Abstract
The ε4 allele of the apolipoprotein E (APOE4) is associated with an increased risk of developing Alzheimer’s disease (AD). Hence, several studies have compared the brain characteristics of APOE4 carriers versus non-carriers in presymptomatic stages to determine early AD biomarkers. The present review provides an overview on APOE4-related brain changes in cognitively normal individuals, focusing on the main neuroimaging biomarkers for AD, i.e. cortical beta-amyloid (Aβ) deposition, hypometabolism and atrophy. The most consistent findings are observed with Aβ deposition as most studies report significantly higher cortical Aβ load in APOE4 carriers compared with non-carriers. Fluorodeoxyglucose-positron emission tomography studies are rare and tend to show hypometabolism in brain regions typically impaired in AD. Structural magnetic resonance imaging findings are the most numerous and also the most discrepant, showing atrophy in AD-sensitive regions in some studies but contradicting results as well. Altogether, this suggests a graded effect of APOE4, with a predominant effect on Aβ over brain structure and metabolism. Multimodal studies confirm this view and also suggest that APOE4 effects on brain structure and function are mediated by both Aβ-dependent and Aβ-independent pathological processes. Neuroimaging studies on asymptomatic APOE4 carriers offer relevant information to the understanding of early pathological mechanisms of the disease, although caution is needed as to whether APOE4 effects reflect AD pathological processes, and are representative of these effects in non-carriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The APOE gene is located on the long arm of chromosome 19 and exists as three main polymorphic alleles (ε2, ε3 and ε4). This gene codes for a lipoprotein called apolipoprotein E that plays a role in the transport of lipids (see Bu 2009; Holtzman et al. 2012 for reviews). The ε4 allele of the APOE (APOE4) is the major known genetic risk factor for late-onset Alzheimer’s disease (AD). By contrast, the APOE2 has a protective effect. A meta-analysis of clinical and autopsy-based studies reported odds ratios for AD of 0.6, for APOE2/2 or 2/3, and 2.6, 3.2, and 14.9 for APOE2/4, 3/4, and 4/4, respectively (Farrer et al. 1997). Recent estimations reported a lifetime risk of AD of 50 – 60 % for APOE4/4 men and women versus 23 – 30 % for APOE4/3 men and women, respectively (Genin et al. 2011). As the number of ε4 alleles rises from 0 to 2, the risk of developing AD became greater from 20 to 90 % and the mean age at onset lowered to 68 from 84 years old (Corder et al. 1993). Yet, carrying the ε4 allele is neither sufficient nor a necessary condition for developing AD (Saunders et al. 1993).
Neuroimaging biomarkers including atrophy, hypometabolism and beta-amyloid (Aβ) load assessed with structural magnetic resonance imaging (MRI), 18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) and Aβ-PET respectively have been integrated in the research criteria for the diagnosis of preclinical AD (Dubois et al. 2010; Sperling et al. 2011). It is well acknowledged that these brain changes occur well before - up to two decades - the onset of symptoms. Refinements are needed, however, regarding the preclinical stages, not only for the use of these biomarkers as diagnostic criteria, but also for the understanding of the sequence of events and pathophysiological mechanisms in AD.
Because APOE4 is the major genetic risk factor for late-onset AD, the comparison of the brain characteristics of APOE4 carriers versus non-carriers in presymptomatic stages represents a great opportunity to study early AD biomarkers. The present review provides an overview on APOE4-related brain changes in cognitively normal individuals, focusing on the main neuroimaging biomarkers for AD, i.e. cortical Aβ deposition, hypometabolism and atrophy.
The Effects of APOE4 on Cortical Aβ Deposition Assessed with PET
Aβ load as assessed using PET with Aβ ligands (PiB, florbetapir, florbetaben, flutemetamol) has been consistently shown to correlate with neuropathological measures of Aβ deposition (Bacskai et al. 2007; Ikonomovic et al. 2008; Leinonen et al. 2008; Clark et al. 2011; 2012). The brain pattern of Aβ deposition evidenced with PET is consistent with that derived from postmortem studies and mainly includes frontal (specifically medial orbito-frontal and anterior cingulate), posterior cingulate and precuneus, temporo-parietal and lateral temporal areas (see Villemagne and Rowe 2013 for review).
Despite the relative recentness of Aβ-PET imaging, there have been numerous studies assessing the effects of APOE polymorphism on Aβ deposition including in presymptomatic stages. That APOE4 influences Aβ deposition is convincingly demonstrated in most studies, although specific aspects may differ from one study to another. Thus, most studies have shown that cognitively normal APOE4 carriers have higher cortical Aβ deposition compared with non-carriers - as illustrated in Fig. 1, or include a greater proportion of Aβ-positive individuals (Reiman et al. 2009; Hinrichs et al. 2010; Morris et al. 2010; Rowe et al. 2010; Villemagne et al. 2011; Jagust et al. 2012; Kantarci et al. 2012; Mielke et al. 2012; Fleisher et al. 2013; Mathis et al. 2013; Murphy et al. 2013; Roe et al. 2013; Mormino et al. 2014; Scheinin et al. 2014). Within cognitively normal elderly, the proportion of Aβ-positive individuals varies from 35 to 49 % amongst APOE4 carriers and from 9 to 37 % amongst non-carriers (see Chételat and Fouquet 2013 for review). Moreover, the effect of APOE4 on Aβ deposition was found to be dose-dependent, i.e. to be proportional to the number of ε4 alleles. Thus, Reiman and colleagues (2009), Protas and colleagues (2013) and Roe and colleagues (2013) showed higher PiB fixation with each additional ε4 allele and Morris and colleagues (2010) observed higher age-related Aβ cortical accumulation with each additional ε4 allele (0, 1 or 2). In some studies, the effect of APOE4 was shown to be region specific but the regions showing a predominant effect differed amongst studies (e.g. the frontal cortex in Reiman et al. 2009 and Scheinin et al. 2014 versus posterior regions in Fleisher et al. 2013).
In cross-sectional studies, a differential effect of age on Aβ deposition according to the APOE4 status has been reported in cognitively normal individuals. Although the slope of the relationship between age and Aβ load was not found to be different between APOE4 carriers and non-carriers (Fleisher et al. 2013; Scheinin et al. 2014; Rodrigue et al. 2012), a linear accumulation was found from 18 to 90 years old in carriers, while it was only observed from 64 years old in the non-carriers (Fleisher et al. 2013). Two other studies yet reported higher accumulation with age (i.e. higher slope) in carriers than in non-carriers, which may be due to methodological differences, i.e. the specification of the APOE2 genotype in the model (Morris et al. 2010) or the inclusion of elderly with subjective memory complaints (Rowe et al. 2010).
Longitudinal Aβ-PET studies show that the presence of the ε4 allele is associated with a higher prevalence of conversion from Aβ-negative to Aβ-positive (Vlassenko et al. 2011) and an earlier age of predicted Aβ-positivity compared with non-carriers (56 versus 76 years old, respectively; Fleisher et al. 2013). In another study, the proportion of APOE4 carriers was higher in the cognitively normal individuals who showed a significant increase in Aβ deposition over time (called “accumulators”) than in the non-accumulators (Villemagne et al. 2013).
Two studies from different cohorts also suggest that APOE4 may modify the relationships between Aβ deposition and concomitant cognitive performances. In the Mayo Clinic Study of Aging (MCSA) cohort, APOE4 was found to modulate the link between Aβ burden and global cognition, with a more detrimental effect of Aβ accumulation in the carriers (Kantarci et al. 2012). In the Australian Imaging, Biomarkers, and Lifestyle (AIBL) cohort (enriched in APOE4 carriers and including older individuals), Lim and colleagues (2013) reported a detrimental effect of Aβ load, notably on memory, only in APOE4 carriers.
Longitudinal studies assessing the modulating effect of APOE4 on the relationship between Aβ load and cognitive decline over time report more inconsistent results. In the AIBL cohort, APOE4 was not found to alter Aβ-related decline in memory assessed over 18 months (Lim et al. 2012; Ellis et al. 2013) or 36 months (Lim et al. 2014). In another study on the AIBL cohort, APOE4 genotype was associated with faster episodic memory decline within accumulators (Villemagne et al. 2013). Finally, when assessing memory decline over time in elderly from the Harvard Aging Brain Study (HABS), MCSA and AIBL longitudinal cohorts together, Mormino and colleagues (2014) found faster memory decline in Aβ-positive APOE4 carriers than in the Aβ-negative or non-carrier subgroups.
In sum, the effect of APOE4 on Aβ imaging appears to be marked and overall consistent amongst studies, although discrepancies exist when focusing on specific aspects of this effect (e.g. a potential region specificity or the relation with rates of cognitive changes). Consistently, APOE4 has been reported as the best predictor of the presence of Aβ in the brain of healthy elderly amongst age, sex, APOE genotype, family history, or cognitive performance (Mielke et al. 2012). Yet, it is interesting to note that one study reported that PiB binding has a high heritability, but that 74 % of the heritable component cannot be explained by APOE4 genotype (Hinrichs et al. 2010).
The Effects of APOE4 on Cortical Metabolism Assessed with FDG-PET
Despite the fact that they started almost 15 years before, FDG-PET studies assessing the effect of APOE4 in asymptomatic elderly are less numerous than Aβ-PET studies. Lower metabolism in APOE4 carriers compared with non-carriers was first reported by Small and colleagues (1995) in elderly with memory complaints and at-least two relatives with AD and by Reiman and colleagues (1996) in cognitively normal elderly, all selected based on their family history of AD. This hypometabolism concerned brain regions typically affected in AD, i.e. mainly posterior cingulate, parietal and temporal areas, but also the prefrontal cortex. Interestingly, the same findings were found in young (20–39 years old) carriers (Reiman et al. 2004). Moreover a gene-dose effect was reported in all these brain regions in late-middle-age individuals, i.e. lower metabolism with higher number of ε4 alleles (Reiman et al. 2005).
Two later studies in independent and larger samples mainly confirmed the presence of hypometabolism in AD-sensitive brain regions such as the posterior cingulate cortex and temporo-parietal regions (Knopman et al. 2014) but also beyond (Jagust and Landau 2012).
Similar effects have also been reported in less healthy populations such as elderly reporting memory difficulties (Rimajova et al. 2008) or anxiety, depression and other health conditions (Langbaum et al. 2010).
However, contradictive findings have also been reported as one study found no difference in individual mean z-scores of metabolism between 45 carriers and 45 age-, sex-, education level-, MMSE-matched non-carriers in the same AD-sensitive regions (Samuraki et al. 2012). Finally, higher metabolism, e.g. in the frontal cortex, was also reported in addition to lower metabolism in AD-sensitive regions in 17 carriers compared with 15 non-carriers (Yi et al. 2014).
As a whole, studies assessing the effect of APOE4 on FDG uptake in cognitively normal subjects are relatively rare and findings altogether are not very consistent. Studies tend to find an effect when assessing specific samples, e.g. in individuals with a family history of AD (Reiman et al. 1996, 2005) or when comparing carriers with slightly but significantly lower scores at the Mini Mental State Examination, to non-carriers amongst which 16 % had an APOE2 allele (Jagust and Landau 2012). Further studies are thus needed to confirm that APOE4 is associated with a lower metabolism in AD-sensitive brain areas and also in other brain regions, but also to specify whether this effect is observed at a particular age range, a question still unresolved to date (Knopman et al. 2014). The effects on brain metabolism appear to be subtler than those on cortical Aβ deposition, although only studies including both FDG- and Aβ-PET imaging could directly address this question (see below “Discussion - Multimodal studies”).
The Effects of APOE4 on Grey Matter Assessed with Structural MRI
Studies assessing the effect of APOE4 on brain structure using structural MRI are both the most numerous and the most inconsistent. Significantly lower grey matter volume in APOE4 carriers compared with non-carriers has been reported in several studies in elderly individuals (Plassman et al. 1997; Tohgi et al. 1997; Lind et al. 2006; Honea et al. 2009; Chen et al. 2012), sometimes only in APOE4 homozygotes (Lemaître et al. 2005), or in young adults (Wishart et al. 2006; O’Dwyer et al. 2012), most often in AD-sensitive brain regions such as the hippocampus or other medial temporal structures.
Other regions are also reported such as the temporal and prefrontal cortex (Wishart et al. 2006) or parietal areas (Honea et al. 2009). Yet, there have also been numerous studies reporting no significant differences, even when assessed throughout the entire cortex, between APOE4 carriers and non-carriers in terms of grey matter volume in elderly (Soininen et al. 1995; Schmidt et al. 1996; Jack et al. 1998; Reiman et al. 1998; Cohen et al. 2001; Han et al. 2007; Filippini et al. 2011; Kukolja et al. 2010; Westlye et al. 2011; Bunce et al. 2012; Protas et al. 2013; Hostage et al. 2013) or in young adults (Dennis et al. 2010; Filippini et al. 2009; Matura et al. 2014). Moreover, some studies even showed greater grey matter volume in elderly APOE4 carriers compared with non-carriers (Honea et al. 2009; Striepens et al. 2011).
A few studies have assessed the effect of APOE4 on the hippocampal histological subfields. Overall, the findings converged to a detrimental effect on the cortical thickness or volume in elderly, although results diverged as regard to the affected subfield. Thus, cortical thickness was found to be lower in elderly APOE4 carriers compared with non-carriers in the subiculum (Burggren et al. 2008; Donix et al. 2010a; Suthana et al. 2010) and CA1 subfield (Kerchner et al. 2014). Lower CA3 and the dentate gyrus volumes were also reported in APOE4 carriers (Mueller et al. 2008; Mueller and Weiner 2009). Moreover, hippocampal cortical thickness (Burggren et al. 2008) but not grey matter volume (Burggren et al. 2008; Mueller et al. 2008; Mueller and Weiner 2009) was found to be lower in APOE4 carriers compared with non-carriers using manual tracing on high-resolution MRI scans. However, no significant effect of APOE4 on cortical thickness was found in the hippocampus when assessed automatically using Freesurfer on T1-weighted scans from 1.5 Tesla MRI, while the same studies reported lower (Fan et al. 2010; Liu et al. 2010a) or greater (Espeseth et al. 2008) cortical thickness in APOE4 carriers compared with non-carriers in other brain areas.
Furthermore several studies argue for a non-linear effect of APOE4 through aging, but once again results are divergent. Lind and colleagues (2006) and Wishart and colleagues (2006) suggest that the effect of APOE4 is more marked in young than in elderly individuals, but more recently Mueller and colleagues (2008) and Mueller and Weiner (2009) reported reverse effects on the hippocampal subfields.
Cognitively normal elderly APOE2 carriers were found to show greater cortical thickness in the temporal cortex (Fan et al. 2010; Fennema-Notestine et al. 2011) compared with APOE3 and in the dorsolateral prefrontal cortex compared with APOE4 (Fan et al. 2010), or greater hippocampal grey matter volume compared with APOE3 (Hostage et al. 2013) or to APOE4 (Alexopoulos et al. 2011) carriers. Yet, again, contradictory findings have been reported, including a lack of difference between APOE2 and APOE3 in hippocampal grey matter volume (Chiang et al. 2010) and cortical thickness (Liu et al. 2010b), or even decreased hippocampal and amygdala grey matter volumes (den Heijer et al. 2002) or hippocampal sulcal cavities widening (Barboriak et al. 2000) in APOE2 equivalent to that found in APOE4 carriers. This latter finding is in line with post-mortem findings of higher AD neuropathology in APOE2 carriers than in APOE3 homozygotes (Berlau et al. 2009).
Regarding longitudinal MRI studies, results in elderly are more consistent. Most studies highlighted a faster rate of grey matter atrophy in APOE4 carriers compared with non-carriers, especially in medial temporal structures (Cohen et al. 2001; Chen et al. 2007; Morra et al. 2009; Donix et al. 2010a; Hua et al. 2010; Risacher et al. 2010; Chiang et al. 2011; Lu et al. 2011; Roussotte et al. 2014), although negative findings have also been reported (Jack et al. 1998; Du et al. 2006; Schuff et al. 2009; Taylor et al. 2014). In contrast, normal elderly APOE2 carriers showed a slower rate of hippocampal grey matter atrophy compared with APOE3 homozygotes (Chiang et al. 2011).
Finally, some studies have reported grey matter differences between APOE4 carriers and non-carriers in children especially in the entorhinal cortex (Shaw et al. 2007) and even in neonates (Dean et al. 2014; Knickmeyer et al. 2014). Although negative findings have recently been reported in adolescents (Khan et al. 2014), these results suggest a part of genetic determination or a neurodevelopmental effect of APOE4. On the other hand, longitudinal studies showing faster rates of atrophy in elderly APOE4 carriers (see above), suggest that APOE4 also has a pathologic effect that tends to exacerbate with time.
Altogether, the effects of APOE4 on brain structure thus seem particularly subtle, if any, which would account for the divergence of findings. Moreover, these effects are probably not specific to AD pathological processes, as atrophy in the same medial temporal areas has also been reported over age in individuals with low risk for AD (i.e. Aβ-negative non-APOE4 carriers; Fjell et al. 2014a; see Fjell et al. 2014b for review).
Discussion
Multimodal Studies
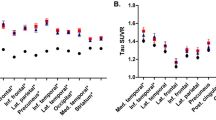
APOE4 is thought to have multiple effects, e.g. to increase Aβ pathology, neurotoxicity, aberrant brain activity, brain atrophy, tangle formation, decrease synaptic function, neurogenesis, glucose metabolism, vascular and mitochondrial functions and lipid and cholesterol metabolism (Liu et al. 2013). While the effects on Aβ plaque burden are well established, those on other pathological processes such as tau-related changes are less clear (Kim et al. 2009). From a neuroimaging standpoint, the review of the literature from mainly single-modality neuroimaging studies described above reveals discrepant - and therefore probably subtle - effects of APOE4 on brain structure, less heterogeneous (but rare) findings for FDG-PET, and a clear effect on Aβ deposition, all mainly occurring in AD-sensitive areas. This graded effect of APOE4 on atrophy, hypometabolism, and Aβ deposition is illustrated in Fig. 2, and is also consistent with several results from multimodal neuroimaging studies. Thus, Chen and colleagues (2012) reported that FDG-PET showed a stronger association to APOE4 compared with structural MRI, and concluded that posterior cingulate hypometabolism appears earlier than any detectable MRI-based structural abnormalities. Similarly, Protas and colleagues (2013) found significant differences between APOE4 carriers and non-carriers in posterior cingulate glucose metabolism, but not in clinical rating, neuropsychological test score, hippocampal volume, or hippocampal glucose metabolism measurements. They concluded that a reduction in posterior cingulate glucose metabolism precedes a reduction in hippocampal volume or metabolism in cognitively normal persons at increased genetic risk for AD.
Schematic representation of the graded effect of APOE4 on structural, functional and molecular cortical changes. APOE4 effects clearly predominate on Aβ deposition (thick arrows), while the effects are more modest on cortical metabolism and volume (thin arrows). This figure also illustrates that APOE4 operates through both Aβ-dependent and Aβ-independent processes
It is thought that there are both Aβ-dependent and Aβ-independent effects of APOE4 acting in concert to exacerbate the pathological and clinical phenotypes of AD (see Huang 2010; Liu et al. 2013 for reviews; see Fig. 2). Multimodal studies confirm this view, showing that the effects of APOE4 on brain structure and function is at least partly independent from its effect on Aβ deposition. Thus, Jagust and Landau (2012) found an effect of APOE4 on both Aβ deposition and FDG-PET metabolism, but showed that the lower metabolism was independent from the presence of Aβ, concluding that APOE genotype, and not aggregated fibrillar forms of Aβ, contributes to reduced glucose metabolism in aging. Similarly, Knopman and colleagues (2014) reported lower metabolism in AD-sensitive regions, within Aβ-positive cognitively normal elderly, or in the APOE4 carriers, but there was no interaction between both terms i.e. the effect of APOE4 on metabolism was not merely a reflection of the effect of Aβ deposition. Amyloidosis and APOE4 carriage rather appear to be additive in their impact on hypometabolism (Knopman et al. 2014). Yi and colleagues (2014) however yield to a different conclusion as they found that hypometabolism in APOE4 carriers largely disappeared when adjusting for global cortical Aβ deposition (see also Lowe et al. 2014) or when only considering the Aβ-negative individuals while hypermetabolism persisted in medial frontal and anterior temporal areas. Yet, the detection of APOE4-related structural and functional changes in children (Shaw et al. 2007) or young adults (Reiman et al. 2004; Knopman et al. 2014), i.e. well before the appearance of Aβ accumulation in the brain (Kok et al. 2009), would also rather argue for the existence of Aβ-independent effects of APOE4 on brain structure and function.
In sum, there are both Aβ-dependent and Aβ-independent effects of APOE4 acting in concert to exacerbate the pathological and clinical phenotypes of AD (see Huang 2010; Huang and Mucke 2012 for reviews).
Methodological Comments
Firstly, it is important to note that the effects of APOE4 on brain aging were often assessed in cross-sectional studies by comparing, within carriers versus non-carriers, the correlation between age and the measure of interest in different individuals (i.e. assessing the interaction between APOE4 and age). However, this cross-sectional approach confuses inter-subject variability with intra-subject variability (Thompson et al. 2011). Consequently, the observed effects of age could reflect an inter-individual or inter-generational cohort effect rather than a genuine effect of age. Only longitudinal studies assessing the effects of age within the same individuals over time to measure intra-individual rates of change would allow to determine specifically the effects of age on cortical Aβ deposition, metabolism, structure and cognition. It is also worth noting that, even if they concern AD-sensitive brain regions, APOE4 effects are not necessarily reflecting AD-related processes (see also above).
Second, the results are partly discrepant regarding the effect of APOE4 especially on brain structure but also on brain metabolism. Methodological differences amongst studies (i.e., acquisition parameters, quantification methods, level of significance, etc.) may explain part of this variability, although no clear methodological factor distinguished discrepant studies (e.g. young versus elderly, homozygotes versus heterozygotes ratio, regions of interest versus whole brain). Besides, part of the conflicting results may also be attributable to the antagonist pleiotropy of APOE4 effects across different stages of the lifespan, such that APOE4 may be beneficial in earlier ages and may confer risk of cognitive decline only later in life (Tuminello and Han 2011). It is also possible that non-intuitive findings are not reported. Moreover, confounding variables are rarely modelled (see Donix et al. 2012 for review). Finally, AD has a complex polygenic background and studying the brain effects associated with the presence of a single genetic risk factor, as the ε4 allele, may lead to variable results. Indeed, unknown gene-gene and gene-environment interactions are likely to modulate the effect of this genetic factor on brain structure and function, potentially resulting in both overestimation and masking of APOE4 effects (see Donix et al. 2012 for review). In this respect, APOE genotype and family history risk were shown to have independent and/or additive contributions to brain structure (Donix et al. 2010b; Honea et al. 2010, 2011) or metabolism (Mosconi et al. 2007, 2009).
APOE4 Effects on Other Neuroimaging Modalities
While this review primarily focuses on the most validated and widely used neuroimaging biomarkers for AD, namely structural MRI, FDG-PET and Aβ-PET, the effect of APOE4 has also been assessed using other neuroimaging modalities. In brief, the results are consistent with the idea that APOE4 cognitively normal elderly, as a group, do present some signs of AD-related features, i.e. damage in white matter tracts connected to the medial temporal lobe usually impaired in AD (see Gold et al. 2012 for review), and higher or lower functional connectivity between AD-sensitive brain areas (see Dennis and Thompson 2014 for review). Besides, fMRI studies frequently report higher activations (often, but not only, within the hippocampus) in APOE4 carriers compared with non-carriers during episodic memory tasks (see Wierenga and Bondi 2007; Trachtenberg et al. 2012 for reviews). For example, Bookheimer et al. (2000) reported higher magnitude and extent of brain activation during memory-activation tasks in regions affected by AD, including the left hippocampal, parietal, and prefrontal regions, in cognitive normal APOE4 carriers compared with non-carriers. This higher activity is usually interpreted as compensatory recruitment to support memory performance, although studies in mice suggest that it may rather reflect a detrimental function of hippocampal inhibitory function (see Gallagher and Koh 2011 for review).
Conclusion
Cognitively normal APOE4 carriers offer a great opportunity to further our understanding of the pathophysiology of AD at the presymptomatic phase of the disease. Studying current neuroimaging biomarkers for preclinical AD with PET and MRI in such individuals allows in vivo evaluation of early AD-related brain changes, although further studies are needed to confirm that APOE4-related brain changes reflect AD pathological process. This evidence-based review argues for a graded effect of APOE4 on brain structural, functional and molecular changes, with a predominant effect on Aβ deposition and subtler effects on cortical metabolism and grey matter volume. It also suggests both Aβ-dependent and Aβ-independent effects of APOE4 on brain structure and function. It is likely that APOE4 not only increases the risk for AD, but also modulates its pathophysiological process at different levels. Future multimodal neuroimaging follow-up studies would tell whether the mechanisms and sequence evidenced in carriers is comparable to those found in non-carriers.
References
Alexopoulos, P., Richter-Schmidinger, T., Horn, M., Maus, S., Reichel, M., Sidiropoulos, C., et al. (2011). Hippocampal volume differences between healthy young apolipoprotein E ε2 and ε4 carriers. Journal of Alzheimer’s Disease, 26(2), 207–210.
Bacskai, B. J., Frosch, M. P., Freeman, S. H., Raymond, S. B., Augustinack, J. C., Johnson, K. A., et al. (2007). Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Archives of Neurology, 64(3), 431–434.
Barboriak, D. P., Doraiswamy, P. M., Krishnan, K. R., Vidyarthi, S., Sylvester, J., & Charles, H. C. (2000). Hippocampal sulcal cavities on MRI: relationship to age and apolipoprotein E genotype. Neurology, 54(11), 2150–2153.
Berlau, D. J., Corrada, M. M., Head, E., & Kawas, C. H. (2009). APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology, 72(9), 829–834.
Bookheimer, S. Y., Strojwas, M. H., Cohen, M. S., Saunders, A. M., Pericak-Vance, M. A., Mazziotta, J. C., et al. (2000). Patterns of brain activation in people at risk for Alzheimer’s disease. The New England Journal of Medicine, 343(7), 450–456.
Bu, G. (2009). Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nature Reviews Neuroscience, 10(5), 333–344.
Bunce, D., Anstey, K. J., Cherbuin, N., Gautam, P., Sachdev, P., & Easteal, S. (2012). APOE genotype and entorhinal cortex volume in non-demented community-dwelling adults in midlife and early old age. Journal of Alzheimer’s Disease, 30(4), 935–942.
Burggren, A. C., Zeineh, M. M., Ekstrom, A. D., Braskie, M. N., Thompson, P. M., Small, G. W., et al. (2008). Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. NeuroImage, 41(4), 1177–1183.
Chen, K., Reiman, E. M., Alexander, G. E., Caselli, R. J., Gerkin, R., Bandy, D., et al. (2007). Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. The American Journal of Psychiatry, 164(6), 916–921.
Chen, K., Ayutyanont, N., Langbaum, J. B. S., Fleisher, A. S., Reschke, C., Lee, W., et al. (2012). Correlations between FDG PET glucose uptake-MRI gray matter volume scores and apolipoprotein E ε4 gene dose in cognitively normal adults: a cross-validation study using voxel-based multi-modal partial least squares. NeuroImage, 60(4), 2316–2322.
Chételat, G., & Fouquet, M. (2013). Neuroimaging biomarkers for Alzheimer’s disease in asymptomatic APOE4 carriers. Revue Neurologique, 169(10), 729–736.
Chiang, G. C., Insel, P. S., Tosun, D., Schuff, N., Truran-Sacrey, D., Raptentsetsang, S. T., et al. (2010). Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normal subjects. Neurology, 75(22), 1976–1981.
Chiang, G. C., Insel, P. S., Tosun, D., Schuff, N., Truran-Sacrey, D., Raptentsetsang, S. T., et al. (2011). Impact of apolipoprotein E4-cerebrospinal fluid β-amyloid interaction on hippocampal volume loss over 1 year in mild cognitive impairment. Alzheimer’s & Dementia, 7(5), 514–520.
Clark, C. M., Schneider, J. A., Bedell, B. J., Beach, T. G., Bilker, W. B., Mintun, M. A., et al. (2011). Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA, the Journal of the American Medical Association, 305(3), 275–283.
Clark, C. M., Pontecorvo, M. J., Beach, T. G., Bedell, B. J., Coleman, R. E., Doraiswamy, P. M., et al. (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurology, 11(8), 669–678.
Cohen, R. M., Small, C., Lalonde, F., Friz, J., & Sunderland, T. (2001). Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology, 57(12), 2223–2228.
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science, 261(5123), 921–923.
Dean, D. C., 3rd, Jerskey, B. A., Chen, K., Protas, H., Thiyyagura, P., Roontiva, A., et al. (2014). Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurology, 71(1), 11–22.
Den Heijer, T., Oudkerk, M., Launer, L. J., van Duijn, C. M., Hofman, A., & Breteler, M. M. B. (2002). Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology, 59(5), 746–748.
Dennis, E. L., & Thompson, P. M. (2014). Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychology Review, 24(1), 49–62.
Dennis, N. A., Browndyke, J. N., Stokes, J., Need, A., Burke, J. R., Welsh-Bohmer, K. A., et al. (2010). Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimer’s & Dementia, 6(4), 303–311.
Donix, M., Burggren, A. C., Suthana, N. A., Siddarth, P., Ekstrom, A. D., Krupa, A. K., et al. (2010a). Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. NeuroImage, 53(1), 37–43.
Donix, M., Burggren, A. C., Suthana, N. A., Siddarth, P., Ekstrom, A. D., Krupa, A. K., et al. (2010b). Family history of Alzheimer’s disease and hippocampal structure in healthy people. The American Journal of Psychiatry, 167(11), 1399–1406.
Donix, M., Small, G. W., & Bookheimer, S. Y. (2012). Family history and APOE-4 genetic risk in Alzheimer’s disease. Neuropsychology Review, 22(3), 298–309.
Du, A.-T., Schuff, N., Chao, L. L., Kornak, J., Jagust, W. J., Kramer, J. H., et al. (2006). Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiology of Aging, 27(5), 733–740.
Dubois, B., Feldman, H. H., Jacova, C., Cummings, J. L., Dekosky, S. T., Barberger-Gateau, P., et al. (2010). Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurology, 9(11), 1118–1127.
Ellis, K. A., Lim, Y. Y., Harrington, K., Ames, D., Bush, A. I., Darby, D., et al. (2013). Decline in cognitive function over 18 months in healthy older adults with high amyloid-β. Journal of Alzheimer’s Disease, 34(4), 861–871.
Espeseth, T., Westlye, L. T., Fjell, A. M., Walhovd, K. B., Rootwelt, H., & Reinvang, I. (2008). Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiology of Aging, 29(3), 329–340.
Fan, M., Liu, B., Zhou, Y., Zhen, X., Xu, C., Jiang, T., et al. (2010). Cortical thickness is associated with different apolipoprotein E genotypes in healthy elderly adults. Neuroscience Letters, 479(3), 332–336.
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. a meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA, the Journal of the American Medical Association, 278(16), 1349–1356.
Fennema-Notestine, C., Panizzon, M. S., Thompson, W. R., Chen, C.-H., Eyler, L. T., Fischl, B., et al. (2011). Presence of ApoE ε4 allele associated with thinner frontal cortex in middle age. Journal of Alzheimer’s Disease, 26(Suppl 3), 49–60.
Filippini, N., MacIntosh, B. J., Hough, M. G., Goodwin, G. M., Frisoni, G. B., Smith, S. M., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106(17), 7209–7214.
Filippini, N., Ebmeier, K. P., MacIntosh, B. J., Trachtenberg, A. J., Frisoni, G. B., Wilcock, G. K., et al. (2011). Differential effects of the APOE genotype on brain function across the lifespan. NeuroImage, 54(1), 602–610.
Fjell, A. M., Westlye, L. T., Grydeland, H., Amlien, I., Espeseth, T., Reinvang, I., et al. (2014a). Accelerating cortical thinning: unique to dementia or universal in aging? Cerebral Cortex, 24(4), 919–934.
Fjell, A. M., McEvoy, L., Holland, D., Dale, A. M., Walhovd, K. B., & Alzheimer’s Disease Neuroimaging Initiative. (2014b). What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Progress in Neurobiology [in press].
Fleisher, A. S., Chen, K., Liu, X., Ayutyanont, N., Roontiva, A., Thiyyagura, P., et al. (2013). Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiology of Aging, 34(1), 1–12.
Gallagher, M., & Koh, M. T. (2011). Episodic memory on the path to Alzheimer’s disease. Current Opinion in Neurobiology, 21(6), 929–934.
Genin, E., Hannequin, D., Wallon, D., Sleegers, K., Hiltunen, M., Combarros, O., et al. (2011). APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Molecular Psychiatry, 16(9), 903–907.
Gold, B. T., Johnson, N. F., Powell, D. K., & Smith, C. D. (2012). White matter integrity and vulnerability to Alzheimer’s disease: preliminary findings and future directions. Biochimica et Biophysica Acta, 1822(3), 416–422.
Han, S. D., Houston, W. S., Jak, A. J., Eyler, L. T., Nagel, B. J., Fleisher, A. S., et al. (2007). Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging, 28(2), 238–247.
Hinrichs, A. L., Mintun, M. A., Head, D., Fagan, A. M., Holtzman, D. M., Morris, J. C., et al. (2010). Cortical binding of pittsburgh compound B, an endophenotype for genetic studies of Alzheimer’s disease. Biological Psychiatry, 67(6), 581–583.
Holtzman, D. M., Herz, J., & Bu, G. (2012). Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2(3), a006312.
Honea, R. A., Vidoni, E., Harsha, A., & Burns, J. M. (2009). Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. Journal of Alzheimer’s Disease, 18(3), 553–564.
Honea, R. A., Swerdlow, R. H., Vidoni, E. D., Goodwin, J., & Burns, J. M. (2010). Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology, 74(2), 113–120.
Honea, R. A., Swerdlow, R. H., Vidoni, E. D., & Burns, J. M. (2011). Progressive regional atrophy in normal adults with a maternal history of Alzheimer disease. Neurology, 76(9), 822–829.
Hostage, C. A., Roy Choudhury, K., Doraiswamy, P. M., Petrella, J. R., & Alzheimer’s Disease Neuroimaging Initiative. (2013). Dissecting the gene dose-effects of the APOE ε4 and ε2 alleles on hippocampal volumes in aging and Alzheimer’s disease. PLoS One, 8(2), e54483.
Hua, X., Hibar, D. P., Lee, S., Toga, A. W., Jack, C. R., Jr., Weiner, M. W., et al. (2010). Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiology of Aging, 31(8), 1463–1480.
Huang, Y. (2010). Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends in Molecular Medicine, 16(6), 287–294.
Huang, Y., & Mucke, L. (2012). Alzheimer mechanisms and therapeutic strategies. Cell, 148(6), 1204–1222.
Ikonomovic, M. D., Klunk, W. E., Abrahamson, E. E., Mathis, C. A., Price, J. C., Tsopelas, N. D., et al. (2008). Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain, 131(Pt 6), 1630–1645.
Jack, C. R., Jr., Petersen, R. C., Xu, Y. C., O’Brien, P. C., Waring, S. C., Tangalos, E. G., et al. (1998). Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Annals of Neurology, 43(3), 303–310.
Jagust, W. J., Landau, S. M., & Alzheimer’s Disease Neuroimaging Initiative. (2012). Apolipoprotein E, not fibrillar β-amyloid, reduces cerebral glucose metabolism in normal aging. The Journal of Neuroscience, 32(50), 18227–18233.
Kantarci, K., Lowe, V., Przybelski, S. A., Weigand, S. D., Senjem, M. L., Ivnik, R. J., et al. (2012). APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology, 78(4), 232–240.
Kerchner, G. A., Berdnik, D., Shen, J. C., Bernstein, J. D., Fenesy, M. C., Deutsch, G. K., et al. (2014). APOE ε4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology, 82(8), 691–697.
Khan, W., Giampietro, V., Ginestet, C., Dell’Acqua, F., Bouls, D., Newhouse, S., Dobson, R., et al. (2014). No differences in hippocampal volume between carriers and non-carriers of the ApoE ε4 and ε2 alleles in young healthy adolescents. Journal of Alzheimer’s Disease, 40(1), 37–43.
Kim, J., Basak, J. M., & Holtzman, D. M. (2009). The role of apolipoprotein E in Alzheimer’s disease. Neuron, 63(3), 287–303.
Knickmeyer, R. C., Wang, J., Zhu, H., Geng, X., Woolson, S., Hamer, R. M., et al. (2014). Common variants in psychiatric risk genes predict brain structure at birth. Cerebral Cortex, 24(5), 1230–1246.
Knopman, D. S., Jack, C. R., Jr, Wiste, H. J., Lundt, E. S., Weigand, S. D., Vemuri, P., et al. (2014). (18) F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiology of Aging [in press].
Kok, E., Haikonen, S., Luoto, T., Huhtala, H., Goebeler, S., Haapasalo, H., & Karhunen, P. J. (2009). Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Annals of Neurology, 65(6), 650–657.
Kukolja, J., Thiel, C. M., Eggermann, T., Zerres, K., & Fink, G. R. (2010). Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience, 168(2), 487–497.
Langbaum, J. B. S., Chen, K., Caselli, R. J., Lee, W., Reschke, C., Bandy, D., et al. (2010). Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Archives of Neurology, 67(4), 462–468.
Leinonen, V., Alafuzoff, I., Aalto, S., Suotunen, T., Savolainen, S., Någren, K., et al. (2008). Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh compound B. Archives of Neurology, 65(10), 1304–1309.
Lemaître, H., Crivello, F., Dufouil, C., Grassiot, B., Tzourio, C., Alpérovitch, A., et al. (2005). No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. NeuroImage, 24(4), 1205–1213.
Lim, Y. Y., Ellis, K. A., Pietrzak, R. H., Ames, D., Darby, D., Harrington, K., et al. (2012). Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology, 79(16), 1645–1652.
Lim, Y. Y., Ellis, K. A., Ames, D., Darby, D., Harrington, K., Martins, R. N., et al. (2013). Aβ amyloid, cognition, and APOE genotype in healthy older adults. Alzheimer’s & Dementia.
Lim, Y. Y., Maruff, P., Pietrzak, R. H., Ames, D., Ellis, K. A., Harrington, K., et al. (2014). Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain, 137(Pt 1), 221–231.
Lind, J., Larsson, A., Persson, J., Ingvar, M., Nilsson, L.-G., Bäckman, L., et al. (2006). Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neuroscience Letters, 396(1), 23–27.
Liu, Y., Paajanen, T., Westman, E., Wahlund, L.-O., Simmons, A., Tunnard, C., et al. (2010a). Effect of APOE ε4 allele on cortical thicknesses and volumes: the addneuromed study. Journal of Alzheimer’s Disease, 21(3), 947–966.
Liu, Y., Paajanen, T., Westman, E., Zhang, Y., Wahlund, L.-O., Simmons, A., et al. (2010b). APOE ε2 allele is associated with larger regional cortical thicknesses and volumes. Dementia and Geriatric Cognitive Disorders, 30(3), 229–237.
Liu, C.-C., Liu, C.-C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology, 9(2), 106–118.
Lowe, V. J., Weigand, S. D., Senjem, M. L., Vemuri, P., Jordan, L., Kantarci, K., et al. (2014). Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology [in press].
Lu, P. H., Thompson, P. M., Leow, A., Lee, G. J., Lee, A., Yanovsky, I., et al. (2011). Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. Journal of Alzheimer’s Disease, 23(3), 433–442.
Mathis, C. A., Kuller, L. H., Klunk, W. E., Snitz, B. E., Price, J. C., Weissfeld, L. A., et al. (2013). In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Annals of Neurology, 73(6), 751–761.
Matura, S., Prvulovic, D., Jurcoane, A., Hartmann, D., Miller, J., Scheibe, M., et al. (2014). Differential effects of the ApoE4 genotype on brain structure and function. NeuroImage, 89, 81–91.
Mielke, M. M., Wiste, H. J., Weigand, S. D., Knopman, D. S., Lowe, V. J., Roberts, R. O., et al. (2012). Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology, 79(15), 1570–1577.
Mormino, E. C., Betensky, R. A., Hedden, T., Schultz, A. P., Ward, A., Huijbers, W., et al. (2014). Amyloid and APOE {varepsilon} 4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology [in press].
Morra, J. H., Tu, Z., Apostolova, L. G., Green, A. E., Avedissian, C., Madsen, S. K., et al. (2009). Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. NeuroImage, 45(1 Suppl), S3–15.
Morris, J. C., Roe, C. M., Xiong, C., Fagan, A. M., Goate, A. M., Holtzman, D. M., et al. (2010). APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of Neurology, 67(1), 122–131.
Mosconi, L., Brys, M., Switalski, R., Mistur, R., Glodzik, L., Pirraglia, E., et al. (2007). Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America, 104(48), 19067–19072.
Mosconi, L., Mistur, R., Switalski, R., Brys, M., Glodzik, L., Rich, K., et al. (2009). Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology, 72(6), 513–520.
Mueller, S. G., & Weiner, M. W. (2009). Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus, 19(6), 558–564.
Mueller, S. G., Schuff, N., Raptentsetsang, S., Elman, J., & Weiner, M. W. (2008). Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4 T. NeuroImage, 42(1), 42–48.
Murphy, K. R., Landau, S. M., Choudhury, K. R., Hostage, C. A., Shpanskaya, K. S., Sair, H. I., et al. (2013). Mapping the effects of ApoE4, age and cognitive status on 18F-florbetapir PET measured regional cortical patterns of beta-amyloid density and growth. NeuroImage, 78, 474–480.
O’Dwyer, L., Lamberton, F., Matura, S., Tanner, C., Scheibe, M., Miller, J., et al. (2012). Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study. PLoS One, 7(11), e48895.
Plassman, B. L., Welsh-Bohmer, K. A., Bigler, E. D., Johnson, S. C., Anderson, C. V., Helms, M. J., et al. (1997). Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology, 48(4), 985–989.
Protas, H. D., Chen, K., Langbaum, J. B. S., Fleisher, A. S., Alexander, G. E., Lee, W., et al. (2013). Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurology, 70(3), 320–325.
Reiman, E. M., Caselli, R. J., Yun, L. S., Chen, K., Bandy, D., Minoshima, S., et al. (1996). Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. The New England Journal of Medicine, 334(12), 752–758.
Reiman, E. M., Uecker, A., Caselli, R. J., Lewis, S., Bandy, D., de Leon, M. J., et al. (1998). Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Annals of Neurology, 44(2), 288–291.
Reiman, E. M., Chen, K., Alexander, G. E., Caselli, R. J., Bandy, D., Osborne, D., et al. (2004). Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of America, 101(1), 284–289.
Reiman, E. M., Chen, K., Alexander, G. E., Caselli, R. J., Bandy, D., Osborne, D., et al. (2005). Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proceedings of the National Academy of Sciences of the United States of America, 102(23), 8299–8302.
Reiman, E. M., Chen, K., Liu, X., Bandy, D., Yu, M., Lee, W., et al. (2009). Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6820–6825.
Rimajova, M., Lenzo, N. P., Wu, J.-S., Bates, K. A., Campbell, A., Dhaliwal, S. S., et al. (2008). Fluoro-2-deoxy-D-glucose (FDG)-PET in APOEepsilon4 carriers in the Australian population. Journal of Alzheimer’s Disease, 13(2), 137–146.
Risacher, S. L., Shen, L., West, J. D., Kim, S., McDonald, B. C., Beckett, L. A., et al. (2010). Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiology of Aging, 31(8), 1401–1418.
Rodrigue, K. M., Kennedy, K. M., Devous, M. D., Sr., Rieck, J. R., Hebrank, A. C., Diaz-Arrastia, R., et al. (2012). β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology, 78(6), 387–395.
Roe, C. M., Fagan, A. M., Grant, E. A., Hassenstab, J., Moulder, K. L., Maue Dreyfus, D., et al. (2013). Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology, 80(19), 1784–1791.
Roussotte, F. F., Gutman, B. A., Madsen, S. K., Colby, J. B., Narr, K. L., Thompson, P. M., et al. (2014). The apolipoprotein E epsilon 4 allele is associated with ventricular expansion rate and surface morphology in dementia and normal aging. Neurobiology of Aging, 35(6), 1309–1317.
Rowe, C. C., Ellis, K. A., Rimajova, M., Bourgeat, P., Pike, K. E., Jones, G., et al. (2010). Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging, 31(8), 1275–1283.
Samuraki, M., Matsunari, I., Chen, W.-P., Shima, K., Yanase, D., Takeda, N., et al. (2012). Glucose metabolism and gray-matter concentration in apolipoprotein E ε4 positive normal subjects. Neurobiology of Aging, 33(10), 2321–2323.
Saunders, A. M., Schmader, K., Breitner, J. C., Benson, M. D., Brown, W. T., Goldfarb, L., et al. (1993). Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet, 342(8873), 710–711.
Scheinin, N. M., Wikman, K., Jula, A., Perola, M., Vahlberg, T., Rokka, J., et al. (2014). Cortical 11C-PIB Uptake is Associated with Age, APOE Genotype, and Gender in « Healthy Aging ». Journal of Alzheimer’s Disease [in press].
Schmidt, H., Schmidt, R., Fazekas, F., Semmler, J., Kapeller, P., Reinhart, B., et al. (1996). Apolipoprotein E e4 allele in the normal elderly: neuropsychologic and brain MRI correlates. Clinical Genetics, 50(5), 293–299.
Schuff, N., Woerner, N., Boreta, L., Kornfield, T., Shaw, L. M., Trojanowski, J. Q., et al. (2009). MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain, 132(Pt 4), 1067–1077.
Shaw, P., Lerch, J. P., Pruessner, J. C., Taylor, K. N., Rose, A. B., Greenstein, D., et al. (2007). Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurology, 6(6), 494–500.
Small, G. W., Mazziotta, J. C., Collins, M. T., Baxter, L. R., Phelps, M. E., Mandelkern, M. A., et al. (1995). Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA, the Journal of the American Medical Association, 273(12), 942–947.
Soininen, H., Partanen, K., Pitkänen, A., Hallikainen, M., Hänninen, T., Helisalmi, S., et al. (1995). Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology, 45(2), 391–392.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the national institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 280–292.
Striepens, N., Scheef, L., Wind, A., Meiberth, D., Popp, J., Spottke, A., et al. (2011). Interaction effects of subjective memory impairment and ApoE4 genotype on episodic memory and hippocampal volume. Psychological Medicine, 41(9), 1997–2006.
Suthana, N. A., Krupa, A., Donix, M., Burggren, A., Ekstrom, A. D., Jones, M., et al. (2010). Reduced hippocampal CA2, CA3, and dentate gyrus activity in asymptomatic people at genetic risk for Alzheimer’s disease. NeuroImage, 53(3), 1077–1084.
Taylor, J. L., Scanlon, B. K., Farrell, M., Hernandez, B., Adamson, M. M., Ashford, J. W., Noda, A., et al. (2014). APOE-epsilon4 and aging of medial temporal lobe gray matter in healthy adults older than 50 years. Neurobiology of Aging [in press].
Thompson, W. K., Hallmayer, J., & O’Hara, R. (2011). Design considerations for characterizing psychiatric trajectories across the lifespan: application to effects of APOE-ε4 on cerebral cortical thickness in Alzheimer’s disease. The American Journal of Psychiatry, 168(9), 894–903.
Tohgi, H., Takahashi, S., Kato, E., Homma, A., Niina, R., Sasaki, K., et al. (1997). Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neuroscience Letters, 236(1), 21–24.
Trachtenberg, A. J., Filippini, N., & Mackay, C. E. (2012). The effects of APOE-ε4 on the BOLD response. Neurobiology of Aging, 33(2), 323–334.
Tuminello, E. R., & Han, S. D. (2011). The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. International Journal of Alzheimer’s Disease, 2011, 726197.
Villemagne, V. L., & Rowe, C. C. (2013). Long night’s journey into the day: amyloid-β imaging in Alzheimer’s disease. Journal of Alzheimer’s Disease, 33(Suppl 1), S349–359.
Villemagne, V. L., Pike, K. E., Chetelat, G., Ellis, K. A., Mulligan, R. S., Bourgeat, P., et al. (2011). Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Annals of Neurology, 69(1), 181–192.
Villemagne, V. L., Burnham, S., Bourgeat, P., Brown, B., Ellis, K. A., Salvado, O., et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurology, 12(4), 357–367.
Vlassenko, A. G., Mintun, M. A., Xiong, C., Sheline, Y. I., Goate, A. M., Benzinger, T. L. S., et al. (2011). Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C] Pittsburgh compound B data. Annals of Neurology, 70(5), 857–861.
Westlye, E. T., Lundervold, A., Rootwelt, H., Lundervold, A. J., & Westlye, L. T. (2011). Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE ε4 carriers: relationships with memory performance. The Journal of Neuroscience, 31(21), 7775–7783.
Wierenga, C. E., & Bondi, M. W. (2007). Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychology Review, 17(2), 127–143.
Wishart, H. A., Saykin, A. J., McAllister, T. W., Rabin, L. A., McDonald, B. C., Flashman, L. A., et al. (2006). Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology, 67(7), 1221–1224.
Yi, D., Lee, D. Y., Sohn, B. K., Choe, Y. M., Seo, E. H., Byun, M. S., et al. (2014). Beta-Amyloid Associated Differential Effects of APOE ε4 on Brain Metabolism in Cognitively Normal Elderly. The American Journal of Geriatric Psychiatry: official journal of the American Association for Geriatric Psychiatry [in press].
Conflicts of Interest
None to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouquet, M., Besson, F.L., Gonneaud, J. et al. Imaging Brain Effects of APOE4 in Cognitively Normal Individuals Across the Lifespan. Neuropsychol Rev 24, 290–299 (2014). https://doi.org/10.1007/s11065-014-9263-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-014-9263-8