Bellon-Fontaine JL, Brisset A, Doubla M, Naïtali (2007) Evidence of temporal postdischarge decontamination of bacteria by gliding electric discharges: application to Hafnia alvei. Appl Environ Microbiol 73:4791–4796. https://doi.org/10.1128/AEM.00120-07
AP, Souto FR, Oliveira N, Carneiro (2016) Polyamide 6.6 modified by DBD plasma treatment for anionic dyeing processes. Intech 13. https://doi.org/10.5772/57353
P, Cools S, Van Vrekhem N, De Geyter R, Morent (2014) The use of DBD plasma treatment and polymerization for the enhancement of biomedical UHMWPE. Thin Solid Films 572:251–259. https://doi.org/10.1016/j.tsf.2014.08.033
F, Judée S, Simon C, Bailly T, Dufour (2018) Plasma-activation of tap water using DBD for agronomy applications: identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res 133:47–59. https://doi.org/10.1016/j.watres.2017.12.035
T, Royintarat P, Seesuriyachan D, Boonyawan EH, Choi W, Wattanutchariya (2019) Mechanism and optimization of non-thermal plasma-activated water for bacterial inactivation by underwater plasma jet and delivery of reactive species underwater by cylindrical DBD plasma. Curr Appl Phys 19:1006–1014. https://doi.org/10.1016/j.cap.2019.05.020
V, Straňák P, Spatenka MT, Tich´y J, Koller V, Kříha V, Scholtz Surfatron plasma-based sterilisation, n.d
M, Dharini S, Jaspin R, Mahendran (2023) Cold plasma reactive species: Generation, properties, and interaction with food biomolecules. Food Chem 405. https://doi.org/10.1016/j.foodchem.2022.134746
C, Hoffmann C, Berganza J, Zhang, Cold Atmospheric Plasma (2013) Methods of production and application in dentistry and oncology. Med Gas Res 3:1–15. https://doi.org/10.1186/2045-9912-3-21
A, Schmidt S, Bekeschus K, Wende B, Vollmar T, von Woedtke (2017) A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp Dermatol 26:156–162. https://doi.org/10.1111/exd.13156
BE, Konelschatz WEAU, Principle, Applications (1997) J PHYS IV FRANCE 7:7
W, Zhou P, Xiao Y, Li L, Zhou (2013) Dielectric properties of BN modified carbon fibers by dip-coating. Ceram Int 39:6569–6576. https://doi.org/10.1016/j.ceramint.2013.01.090
H-J, Lee S, Jung H-S, Jung S-H, Park W-H, Choe J-S, Ham C, Jo (2012) Evaluation of a dielectric barrier discharge plasma system for inactivating pathogens on cheese slices. J Anim Sci Technol 54:191–198. https://doi.org/10.5187/jast.2012.54.3.191
S, Mohades AM, Lietz MJ, Kushner (2020) Generation of reactive species in water film dielectric barrier discharges sustained in argon, helium, air, oxygen and nitrogen. J Phys D Appl Phys 53. https://doi.org/10.1088/1361-6463/aba21a
A, Fridman S, Nester LA, Kennedy A, Saveliev O, Mutaf-Yardimci PII: S0360-1285(98)00021-5, (n.d.)
A (2008) Fridman, Plasma chemistry, Cambridge university press,
R, Burlica MJ, Kirkpatrick BR, Locke (2006) Formation of reactive species in gliding arc discharges with liquid water. J Electrostat 64:35–43. https://doi.org/10.1016/j.elstat.2004.12.007
J, Pawłat P, Terebun M, Kwiatkowski B, Tarabová Z, Kovaľová K, Kučerová Z, Machala M, Janda K, Hensel (2019) Evaluation of oxidative species in Gaseous and Liquid Phase generated by Mini-Gliding Arc Discharge, plasma Chemistry and plasma Processing. https://doi.org/10.1007/s11090-019-09974-9
B, Riviere J-M, Mermet D, Deruaz (1989) Behaviour and Analytical Applications of a Modulated Power Microwave-induced Plasma (Surfatron),
K, Kutasi D, Popović N, Krstulović S, Milošević (2019) Tuning the composition of plasma-activated water by a surface-wave microwave discharge and a kHz plasma jet. Plasma Sources Sci Technol 28. https://doi.org/10.1088/1361-6595/ab3c2f
U, Kogelschatz B, Eliasson W, Egli U, Kogelschatz B, Eliasson WEDD, Principle (1997) Dielectric-Barrier Discharges. Principle and Applications To cite this version: HAL Id: jpa-00255561,
F, Gasi G, Petraconi E, Bittencourt SR, Lourenço AHR, Castro F, de Miranda S, Essiptchouk AM, Nascimento L, Petraconi A, Fraga MA, Pessoa RS (2020) Plasma treatment of Polyamide Fabric Surface by Hybrid Corona-Dielectric Barrier Discharge: material characterization and Dyeing/Washing processes. Mater Res 23:1–9. https://doi.org/10.1590/1980-5373-mr-2019-0255
S, Portugal B, Choudhury D, Cardenas (2022) Advances on aerodynamic actuation induced by surface dielectric barrier discharges. Front Phys 10. https://doi.org/10.3389/fphy.2022.923103
PIV, France E, Abb (1997) Dielectric-barrier discharges. Principle and Applications, 7
S, Wu Y, Cao X, Lu (2016) The state of the art of Applications of Atmospheric-Pressure nonequilibrium plasma jets in Dentistry. IEEE Trans Plasma Sci 44:134–151. https://doi.org/10.1109/TPS.2015.2506658
R, Laurita D, Barbieri M, Gherardi V, Colombo P, Lukes (2015) Chemical analysis of reactive species and antimicrobial activity of water treated by nanosecond pulsed DBD air plasma. Clin Plasma Med 3:53–61. https://doi.org/10.1016/j.cpme.2015.10.001
UK, Ercan B, Sen AD, Brooks SG, Joshi (2018) Escherichia coli cellular responses to exposure to atmospheric-pressure dielectric barrier discharge plasma-treated N-acetylcysteine solution. J Appl Microbiol 125:383–397. https://doi.org/10.1111/jam.13777
V, Luvita AT, Sugiarto S, Bismo (2022) Characterization of dielectric barrier discharge reactor with nanobubble application for industrial water treatment and depollution. S Afr J Chem Eng 40:246–257. https://doi.org/10.1016/j.sajce.2022.03.009
M, Vadivel S, Shobana SA, Narayan L, Mitu JD, Raja M, Sankarganesh (2017) Optimization of process conditions and characterization of ethylene-propylene-diene rubber with bismaleimide. Bul Chem Commun 49:26–30
A, Pedro F, Ribeiro N, Carneiro (2011) Polyamide 6.6 modified by DBD plasma treatment for anionic dyeing processes, Textile Dyeing. https://doi.org/10.5772/21755
YD, Korolev (2015) Low-current discharge plasma jets in a gas flow. Application of plasma jets. Russ J Gen Chem 85:1311–1325. https://doi.org/10.1134/S1070363215050473
G, Kamgang-Youbi JM, Herry T, Meylheuc JL, Brisset MN, Bellon-Fontaine A, Doubla M, Naïtali (2009) Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett Appl Microbiol 48:13–18. https://doi.org/10.1111/j.1472-765X.2008.02476.x
G, Kamgang-Youbi JM, Herry MN, Bellon-Fontaine JL, Brisset A, Doubla M, Naïtali (2007) Evidence of temporal postdischarge decontamination of bacteria by gliding electric discharges: application to Hafnia alvei. Appl Environ Microbiol 73:4791–4796. https://doi.org/10.1128/AEM.00120-07
ACOC, Doria CPC, Sorge TB, Santos J, Brandão PAR, Gonçalves HS, Maciel S, Khouri RS, Pessoa (2015) Application of post-discharge region of atmospheric pressure argon and air plasma jet in the contamination control of Candida albicans biofilms. Res Biomed Eng 31(4). https://doi.org/10.1590/2446-4740.01215
ACOC, Doria FR, Figueira JSB, de Lima JAN, Figueira AHR, Castro BN, Sismanoglu G, Petraconi HS, Maciel S, Khouri RS, Pessoa Inactivation of Candida albicans biofilms by atmospheric gliding arc plasma jet: effect of gas chemistry/flow and plasma pulsing, Plasma Res Express 1 015001. https://doi.org/10.1088/2516-1067/aae7e1
GG, Bălan I, Roşca EL, Ursu F, Doroftei AC, Bostănaru E, Hnatiuc V, Năstasă V, Şandru G, Ştefănescu A, Trifan M, Mareş (2018) Plasma-activated water: a new and effective alternative for duodenoscope reprocessing. Infect Drug Resist 11:727–733. https://doi.org/10.2147/IDR.S159243
MJ, Traylor MJ, Pavlovich S, Karim P, Hait Y, Sakiyama DS, Clark DB, Graves (2011) Long-term antibacterial efficacy of air plasma-activated water. J Phys D Appl Phys 44. https://doi.org/10.1088/0022-3727/44/47/472001
YM, Zhao S, Ojha CM, Burgess DW, Sun BK, Tiwari (2021) Inactivation efficacy of plasma-activated water: influence of plasma treatment time, exposure time and bacterial species. Int J Food Sci Technol 56:721–732. https://doi.org/10.1111/ijfs.14708
X, Lu GV, Naidis M, Laroussi S, Reuter DB, Graves K, Ostrikov (2016) Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys Rep 630:1–84. https://doi.org/10.1016/j.physrep.2016.03.003
YM, Zhao A, Patange DW, Sun B, Tiwari (2020) Plasma-activated water: physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr Rev Food Sci Food Saf 19:3951–3979. https://doi.org/10.1111/1541-4337.12644
R, Ma G, Wang Y, Tian K, Wang J, Zhang J, Fang (2015) Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J Hazard Mater 300:643–651. https://doi.org/10.1016/j.jhazmat.2015.07.061
Q, Xiang L, Fan Y, Li S, Dong K, Li Y, Bai (2020) A review on recent advances in plasma-activated water for food safety: current applications and future trends. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2020.1852173
A, Soni J, Choi G, Brightwell (2021) Plasma-activated water (PAW) as a disinfection technology for bacterial inactivation with a focus on fruit and vegetables, Foods. 10 https://doi.org/10.3390/foods10010166
J, Wang R, Han X, Liao T, Ding (2021) Application of plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. Lwt 137:110465. https://doi.org/10.1016/j.lwt.2020.110465
Y, Li J, Pan G, Ye Q, Zhang J, Wang J, Zhang J, Fang (2017) In vitro studies of the antimicrobial effect of non-thermal plasma-activated water as a novel mouthwash, Eur J Oral Sci. 125 463–470. https://doi.org/10.1111/eos.12374
, DIN SPEC 91315 (2014), General Requirements For Plasma Sources In Medicine, pp. 1–16
L, Eisner RM, Brown D, Modi (2004) Leakage Current Standards Simplified. MDDI in Regulatory and Compliance,
KG, Kostov et al (2010) “Bacterial sterilization by a dielectric barrier discharge (DBD) in air,” Surface and Coatings Technology, vol. 204, no. 18, pp. 2954–2959, doi: https://doi.org/10.1016/j.surfcoat.2010.01.052
TMC, Nishime “Development and characterization of extended and flexible plasma jets,” PhD thesis, São Paulo State University–UNESP, Guaratinguetá, São Paulo, Brazil, 2019. [Online]. Available: https://repositorio.unesp.br/bitstream/handle/11449/190654/nishime_tmc_dr_guara_int.pdf?sequence=4&isAllowed=y
KG, Kostov M, Machida V, Prysiazhnyi, Honda RY (Apr. 2015) Transfer of a cold atmospheric pressure plasma jet through a long flexible plastic tube. Plasma Sources Sci Technol 24(2):025038. https://doi.org/10.1088/0963-0252/24/2/025038
Y, Xia et al (Jan. 2016) The transfer of atmospheric-pressure ionization waves via a metal wire. Phys Plasmas 23(1):013509. https://doi.org/10.1063/1.4940332
O, Bastin et al (2020) Optical and electrical characteristics of an endoscopic DBD plasma jet. PMED 10(2):71–90. https://doi.org/10.1615/PlasmaMed.2020034526
KG, Kostov TMC, Nishime M, Machida AC, Borges V, Prysiazhnyi, Koga-Ito CY (2015) Study of Cold Atmospheric plasma jet at the end of Flexible Plastic Tube for Microbial Decontamination. Plasma Processes Polym 12(12):1383–1391. https://doi.org/10.1002/ppap.201500125
TMC, Nishime AC, Borges CY, Koga-Ito M, Machida LRO, Hein, Kostov KG (2017) “Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms,” Surface and Coatings Technology, vol. 312, pp. 19–24, Feb. doi: https://doi.org/10.1016/j.surfcoat.2016.07.076
AC, Borges et al (2017) “Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits,” Clinical Plasma Medicine, vol. 7–8, pp. 9–15, doi: https://doi.org/10.1016/j.cpme.2017.06.002
AC, Borges G, de Lima MG, Nishime TMC, Gontijo AVL, Kostov KG, Koga-Ito CY (2018) “Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study,” PLOS ONE, vol. 13, no. 6, p. e0199832, Jun. doi: https://doi.org/10.1371/journal.pone.0199832
AC, Borges et al (2019) “Cold Atmospheric Pressure Plasma Jet Reduces Trichophyton rubrum Adherence and Infection Capacity,” Mycopathologia, vol. 184, no. 5, pp. 585–595, doi: https://doi.org/10.1007/s11046-019-00375-2
LDP, Leite et al (2021) “Effect of Cold Atmospheric Plasma Jet Associated to Polyene Antifungals on Candida albicans Biofilms,” Molecules, vol. 26, no. 19, Art. no. 19, doi: https://doi.org/10.3390/molecules26195815
G, de Lima MG et al (2021) Cold Atmospheric plasma jet as a possible adjuvant therapy for Periodontal Disease. Molecules 26 18, Art. no. 18, Jan. https://doi.org/10.3390/molecules26185590
MAC, de Oliveira et al (2021) “Inhibitory Effect of Cold Atmospheric Plasma on Chronic Wound-Related Multispecies Biofilms,” Applied Sciences, vol. 11, no. 12, Art. no. 12, doi: https://doi.org/10.3390/app11125441
G (2022) de Morais Gouvêa Lima, “Cold Atmospheric Pressure Plasma Is Effective against P. gingivalis (HW24D-1) Mature Biofilms and Non-Genotoxic to Oral Cells,” Applied Sciences, vol. 12, no. 14, Art. no. 14, doi: https://doi.org/10.3390/app12147247
F, do Nascimento KG, Kostov M, Machida, Flacker A (2021) “Properties of DBD Plasma Jets Using Powered Electrode With and Without Contact With the Plasma,” IEEE Transactions on Plasma Science, vol. 49, no. 4, pp. 1293–1301, Apr. doi: https://doi.org/10.1109/TPS.2021.3067159
F, do Nascimento T, Gerling, Kostov KG (Apr. 2023) On the gas heating effect of helium atmospheric pressure plasma jet. Phys Scr 98(5):055013. https://doi.org/10.1088/1402-4896/accb17
F, do Nascimento BS, Leal A, Quade, Kostov KG (Jan. 2022) Different radial modification profiles observed on APPJ-Treated polypropylene surfaces according to the Distance between plasma outlet and target. Polymers 14 no. 21, Art. no. 21. https://doi.org/10.3390/polym14214524
F, do Nascimento A, Quade MA, Canesqui, Kostov KG (2023) “Different configurations of transferred atmospheric pressure plasma jet and their application to polymer treatment,” Contributions to Plasma Physics, vol. 63, no. 1, p. e202200055, doi: https://doi.org/10.1002/ctpp.202200055
Rao Y, Shang W, Yang Y, Zhou R, Rao X (2020) Fighting mixed-species microbial Biofilms with Cold Atmospheric plasma. Front Microbiol 11:11. https://doi.org/10.3389/fmicb.2020.01000
KG, Kostov V, Rocha CY, Koga-Ito MA, Algatti RY, Honda ME, Kayama RP, Mota (2010) Surf Coat Technol 204:18
TMC, Nishime AC, Borges CY, Koga-Ito M, Munemasa LRO, Hein KG, Kostov (2017) Surf Coat Technol 1:19
AC, Borges TMC, Nishime SM, Rovetta GMG, Lima KG, Kostov GP, Thim BR, Menezes JPB, Machado CY (2019) Koga-Ito Mycopathologia 184:585
Z, Wang M, Zhu D, Liu L, Liu X, Wang J, Chen L, Guo Y, Liu M, Hou M, Rong (2023) N2O5 in air discharge plasma: energy-efficient production, maintenance factors and sterilization effects. J Phys D Appl Phys 56. https://doi.org/10.1088/1361-6463/acb65f
M, Moreau N, Orange MGJ, Feuilloley (2008) Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol Adv 26:610–617. https://doi.org/10.1016/j.biotechadv.2008.08.001
Sakudo A, Yagyu Y, Onodera T (2019) Disinfection and sterilization using plasma technology: Fundamentals and Future Perspectives for Biological Applications. Int J Mol Sci 20(20):5216. https://doi.org/10.3390/ijms20205216
Chen Z, Garcia G Jr, Arumugaswami V, Wirz RE (2020) Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys Fluids (1994) 32(11):111702. https://doi.org/10.1063/5.0031332
Volotskova O, Dubrovsky L, Keidar M, Bukrinsky M (2016) Cold Atmospheric plasma inhibits HIV-1 replication in Macrophages by Targeting both the Virus and the cells. PLoS ONE 11(10):e0165322. https://doi.org/10.1371/journal.pone.0165322
Ciofu O, Moser C, Jensen P, Høiby N (2022) Tolerance and resistance of microbial biofilms. In Nature Reviews Microbiology (Vol. 20, Issue 10, pp. 621–635). Nature Research. https://doi.org/10.1038/s41579-022-00682-4
Eming SA, Martin P, Tomic-Canic M (2014) Wound repair and regeneration: mechanisms, signaling and translation. Sci Transl Med 6(265):265sr6. https://doi.org/10.1126/scitranslmed.3009337
MAC, Oliveira GMG, Lima TMC, Nishime AVL, Gontijo MBRC, Menezes MV, Caliari KG, Kostov CY (2021) Koga-Ito, Applied Sciences, 11, 5441
Lee MH, Lee YS, Kim HJ, Han CH, Kang SU, Kim CH (2019) Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-49938-9
Xiong Q, Wang X, Yin R, Xiong L, Chen Q, Zheng MX, Xu L, Huang QH, Hamblin MR (2018) Surface treatment with non-thermal humid argon plasma as a treatment for allergic contact Dermatitis in a mouse model. Clin Plasma Med 12:10–16. https://doi.org/10.1016/j.cpme.2018.09.002
VN, Vasilets (2019) Plasmachemical generation of nitric oxides in air plasmas for medical applications. ChemChemTech 62:4–13. https://doi.org/10.6060/ivkkt.20196205.5958
MD, Nguyen QT, Do TT, Luong NT, Le TT, Nguyen TP, Bui HT, Do H, Metelmann C, Seebauer TT, Vu (2020) Cold atmospheric plasma treatment on failed finger perforator flap: a case report. Clin Plasma Med 19–20. https://doi.org/10.1016/j.cpme.2020.100105
Benova E, Topalova Y, Marinova P, Todorova Y, Atanasova M, Bogdanov T, Yotinov I Surface-wave-sustained plasma for model biological systems treatment. In Proceedings of the XXXIII International Conference on Phenomena in Ionized Gases (ICPIG), Estoril, Portugal, 9–14 July 2017; Alves, L.L., Tejero-del-Caz, A., Eds.; Instituto de Plasmas e Fusão Nuclear, Instituto Superior Técnico, Universidade de Lisboa: Lisboa, Portugal, 2017. Topic Number: 17. p. 87
Tsvetkov V, Hinkov A, Todorov D, Benova E, Tsonev I, Bogdanov T, Shishkov S, Shishkova K (2019) Effect of plasma-activated medium and water on replication and extracellular virions of herpes simplex Virus-1. Plasma Med 9:205–216
Arndt S, Schmidt A, Karrer S, VonWoedtke T (2018) Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clin Plasma Med 9:24–33
Bogdanov T, Tsonev I, Traikov LL (2020) Microwave plasma torch for wound treatment. J Phys Conf Ser 1598:012001
Krcma F, Tsonev I, Smejkalová K, Truchlá D, Kozáková Z, Zhekova M, Marinova P, Bogdanov T, Benova E (2018) Microwave micro torch generated in argon based mixtures for biomedical applications. J Phys D Appl Phys 51:414001
Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, Tagami J, Twetman S, Tsakos G, Ismail A (2017) Dental caries. Nat Reviews Disease Primers 3. https://doi.org/10.1038/nrdp.2017.30
Jungbauer G, Moser D, Müller S, Pfister W, Sculean A, Eick S (2021) The Antimicrobial Effect of Cold Atmospheric plasma against Dental Pathogens-A systematic review of In-Vitro Studies. Antibiot (Basel) 10(2):211. https://doi.org/10.3390/antibiotics10020211
GMG, Lima CFL, Carta AC, Borges TMC, Nishime CAV, Silva MV, Caliari MP, Mayer KG, Kostov (2022) C.Y. Koga-Ito, Applied Sciences, 12, 7247
Schaudinn C, Jaramillo D, Freire MO, Sedghizadeh PP, Nguyen A, Webster P, Costerton JW, Jiang C (2013) Evaluation of a nonthermal plasma needle to eliminate ex vivo biofilms in root canals of extracted human teeth. Int Endod J 46(10):930–937. https://doi.org/10.1111/iej.12083
KG, Kostov AC, Borges CY, Koga-Ito TMC, Nishime V, Prysiazhnyi RI, Honda (2015) IEEE Trans Plasma Sci 43:770
AC, Borges TMC, Nishime KG, Kostov GMG, Lima AVL, Gontijo JNMM, Carvalho RI, Honda CY (2017) Koga-Ito. Clin Plasma Med 7:9
AC, Borges GMG, Lima TMC, Nishime AVL, Gontijo KG, Kostov CY (2018) Koga-Ito Plos One 13:0199832
Ebrahimi-Shaghaghi F, Noormohammadi Z, Atyabi SM, Razzaghi-Abyaneh M (2021) Inhibitory effects of cold atmospheric plasma on the growth, virulence factors and HSP90 gene expression in Candida albicans. Arch Biochem Biophys 700:108772
Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 3:17038. https://doi.org/10.1038/nrdp.2017.38
GMG, Lima AC, Borges TMC, Nishime GF, Santana-Melo KG, Kostov MP, Mayer CY (2021) Koga-Ito Molecules 26:5590
Liu Z, Du X, Xu L, Shi Q, Tang X, Cao Y, Song K (2023 Feb) The therapeutic perspective of cold atmospheric plasma in periodontal disease. Oral Dis 24. https://doi.org/10.1111/odi.14547
Seebauer C, Freund E, Hasse S, Miller V, Segebarth M, Lucas C, Kindler S, Dieke T, Metelmann HR, Daeschlein G, Jesse K, Weltmann KD, Bekeschus S (2021) Effects of cold physical plasma on oral lichen planus: an in vitro study (Effects of CAP on OLP). Oral Dis 27(7):1728–1737. https://doi.org/10.1111/odi.13697
Semmler ML, Bekeschus S, Schäfer M, Bernhardt T, Fischer T, Witzke K, Seebauer C, Rebl H, Grambow E, Vollmar B, Nebe JB, Metelmann HR, Woedtke TV, Emmert S, Boeckmann L (2020) Molecular Mechanisms of the efficacy of Cold Atmospheric pressure plasma (CAP) in Cancer Treatment. Cancers (Basel) 12(2):269. https://doi.org/10.3390/cancers12020269
Murillo D, Huergo C, Gallego B, Rodríguez R, Tornín J (2023) Exploring the Use of Cold Atmospheric plasma to Overcome Drug Resistance in Cancer. Biomedicines 11(1):208. https://doi.org/10.3390/biomedicines11010208
Bugshan A, Farooq I (2020) Oral squamous cell carcinoma: metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Res 9:229. https://doi.org/10.12688/f1000research
Metelmann HR, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB et al (2018) Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin Plasma Med 9:6–13. https://doi.org/10.1016/j.cpme.2017.09.001
Li X, Rui X, Li D, Wang Y, Tan F (2022) Plasma oncology: adjuvant therapy for head and neck cancer using cold atmospheric plasma. Front Oncol 12:994172. https://doi.org/10.3389/fonc.2022.994172
Wagner G, Eggers B, Duddeck D, Kramer FJ, Bourauel C, Jepsen S, Deschner J, Nokhbehsaim M (2022) Influence of cold atmospheric plasma on dental implant materials - an in vitro analysis. Clin Oral Investig 26(3):2949–2963. https://doi.org/10.1007/s00784-021-04277-w
Kochar SP, Reche A, Paul P (2022) The etiology and management of Dental Implant failure: a review. Cureus 14(10):e30455. https://doi.org/10.7759/cureus.30455PMID: 36415394; PMCID: PMC9674049
Nam SH, Lee HW, Cho SH, Lee JK, Jeon YC, Kim GC (2013) High-efficiency tooth bleaching using non-thermal atmospheric pressure plasma with low concentration of hydrogen peroxide. J Appl Oral Sci 21(3):265–270. https://doi.org/10.1590/1679-775720130016PMID: 23857658; PMCID: PMC3881910
KG, Kostov TMC, Nishime M, Machida AC, Borges V, Prysiazhnyi CY (2015) Koga-Ito Plasma Processes and Polymers 12:1383
Chiappim W, Sampaio A, da Miranda G, Fraga F, Petraconi M, da Silva Sobrinho G, Kostov A, Koga-Ito K, Pessoa C (2021) Antimicrobial effect of plasma‐activated tap water on staphylococcus aureus, escherichia coli, and Candida albicans. Water (Switzerland) 13:1480. https://doi.org/10.3390/w13111480
W, Chiappim AG, Sampaio F, Miranda M, Fraga G, Petraconi A, Silva Sobrinho P, Cardoso KG, Kostov CY, Koga-Ito (2021) R Pessoa Plasma Processes and Polymers 210001:14
AG, Sampaio W, Chiappim NV, Moreira B, Botan Neto R, Pessoa CY (2022) Koga-Ito. Int J Mol Sci 23:13893
Mai-Prochnow A, Zhou R, Zhang T et al (2021) Interactions of plasma-activated water with biofilms: inactivation, dispersal effects and mechanisms of action. npj Biofilms Microbiomes 7:11. https://doi.org/10.1038/s41522-020-00180-6
Naïtali M, Kamgang-Youbi G, Herry J‐M, Bellon‐Fontaine M‐N, Brisset J‐L (2010) Combined effects of long‐living chemical species during microbial inactivation using atmospheric plasma‐treated water. Appl Environ Microbiol 76:7662–7664. https://doi.org/10.1128/AEM.01615-10
Shen J, Tian Y, Li Y, Ma R, Zhang Q, Zhang J, Fang J (2016) Bactericidal Effects against S. aureus and Physicochemical Properties of plasma activated Water stored at different temperatures. Sci Rep 6:28505. https://doi.org/10.1038/srep28505
Pan J, Li YL, Liu CM, Tian Y, Yu S, Wang KL, Zhang J, Fang J (2017) Investigation of Cold Atmospheric plasma-activated water for the Dental Unit Waterline System Contamination and Safety evaluation in Vitro. Plasma Chem Plasma Process 37:1091–1103. https://doi.org/10.1007/s11090-017-9811-0
Bălan GG, Roşca I, Ursu EL, Doroftei F, Bostănaru AC, Hnatiuc E, Năstasă V, Şandru V, Ştefănescu G, Trifan A et al (2018) Plasma-activated water: a new and effective alternative for duodenoscope reprocessing. Infect Drug Resist 11:727–733. https://doi.org/10.2147/IDR.S159243
Gabrilska RA, Rumbaugh KP (2015) Biofilm models of polymicrobial infection. Future Microbiol 10:1997–2015. https://doi.org/10.2217/fmb.15.109
Li Y, Pan J, Ye G, Zhang Q, Wang J, Zhang J, Fang J (2017) In vitro studies of the antimicrobial effect of non-thermal plasma‐activated water as a novel mouthwash. Eur J Oral Sci 125:463–470. https://doi.org/10.1111/eos.12374
Hong Q, Dong X, Yu H, Sun H, Chen M, Wang Y, Yu Q (2021) The Antimicrobial Property of plasma activated liquids (PALs) against oral Bacteria streptococcus mutans. Dental 3:1–7. https://doi.org/10.35702/dent.10007
Laurita R, Barbieri D, Gherardi M, Colombo V, Lukes P (2015) Chemical analysis of reactive species and antimicrobial activity of water treated by nanosecond pulsed DBD air plasma. Clin Plasma Med 3:53–61. https://doi.org/10.1016/j.cpme.2015.10.001
Li Y, Pan J, Wu D, Tian Y, Zhang J, Fang J (2019) Regulation of Enterococcus faecalis Biofilm formation and Quorum sensing related virulence factors with ultra-low dose reactive species produced by plasma activated Water. Plasma Chem Plasma Process 39:35–49. https://doi.org/10.1007/s11090-018-9930-2
G-J, Júnior E, Fardin AC, Gaetti‐Jardim EC, de Castro AL, Schweitzer CM, Avila‐Campos MJ (2010) Microbiota associated with chronic osteomyelitis of the jaws. Brazilian J Microbiol 41:1056–1064. https://doi.org/10.1590/S1517-83822010000400025
Dobrynin D, Fridman G, Friedman G, Fridman A (2009) Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys 11. https://doi.org/10.1088/1367-2630/11/11/115020
Nastasa V, Pasca A-S, Malancus R‐N, Bostanaru A‐C, Ailincai L‐I, Ursu E‐L, Vasiliu A‐L, Minea B, Hnatiuc E, Mares M (2021) Toxicity Assessment of Long‐Term exposure to non‐thermal plasma activated Water in mice. Int J Mol Sci 22:11534. https://doi.org/10.3390/ijms222111534
Lee MH, Lee YS, Kim HJ, Han CH, Kang SU, Kim CH (2019) Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-49938-9
Lou BS, Hsieh JH, Chen CM, Hou CW, Wu HY, Chou PY, Lai CH, Lee JW (2020) Helium/Argon-Generated Cold Atmospheric plasma facilitates cutaneous Wound Healing. Front Bioeng Biotechnol 8:1–11. https://doi.org/10.3389/fbioe.2020.00683
Zhang Y, Xiong Y, Xie P, Ao X, Zheng Z, Dong X, Li H, Yu Q, Zhu Z, Chen M et al (2018) Non-thermal plasma reduces periodontitis-induced alveolar bone loss in rats. Biochem Biophys Res Commun 503:2040–2046. https://doi.org/10.1016/j.bbrc.2018.07.154
Eggers B, Marciniak J, Memmert S, Kramer FJ, Deschner J, Nokhbehsaim M (2020) The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology 108:607–616. https://doi.org/10.1007/s10266-020-00487-y
Rezaei F, Vanraes P, Nikiforov A, Morent R, Geyter N (2019) De Appl Plasma-Liquid Systems: Rev Mater 12:2751
Nakamura K, Peng Y, Utsumi F, Tanaka H, Mizuno M, Toyokuni S, Hori M, Kikkawa F, Kajiyama H (2017) Novel intraperitoneal treatment with non-thermal plasma-activated medium inhibits metastatic potential of ovarian Cancer cells. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-05620-6
Tanaka H, Bekeschus S, Yan D, Hori M, Keidar M, Laroussi M, Rns ROS, Rns ROS, Rns ROS (2021) Plasma-treated solutions (PTS) in Cancer Therapy. Cancers 13:1737
Shi L, Yu L, Zou F, Hu H, Liu K, Lin Z (2017) Gene expression profiling and functional analysis reveals that p53 pathway-related gene expression is highly activated in cancer cells treated by cold atmospheric plasma‐activated medium. PeerJ 5:e3751. https://doi.org/10.7717/peerj.3751
Funding
The authors acknowledge financial support from The São Paulo State Research Foundation (FAPESP, grants 2016/07196-6 and 2019/05856-7; fellowships 21/14181-3, 21/00046 − 7, 20/09481-5, and 23/02268-2) and Brazilian National Council for Scientific and Technological Development (309762/2021-9).
Author information
Authors and Affiliations
Contributions
CKI, RSP, KK, NM, FM, NFAN, and FN wrote the main manuscript text and prepared the Figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koga-Ito, C.Y., Kostov, K.G., Miranda, F.S. et al. Cold Atmospheric Plasma as a Therapeutic Tool in Medicine and Dentistry. Plasma Chem Plasma Process 44, 1393–1429 (2024). https://doi.org/10.1007/s11090-023-10380-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10380-5