Abstract
The disinfection of the inner surface of a medical device has long been a challenge for the central sterile supply departments. Dental unit waterline system (DUWLs) foster the attachment of microorganisms and development of biofilm, which lead to continuous contamination of the outlet water from dental units; this contamination may be responsible for a potential risk of infection due to the exposure of patients and medical staff. The present study investigated the disinfection effects of cold atmospheric plasma-activated water (CAPAW) on DUWLs using a model of 5-day-old Enterococcus faecalis biofilm. The results showed that the colony-forming unit was reduced from 107 to 0 after 5 min of treatment. The physicochemical properties of CAPAW were evaluated, including the pH value, oxidation reduction potential, and NO radical. The results showed that the inactivation mechanisms were mainly triggered by the reactive oxygen/nitrogen species. Additionally, CAPAW had a metal corrosion rate same as that of deionized water. We conclude that CAPAW can be applied as an appropriate alternative disinfectant against biofilm contamination of DUWLs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The disinfection of the inner surface of equipment such as dental unit waterline system (DUWLs), ventilator circuits, and catheters is challenging for nursing staff and central sterile supply departments. The water used for dental units is frequently contaminated and can be a source of cross-infection [1]. The two main sources of microbes are municipal water piped into a dental unit and suck-back from patient’s saliva [2]. These bacteria are a potential risk for immunocompromised patients, and the number of reported cases has been underestimated [3, 4]. Thus, the American Dental Association has suggested that the output water from DUWLs must contain less than 500 colony-forming units per milliliter (CFUs/mL) [5, 6] for all dental procedures. However, many studies have shown that DUWLs are highly contaminated by planktonic pathogens released from biofilms and that the contamination level can reach 105 CFU/mL in a week [7]. Other studies have shown that these systems are extensively colonized by microorganisms in the form of biofilms embedded in extracellular polymeric substances (EPS) [8–10]. The microbes detected in the output water of DUWLs include various bacteria, fungi, and free-living amoebae [11]. Although Enterococcus species are not the dominant bacteria in DUWLs, they are often isolated from DUWLs and are widely known as surface-associated bacteria, opportunistic pathogens causing infections and carrying higher drug-resistant properties than most of the other bacteria isolated from DUWLs [12, 13]. In the oral environment, Enterococcus faecalis (E. faecalis) is the key bacterium that leads to root canal treatment failure and retreatment [14]. The frequency of E. faecalis in oral rinse can reach 11% when receiving endodontic treatment; thus, this bacterium could retract into DUWLs and lead to cross-infection [15]. Root-canal-treated teeth are approximately nine times more likely to harbor E. faecalis than cases of primary infections [16]. In addition, E. faecalis tends to transfer antibiotic resistance to other pathogens via horizontal routes [17]. Owing to its considerable importance, E. faecalis biofilm was selected for the evaluation of the antimicrobial efficiency of broad-spectrum antibiotics.
Traditional disinfection methods, such as waterline flushing for 30 s between consecutive patients [6], independent water reservoir systems, or continuous chemical disinfection, have been used to control contamination [18]. However, waterline flushing cannot reduce the biofilm attached to the lumen surface, and the residue of the detergents and disinfectants proposed for chemical treatment may pose an additional risk to patients. Different biocides are used for the disinfection of DUWLs, including sodium hypochlorite (NaClO), chlorhexidine gluconate, peroxide (H2O2), peracetic acid, ethanol, and glutaraldehyde [19, 20]. However, some chemical biocides are not compatible with the materials (e.g., steel fittings and various washers) used in DUWLs [21]. Additionally, water treated with chemical disinfectants may become toxic after treatment and may compromise environmental safety, which is a crucial issue for economic and public health. Thus, an ideal decontaminant with a broad antimicrobial spectrum, safe for patients, and friendly to the materials of DUWLs and the environment must be identified.
Cold atmospheric plasma (CAP) is a novel technology for the disinfection of medical devices or instruments, which consists of partially ionized gas, molecules, charged particles, negative/positive ions, electrons, photons, and free radicals [22]. CAP-activated water (CAPAW) is an acidified solution that contains reactive oxygen/nitrogen species (ROS/RNS), and has been shown to exhibit strong antimicrobial activity [23]. Among the various active constituents of CAPAW, the superoxide radical \(\left( {{\text{O}}_{2}^{ - } } \right)\), hydroxyl radical (OH·), oxygen (O), Ozone (O3), and hydrogen peroxide (H2O2) are known as the main ROS, which play an important role in the inactivation process [24–26]. Furthermore, RNS are bactericidal and can convert to other non-radical forms in water, including nitrites \(\left( {{\text{NO}}_{2}^{ - } } \right)\), nitrates \(\left( {{\text{NO}}_{3}^{ - } } \right)\), and peroxynitrites (ONOO−) [27, 28]. In previous studies, CAPAW has been applied to inactivate a range of planktonic bacteria, but not biofilms [17, 29].
The aim of this study was to systematically investigate the inactivation efficacy, the main antimicrobial mechanisms, and the capability of CAPAW treatment to remove biofilm contamination from DUWLs.
Experimental Setup and Methods
Sample Preparation and Biofilm Formation
DUWLs tubes (KaVo, Biberach, Germany) were purchased and divided into 100 coupons to cultivate the E. faecalis biofilm examined in the study. All tubes were cleaned with deionized water and sterilized with ethylene oxide. Each sample had an inner diameter of 2 mm and a total length of 21 mm. E. faecalis (ATCC 29212) was grown on brain heart infusion agar (BHI) (Land Bridge Technology Co., Ltd, China) plates in an aerobic environment at 37 °C. A single colony was inoculated in 1 mL of sterile BHI broth at 37 °C. All tubes were placed into 1.5-mL Eppendorf tubes with 1 mL BHI broth containing 108 CFU of E. faecalis. The E. faecalis biofilm was incubated anaerobically at 37 °C for 5 days, and fresh sterile BHI broth was refreshed every alternate day to maintain bacterial viability.
Scanning Electron Microscopy
To verify biofilm formation on the inner surface of the tube, scanning electron microscopy (SEM, S-4800, Hitachi, Japan) was used. Dental unit tube coupons were rinsed with phosphate-buffered saline (PBS) to remove planktonic bacteria, immersed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate (pH = 7.4) for 48 h at 4 °C, and subjected to a gradient of dehydration in 30, 50, 70, 90, and 100% ethanol. Subsequently, the tubes were divided longitudinally, dried naturally, fixed with electrically conductive silicone, and sputter-coated with gold–palladium before examination.
CAPAW Treatment
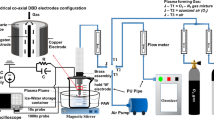
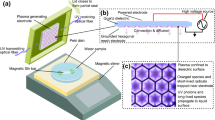
Figure 1a shows the schematic diagram of an air plasma generator, which was designed based on a dielectric barrier structure with hollow electrodes. A more detailed description of the cold plasma device is provided in our previous papers [30, 31]. The system was mainly composed of copper electrodes and a quartz dielectric tube with an inlet diameter of 1.5 mm. Compressed air with a flow rate of 260 L/h was pumped into the quartz tube as the working gas. A copper foil, operating as a high-voltage electrode, was connected to an excitation voltage with a peak voltage of 25 kV and a power source of 20 kHz. The input power was approximately 25 W, which was measured with an electric parameter tester (PM9801, Napui Electronic Technology Co., Ltd, Dongguan, China). Continuous plasma was generated at the end of the plasma jet, reaching a length of 7 mm. As shown in Fig. 1b, the CAPAW was produced by placing the plasma jet beneath the water surface. Ten milliliters of distilled water were injected into the Eppendorf tube. The distance between the end of the plasma microjet (PMJ) and the liquid surface was 10 mm.
Assessment of Antibacterial Activity
Colony count assays (CFU) were employed to demonstrate the bactericidal efficacy for each group. As shown in Table 1, 80 E. faecalis biofilm tubing samples (n = 10/group) were used to evaluate the disinfection effectiveness of the CAPAW. After 5 days of incubation, biofilms were formed on the lumen surface of the tube. All external tubing surfaces were disinfected with 75% alcohol wipes and cut by approximately 5 mm at each end. The negative control group G1 did not receive any treatment. The experimental groups G2–G6 were treated with CAPAW for 1, 2, 3, 4, and 5 min, respectively. The positive groups G7–G8 were disinfected with chemicals (H2O2 and NaClO) for 5 min [18]. The biofilm formed on the lumen surface was collected with a broach and churned for 1 min; then, two #30 sterile paper points were used to collect the bacteria for 1 min. The paper points were transferred into 1 mL of PBS and the process was repeated three times. The centrifuge tubes were shaken vigorously for 1 min. The number of E. faecalis bacteria was determined using an EasySpiral instrument (Interscience, France).
Confocal Laser Scanning Microscopy Analysis
The viability of the biofilms formed on the luminal surfaces of the samples after CAPAW treatment was analyzed using a Zeiss confocal laser scanning microscopy (CLSM) system equipped with an Ar ion laser (488 nm) and a He–Ne laser (543 nm). Fifteen samples with biofilms, including non-treated samples and specimens treated with CAPAW for 1, 3, and 5 min, were prepared to examine the antimicrobial efficiency of CAPAW treatment. All samples were slit axially and immediately stained with a LIVE/DEAD® BackLight™ Viability Kit (Invitrogen, Carlsbad, CA). When observed with CLSM, live bacteria with intact membranes show green fluorescence, whereas dead bacteria with damaged membranes exhibit red fluorescence. The entire luminal surface was examined using a ×10 lens and imaged with the ZenLightEdition 2009 software (Zeiss).
Physicochemical Property Evaluation of CAPAW
The pH and oxidation reduction potential (ORP) value of water were measured immediately after CAP treatment for 1, 2, 3, 4, and 5 min to evaluate its physical and chemical properties. The ORP was employed to evaluate the general level of total oxidants and total reductants in the water. The ORP was measured in each sample using a redox-sensitive electrode (LE501, Mettler Toledo, Switzerland) and the pH value was monitored by a microprocessor pH-meter (LE438, Mettler Toledo, Switzerland) at room temperature.
Nitrates (NO3 −) are the main RNS that are highly related with the germicidal mechanisms of CAPAW [32]. After CAPAW treatment for 1, 2, 3, 4, and 5 min, 200 μL of 10 g/L sulphanilamide was added into the Eppendorf centrifuge tube with 10 mL of CAPAW and the mixture was incubated for 2 min. Subsequently, 200 μL of 1.0 g/L N-(1-naphthy1)-ethylenediamine hydrochloride was added, followed by incubation for 20 min at room temperature. The concentration of NO3 − was determined by measuring the absorbance at 220 nm with a Nanodrop 8000 spectrometer (Thermo Scientific, USA) at room temperature.
Main Reactive Species Measurement
To identify the major reactive species generated in the water by the cold plasma after CAPAW treatment for 1, 2, 3, 4, and 5 min, optical emission spectrometry (OES) was applied using a fiber optic spectrometer (AVANTES, USA). The dispersed emission spectra were recorded in the 250–1000 nm range by a 2048-pixel charge-coupled device (CCD) detector array. To acquire the emission spectrum at the bottom of the tube, a quartz tube was placed at a distance of 5 mm away from the nozzle. Details of the measurement method have been provided in our earlier paper [33]. NO has been confirmed to exert antimicrobial activity, induce ROS generation, and cause oxidative damage of cells [34]. Electron spin resonance (ESR) spectroscopy, which has been widely used for free-radical detection, was performed using a Bruker ER-200D-SRC/E-500 spectrometer operating at room temperature to detect free NO radicals after CAPAW treatment for 1, 2, 3, 4, and 5 min. N-Methyl-D-glucamine dithiocarbamate (MGD) and FeSO4·7H2O were used to trap free NO radicals released in the water, which selectively produce NO–Fe2+(MGD)2 and exhibit a characteristic ESR feature. The tests were conducted with a central magnetic field of 3369.850 G, a sweep width of 100.0 G, a frequency of 9.628 GHz, a modulation frequency of 100 kHz, and a power of 7.971 mW.
Metal Corrosion Test
We investigated the capability of CAPAW to induce metal corrosion of DUWLs. We selected stainless steel (1Cr18Ni9Ti, 7 750 kg/m3) and copper (H62, 8 400 kg/m3), according to the National Standard of China GB/10124-88 [35]. All samples (diameter = 24.0 mm, thickness = 1.0 mm) with a hole in their center and a surface area of approximately 9.8 cm2 were used. To remove the oxide layer, all metal samples were ground and polished with wet grinding paper with a grain size of up to #120, while the oily layer was eliminated using 100% ethanol. Then, the samples were dehydrated at 50 °C for 1 h, cooled to ambient temperature, and weighed (corrected to 0.1 mg). Subsequently, all copper and stainless steel discs (n = 3/group) were immersed into 200 mL of 5 min CAPAW and deionized water for 72 h (t). Then, all samples were rinsed with distilled water, dehydrated, cooled, and weighed again (mt). The formula that provides the rate (R) of metal erosion is as follows:
R is the rate of erosion (mm/a), m is the mass before experiment (g), m t is the mass after experiment (g), m k is the mass reduction with chemical cleaning method (g), S is the superficial area (cm2), t is the processing time (h), d is the density of disc (kg/m3). R < 0.005 and is non-corrosive; 0.005 ≤ R ≤ 0.01 and is basic non-corrosive, 0.01 < R ≤ 0.1 and is slightly corrosive, 0.1 < R ≤ 1.0 and is moderately corrosive, R > 1.0 and is severely corrosive.
Statistical Analysis
Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., USA) for Windows. Analysis of variance (ANOVA) was conducted to evaluate the disinfection efficiency of different groups using Duncan’s multiple-range post hoc tests. Statistical significance was considered for p < 0.05.
Results and Discussion
Determination of Bacterial Viability
As shown in Fig. 2, after 5 days of incubation, the total CFU count reached 106. After 1–3 min of CAPAW treatment, the CFU count was reduced significantly compared to that of the negative group (G1) (p < 0.05). When the treatment time was prolonged to 5 min, no bacteria were detected on the plate (G6), as shown Fig. 3b. The bactericidal effects of CAPAW treatment for 4 and 5 min were better than those seen in the positive groups (G7, G8) (p < 0.05). Therefore, the CAPAW treatment demonstrated antimicrobial efficiency in the E. faecalis biofilm model, which makes it a potentially promising technology to control DUWLs contamination.
The inactivation results of CAPAW treatment and chemical agents. A statistical difference (p < 0.05) was observed for all the CAPAW treatment groups (G2–G6), the 1% H2O2 group, and the 10 mg/L NaClO group compared with the negative group (G1). Besides, the antimicrobial activity of G3–G6 was superior to that of chemical agents (p < 0.05)
The chemical agent groups (G7, G8), 1% H2O2, and 10 mg/L NaClO showed germicidal effects similar to that of the 3-min CAPAW treatment. However, peroxide-based disinfectants can cause occlusion and even blockage of DUWLs [36]. Meanwhile, chlorine-based products, like sodium hypochlorite, chlorine dioxide, and chlorhexidine, could produce by-products such as toxic trihalomethanes in the presence of organic matter. An important characteristic of biofilms relevant to clinical settings is their enhanced tolerance to chemicals and to antimicrobial agents [37]. Additionally, the limitations of chemical antimicrobial agents include improper drug use [38].
CLSM was performed to examine the inactivation induced by CAPAW treatment. The control group is dominated by live biofilm cells after 5 days of incubation (Fig. 3a). Figure 3c shows that the application of CAPAW treatment for 5 min killed all bacteria in the biofilm (red fluorescence), whereas Fig. 3d demonstrates that all layers of the biofilm were killed. This result further verifies the inactivation of E. faecalis biofilm treated with CAPAW.
SEM was applied to observe the morphological changes in the biofilm. Typical SEM photographs of the 5-day E. faecalis model were acquired before and after CAPAW treatment. As shown in Fig. 4a, bacteria embedded with EPS were attached to the surface and the cells exhibited a normal cell shape (Fig. 4b). To better understand the effect of CAPAW treatment on the biofilm, samples treated with CAPAW for 5 min was observed with the SEM. At the CAPAW group, the 3D structure of the biofilm was destroyed to a greater extent. Moreover, the bacterial surfaces changed from smooth to severely deformed. Damages to the bacterial cell membrane could be caused by ROS in CAPAW, which could oxidize unsaturated fatty acids in the lipid bilayer of the cell wall, cleave peptide bonds, and oxidize amino acid side chains [39].
SEM results of the control (non-treated) group and the CAPAW 5-min group at magnifications of ×1000 (a, c) and ×5000 (b, d). a, b The lumen surface of the tubing after 5 days of incubation, exhibiting the biofilm attached to the surface and the normal cell morphology (as shown by the yellow arrow in (b). c, d Group treated with CAPAW for 5 min; the dimensional structure of the biofilm and the normal morphology are destroyed, as shown by the yellow arrow in (d)
Physical and Chemical Property Evaluation of CAPAW
We measured the physicochemical characteristics (pH, ORP, NO3 −) of CAPAW (n = 5/group). As shown in Fig. 5a,b, the pH of CAPAW decreased from 6.76 to 2.21, while the ORP reached approximately 600 mV from the initial 160 mV in 5 min. During the first minute, we observed a quick decrease of the pH value from 6.87 to 3.8 and the ORP value increased enormously from 163.8 to 382.8. Subsequently, the pH and ORP reached steady values of approximately 3 and 600, respectively, after 3 min of CAPAW treatment. Although plasma treatment can lead to the decrease of pH [34, 40], the source of acidity remains unclear. The acidification of water by plasma, when air is used as the working gas, is mostly due to the production of nitric acid and an acid that consists of a hydrogen cation (H+) and a superoxide anion \(\left( {{\text{O}}_{2}^{ - \cdot } } \right)\) [41, 42]:
Although the pH value is an important inactivation factor, it has limited impact on E. faecalis biofilms, despite its reduction to 2.5 [43]. However, it must be noted that a rapidly decreasing pH value promotes more effective reaction chemistry, such as that of hydroperoxyl radicals (HOO·), which can denature proteins inside the cell by chemical modification and cause bacterial inactivation [25, 44]. Biofilms have a highly sophisticated defense system to maintain homeostasis, including a stable intracellular pH [45–47]. The gel-like EPS could protect the deeper layers of cells from disinfectants by permitting only limited diffusion of chemicals into the biofilm [48, 49]. However, additional reactive species could cause oxidative membrane changes and destroy the defense system. Moreover, an acidic environment catalyzes a more efficient reaction in reactive species and helps destroy biofilm structures composed of EPS [50]. Thus, acidic conditions act synergistically with antimicrobial effects. This conclusion has been verified by Satoshi Ikawa et al. [51] and is likely to result from HOO· radicals formed by the protonation of \({\text{O}}_{2}^{ - \cdot}\), which is an electrically neutral species that can easily penetrate the cell membrane and damage intracellular components [40].
The ORP value expresses the general level of total oxidants and total reductants in CAPAW. It is an important factor that affects microbial inactivation, as a high ORP can break down the outer and inner membranes of bacteria and inactivate the defense system of microbes [52, 53].
Nitrate is an important secondary species, which has been positively correlated with bactericidal effects in recent studies. It is a long-lived free radical, and the concentration of NO3 − in CAPAW increases with processing time (Fig. 4c). In this study, the concentration of NO3 − increased from 7.857 to 35.405 mg/L within 5 min. Although NO2 − is an important antibacterial agent, its concentration was relatively low (data not shown) in this study because under acidic conditions (pH < 3.5), a series of reactions occurs and eventually produces NO3 − [34, 54, 55]. These results are in agreement with other recent studies [32, 56]. Additionally, NO contributes to the generation of NO3 − in the water.
As shown in Fig. 6, the emission spectra are dominated by N2, as N2 is the major component of air (78%). The figure displays the N2 + first negative system at 400–520 nm, the N2 first positive system at 550–730 nm, and the second positive system at 300–420 nm. Additionally, N and O emission lines can be detected at 730–900 nm. The dissociation of O2 and N2 generates O and N, respectively, which are excited from the ground state by electron impact:
As shown in Fig. 6b, OH was detected (306–316 nm), which possesses strong oxidization, can easily attack unsaturated fatty acids on the cell membrane, and interfere with intracellular materials such as protein, lipids, and DNA [57]. Therefore, OH· radicals existing in CAPAW may be vital agents contributing to the disinfection process.
The direct spin-trapping reaction between Fe2+(MGD)2 and NO produces the spin adduct NO–Fe2+(MGD)2, which is characterized by a tripling ESR spectrum with a peak intensity ratio of 1:1:1. As shown in Fig. 7, abundant NO–Fe2+ (MGD)2 signals were detected in the CAPAW-treated groups, which suggests that the NO radical was generated. However, the NO concentration in the group treated with CAPAW for 2 min was higher than in that treated for 1 min and the treatment time had little influence on the concentrations observed in groups 3 min to 5 min. The relevant chemical reactions in the water are:
No significant difference was observed between the treatment groups; this indicates that the NO radical might not be the key factor affecting their different disinfection abilities [20]. However, NO can induce ROS/RNS generation and cause oxidative damage to proteins during the antimicrobial process, as shown in Fig. 4d thus inactivating the bacteria in the biofilm. The evolution of NO can be described by the following sequences:
In conclusion, the antimicrobial mechanisms of CAPAW on biofilm are mainly attributed to ROS/RNS, including H+, NO, OH, NO3 −, and several chemical reaction intermediates.
Metal Corrosion Analysis
Besides their antimicrobial effect, chemical agents used to control DUWLs contamination must maintain a balance between efficiency, safety to patients, and material compatibility. As shown in Table 2, CAPAW and H2O have the same corrosion effect on copper (R1) and stainless steel (R0). Copper is more susceptible to corrosion than stainless steel. In published studies, even 1% H2O2 induced corrosion in the system, while NaClO solution also exhibited a high corrosive ability in DUWLs components [45]. In this study, CAPAW was basic non-corrosive to copper discs (R1) and non-corrosive to stainless steel discs (R0) compared with the distilled-water group. In acidified water, copper has a high standard potential, implying that it cannot be corroded with decreasing pH. However, the oxidization agents in CAPAW oxidize copper to Cu2+ and then form cupric nitrate with nitric acid [58]. Besides, in our previous studies, CAPAW was proven to be a green disinfection product, which is a promising alternative to traditional sanitizers applied in the food industry [53, 59].
Conclusion
In summary, this study systematically investigated the application of CAPAW to control biofilm contamination in DUWLs. The components and main mechanisms of CAPAW as well as its causticity to DUWLs components were carefully investigated. Overall, this study adopted a novel technology to control DUWLs contamination. However, some limitations exist and a future study will focus on evaluating the efficacy of CAPAW on natural DUWLs biofilms, combining the system with a dental unit, and investigating potential adverse effects of CAPAW on waterline tubing.
References
Toroglu MS, Haytac MC, Koksal F (2001) Angle Orthod 71:299–306
Panagakos FS, Lassiter T, Kumar E (2001) J N J Dent Assoc 72:20–25
Martin MV (1987) Br Dent J 163:152–154
Ricci ML, Fontana S, Pinci F, Fiumana E, Pedna MF, Farolfi P, Sabattini MA, Scaturro M (2012) Lancet 379:684
American Dental Association, Statement on Dental Unit Waterlines (2012). http://www.ada.org/1856.aspx
Centers for Disease Control and Prevention, Guidelines for Infection Control in Dental Health-Care Settings (2003) MMWR 52 (No. RR-17)
Barbeau J, Tanguay R, Faucher E, Avezard C, Trudel L, Côté L, Prévost AP (1996) Appl Environ Microbiol 62:3954–3959
Molinari JA (1999) Compend Contin Educ Dent 20:358–362
Szymanska J, Sitkowska J (2008) Dutkiewicz. J Ann Agric Environ Med 15:173–179
Uzel A, Cogulu D, Oncag O (2008) Int J Dent Hyg 6:43–47
Arvand M, Hack A (2013) Eur J Microbiol Immunol (Bp) 3:49–52
Wirthlin MR, Marshall GW Jr, Rowland RW (2003) J Periodontol 74:1595–1609
Michałkiewicz M, Ginter-Kramarczyk D, Kruszelnicka IK (2015) Med Pr 66:763–770
Stuart CH, Schwartz SA, Beeson TJ, Owatz CB (2006) J Endod 32:93–98
Sedgley CM, Lennan SL, Clewell DB (2004) Mol Oral Microbiol 19:95–101
Rôças IN, Siqueira JF Jr, Santos KR (2004) J Endodont 30:315–320
Meiller TF, Depaola LG, Kelley JIJI, Baqui AAMA, Turng BF, Falkler WA (1999) J Am Dent Assoc 130:65–72
Lin SM, Svoboda KK, Giletto A, Seibert J, Puttaiah R (2011) Eur J Dent 5:7–59
Liaqat I, Sabri AN (2008) Curr Microbiol 56:619–624
Montebugnoli L, Chersoni S, Prati C, Dolei G (2004) J Hosp Infect 56:297–304
Plamondon TJ, Mills SE, Sherman LR, Nemeth J, Puttaiah R (1996) J Dent Res 75:414
Isbary G, Shimizu T, Li YF, Stolz W, Thomas HM, Morfill GE, Zimmermann JL (2013) Expert Rev Med Devic 1:367–377
Traylor MJ, Pavlovich MJ, Karim S, Hait P, Sakiyama Y, Clark DS, Graves DB (2011) J Phys D Appl Phys 44:472001
Pavlovich MJ, Chang HW, Sakiyama Y, Clark DS, Graves DB (2013) J Phys D Appl Phys 46:145202
Khlyustova A, Khomyakova N, Sirotkin N, Marfin Y (2016) Plasma Chem Plasma P 36:1–10
Brisset JL, Pawlat J (2016) Plasma Chem Plasma P 36:355–381
Fridman G, Dobrynin D, Friedman G, Friedman A (2009) New J Phys 11:115020
Zhu AM, Sun Q, Niu JH, Song ZM (2005) Plasma Chem Plasma P 2:371–386
Kamgang-Youbi G, Herry JM, Meylheuc T, Brisset JL, Bellon-Fontaine MN, Doubla A, Naïtali M (2009) Lett Appl Microbiol 48:13–18
Yu S, Chen QZ, Liu JH, Wang KL, Jiang Z, Sun ZL, Zhang J, Fang J (2015) Appl Phys Lett 106:244101
Yu S, Wang K, Zuo S, Liu J, Zhang J, Fang J (2015) Phys Plasmas 22:1284
Schnabel U, Andrasch M, Weltmann KD, Ehlbeck J (2014) Plasma Process Polym 11:110–116
Ma RN, Feng HQ, Liang YD, Zhang Q, Tian Y, Su B, Zhang J, Fang J (2013) J Phys D Appl Phys 46:285401
Oehmigen K, Hähnel M, Brandenburg R, Wilke C, Weltmann KD, Woedtke TV (2010) Plasma Process Polym 7:250–257
The National Standard GB/10124-88 of the People’s Republic of China (2008)
Tuttlebee CM, O’Donnell MJ, Keane CT, Russell RJ, Sullivan DJ, Falkiner F, Coleman DC (2002) J Hosp Infect 52:192–205
Gilbert P, Das JR, Jones MV, Allison DG (2001) J Appl Microbiol 91:248–254
Chate RA (2006) Brit Dent J 201:565–569
Venkatadri R, Peters RW (1993) Hazard Waste Hazard Mater 10:107–149
Ikawa S, Kitano K, Hamaguchi S (2010) Plasma Process Polym 7:33–42
Park G, Ryu YH, Hong YJ, Choi EH, Uhm HS (2012) Appl Phys Lett 100:1–4
Shainsky N, Dobrynin D, Ercan U, Joshi SG, Ji H, Brooks A, Fridman G, Cho Y, Fridman A (2012) Plasma Process Polym. doi:10.1002/ppap.201100084
Pereira CI, Matos D, Romão MVS, Crespo MTB (2009) Appl Environ Microbiol 75:1904–1907
Zhang Q, Zhuang J, von Woedtke T, Kolb JF, Zhang J, Fang J, Weltmann KD (2014) Appl Phys Lett 105:1–4
Puttaiah R, Karpay RI, Fabre C, Sherman L, Nemeth JF, Mills SE, Plamondon TJ (1998) Microchem J 59:333–340
Flahaut S, Hartke A, Giard JC, Benachour A, Boutibonnes P, Auffray Y (1996) FEMS Microbiol Lett 138:49–54
Cotter PD, Hill C (2003) Microbiol Mol Biol R 67:429–453
Gordon CA, Hodges NA, Marriott C (1998) J Antimicrob Chemother 22:667–674
Xue Z, Sendamangalam VR, Gruden CL, Seo Y (2012) Environ Sci Technol 46:13212–13219
Brisset JL, Benstaali B, Moussa D, Fanmoe J, Njoyim-Tamungang E (2011) Plasma Sour Sci Technol 20:034021
Ikawa S, Tani A, Nakashima Y, Kitano K (2016) J Phys D Appl Phys 49:425401
McPherson LL (1993) Water Eng Manag 140:29–31
Zhang Q, Ma RN, Tian Y, Su B, Wang KL, Yu S, Zhang J, Fang J (2016) Environ Sci Technol 50:3184–3192
Park JY, Yin NL (2002) J Phys Chem 92:6294–6302
Brisset JL, Hnatiuc E (2012) Plasma Chem Plasma P 32:655–674
Van Gils CAJ, Hofmann S, Boekema BKHL, Brandenburg R (2013) J Phys D Appl Phys 46:175203
Cheeseman KH, Slater TF (1993) Br Med Bull 49:481–493
Brisset JL, Moussa D, Doubla A, Hnatiuc E, Hnatiuc B, Kamgang-Youbi GK, Herry JM, Naïtali M, Bellon-Fontaine MN (2008) Ind Eng Chem Res 47:5761–5781
Ma RN, Wang GM, Tian Y, Wang KL, Zhang J, Fang J (2015) J Hazard Mater 300:643–651
Acknowledgements
This study was supported by the 985 program of Peking University. The authors are highly thankful to Peking University for providing financial assistance of this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, J., Li, Y.L., Liu, C.M. et al. Investigation of Cold Atmospheric Plasma-Activated Water for the Dental Unit Waterline System Contamination and Safety Evaluation in Vitro. Plasma Chem Plasma Process 37, 1091–1103 (2017). https://doi.org/10.1007/s11090-017-9811-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9811-0