Abstract
Miltirone is a phenanthrene-quinone derived from Salvia miltiorrhiza Bunge with anti-inflammatory and anti-oxidant effects. Our study aimed to explore the protective effect of miltirone on 1-methyl-4-phenylpyridinium (MPP+)-induced cell model of Parkinson’s disease (PD). PharmMapper database was employed to predict the targets of miltirone. PD-related genes were identified using GeneCards database. The overlapping genes between miltirone and PD were screened out using Venn diagram. KEGG analysis was performed using DAVID and KOBAS databases. Cell viability, reactive oxygen species (ROS) generation, apoptosis, and caspase-3 activity were detected by CCK-8 assay, a ROS assay kit, TUNEL, and caspase-3 activity assay, respectively. Effect of miltirone on the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway was explored by western blot analysis. A total of 214 targets of miltirone and 372 targets related to PD were attained, including 29 overlapping targets. KEGG analysis demonstrated that the 29 overlapping targets were both significantly enriched in the PI3K/Akt pathway. MPP+ stimulation reduced the cell viability in SH-SY5Y cells and neuronal primary cultures derived from human brain. Miltirone or N-acetylcysteine (NAC) attenuated MPP+-induced reduction in cell viability, ROS production, SOD activity reduction, apoptosis, and increase of caspase-3 activity. Additionally, miltirone recuperated MPP+-induced inactivation of the PI3K/Akt pathway. Moreover, treatment with LY294002, an inhibitor of the PI3K/Akt pathway, reversed the inhibitory effect of miltirone on MPP+-induced ROS generation and apoptosis in SH-SY5Y cells and neuronal primary cultures. In conclusion, miltirone attenuated ROS-dependent apoptosis in MPP+-induced cellular model of PD through activating the PI3K/Akt pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is ranked as the second most common age-related neurodegenerative disorder occurring in middle-aged and elderly people [1]. The incidence of PD is increasing with an aging population, posing a serious threat to the aged people [2]. Clinically, PD is pathologically characterized by the progressive and selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), leading to the lack of dopamine (DA) in the striatum [3]. Several biochemical processes and molecular mechanisms have been recognized as the important contributors to the pathophysiology of PD, such as increased oxidative stress, neuroinflammation, and activation of the apoptotic cascade [4, 5]. Accumulating evidence suggests that excessive production of reactive oxygen species (ROS) in response to oxidative stress appears to cause the impairment of cellular function and contributes to the apoptotic process in PD [6,7,8]. Thus, the novel neuroprotective agents with the potential to alleviate ROS-induced neuronal apoptosis might be supposed to be beneficial to prevent PD progression. 1-Methyl-4-phenylpyridinium (MPP+) is selectively toxic to dopaminergic neurons, and has been commonly used as an in vitro etiologic model of PD [9].
Numerous studies have reported that natural compounds as an alternative source of drugs or herbal extracts are effective for the treatment of various diseases, including PD. Miltirone, one of the tanshinones firstly identified in the 1970s, is a naturally occurring derivative of phenanthrene-quinone derived from the roots of Salvia miltiorrhiza Bunge, a traditional Chinese herbal medicine (Danshen) widely applied either individually or synergistically with other therapies in China [10]. Miltirone was confirmed to exhibit extensive pharmacological activities, such as anti-inflammatory, anti-oxidant, anti-cancer and anti-proliferative activities [11, 12]. However, there is no available information concerning the effect of miltirone on PD.
Based on the concept of “Disease-Gene-Target-Medicine”, network pharmacology is a novel interactive network using various publicly available bioinformatics resources to explore the scientific basis and therapeutic mechanism of Traditional Chinese medicine (TCM) formulae [13]. To uncover the molecular mechanism of miltirone against PD, we used the network pharmacology to analyze the drug targets and key biological pathways of miltirone in PD therapy. Furthermore, the current study used MPP+-induced SH-SY5Y cells and neuronal primary cultures as cellular models of PD to examine the effects and mechanism of miltirone in MPP+-induced neuroapoptosis.
Methods
Miltirone Structural Information
The molecular structure information of miltirone was obtained from NCBI PubChem (https://www.ncbi.nlm.nih.gov/pccompound). Absorption, distribution, metabolism, and excretion (ADME) screening criteria from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) (http://lsp.nwu.edu.cn/tcmsp.php) database [14] for miltirone mainly included oral bioavailability (OB), drug-likeness (DL), and blood–brain barrier (BBB).
Screening of the Target Genes of Miltirone and PD
The potential targets of miltirone were identified using the PharmMapper database (http://lilab.ecust.edu.cn/pharmmapper/) [15]. Known targets associated with PD were acquired from the currently available database GeneCards (http://www.genecards.org) with the keyword of “Parkinson’s disease” [16]. Finally, Venn diagram (http://bioinformatics.psb.ugent.be/Webtools/Venn/) was used to identify the overlapping genes relating to miltirone and PD.
Protein–Protein Interaction (PPI) Network Establishment
The 29 overlapping targets between miltirone and PD were imported into the STRING 11.0 (https://string-db.org/) to perform PPI analysis, with the species set to “Homo sapiens” and a confidence score > 0.4.
Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
KEGG pathway enrichment analysis of the overlapping genes between miltirone and PD was carried out on the functional annotation tool of Database for Annotation, Visualization, and Integrated Discovery (DAVID) ver. 6.8 (https://david.ncifcrf.gov/) [17] and KOBAS 3.0 website (http://kobas.cbi.pku.edu.cn/) [18], with the “Homo sapiens” setting. The top 10 pathway terms with P value < 0.05 were selected.
Cell Culture and Treatment
The human neuroblastoma cell line (SH-SY5Y cells) were obtained from the ATCC (Manassas, VA, USA; CRL-2266) and cultured in Dulbecco’s modified Eagle medium /F12 (DMEM/F12) (Gibco, CA, USA) complemented with 10% heat inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT, USA), and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). Neuronal primary cultures derived from human brain were purchased from ScienCell (Carlsbad, CA, USA; #1520-5) and maintained in complete neurobasal® medium (ScienCell) containing 2% B27 supplement (Thermo Fisher Scientific Inc., MA, USA) and 2 mM glutamine (Thermo Fisher Scientific Inc.) on poly-l-lysine-coated plates. SH-SY5Y cells and neuronal primary cultures were maintained in a humidified 5% CO2-air incubator at 37 °C. To establish an experimental PD model in vitro, SH-SY5Y cells and neuronal primary cultures were stimulated with MPP+ at various doses (0, 50, 100, 200, 400, and 800 μM) (Sigma, St. Louis, MO, USA) for 24 h. Additionally, SH-SY5Y cells and neuronal primary cultures were exposed to a series of concentrations of miltirone (0, 0.25, 0.5, 1, 2, 4, and 8 μM) (Sigma). To eliminate ROS, cells were treated with 5 mM N-acetylcysteine (NAC), a scavenger of ROS. To suppress the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, cells were treated with 10 μM LY294002 (an inhibitor of PI3K/Akt pathway).
Cell Viability Determination
Cell Counting Kit-8 (CCK-8) assay exhibits superior detection sensitivity than other tetrazolium salts-based assays, e.g., 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) [19], and is widely applied for the evaluation of cell viability and cytotoxicity. SH-SY5Y cells and neuronal primary cultures were inoculated in 96-well plates. Following 24 h of incubation, 10 μL CCK-8 solution (Solarbio, Beijing, China) was added to each well and the cells were maintained for another 2 h. Finally, the optical density at 450 nm was recoded using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Examination of Caspase-3 Activity
Caspase-3 activity was examined using a Caspase-3 Activity Kit (Beyotime, Shanghai, China) based on the manufacturer’s instructions. Following incubation with the indicated treatments, cell lysate was mixed with the reaction buffer containing 2 mM caspase-3 substrate (Ac-DEVD-pNA), followed by culturing for 2 h at 37 °C. The absorbance at 405 nm was detected using a SpectraMax M5microplate reader (Molecular Devices).
Measurement of ROS Level
Intracellular ROS level was measured using an ROS assay kit (Beyotime) containing a fluorescent probe-2′, 7′-dichlorofluorescin diacetate (DCFH-DA). Briefly, cells were washed with PBS twice, and then cultured with 10 μM DCFH-DA for 30 min in darkness at 37 °C. The fluorescence intensity was estimated by a Shimadzu spectrofluorometer (Kyoto, Japan) at a 485/525 nm of excitation/emission wavelength.
Detection of Superoxide Dismutase (SOD) Activity
SH-SY5Y cells and neuronal primary cultures were added in 96-well plates (2 × 104/well), and then suffered from indicated treatment, followed by lysis for detection of SOD activity using an SOD assay kit (Abcam) according to the manufacturer’s protocols.
Terminal Transferase-Mediated dUTP Nick End Labeling (TUNEL) Assay
Apoptosis was evaluated using the one-step TUNEL kit (Roche Molecular Biochemicals, Indianapolis, IN, USA). The treated SH-SY5Y cells and neuronal primary cultures were cultured on the 4-well glass coverslips, fixed with freshly prepared fixation solution containing 4% paraformaldehyde for 15 min and penetrated with 0.1% Triton X-100 for 5 min. The cells were further labeled with TUNEL reagent mixture in a humidified incubator at 37 °C for 1 h, followed by nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min in the dark. The TUNEL-positive cells were visualized using a Leica fluorescence microscope (Buffalo Grove, IL, USA). Apoptosis rate was determined by calculating the percentage of the number of TUNEL-positive cells to total number of cells in three random fields.
Western Blot Analysis
Protein lysates from SH-SY5Y cells and neuronal primary cultures were obtained using radioimmunoprecipitation (RIPA) lysis buffer (Beyotime). The supernatant was collected after centrifugation at 14,000 rpm for 20 min and protein concentration was quantified using the bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific Inc.). Equivalent amount of protein samples (40 μg) was then subjected to 10% SDS-PAGE and subsequently transferred onto a 0.45 µm PVDF membrane, followed by the blockage of nonspecific binding using 5% non-fat milk (Sigma) for 2 h. Then, the membranes were further incubated at 4 °C overnight with the following monoclonal antibodies against phosphatidylinositol 3-kinase (PI3K), phospho-PI3K, protein kinase B (Akt), phospho-Akt (p-Akt) and β-actin (all from Cell Signaling Technology, Danvers, MA, USA), followed by incubation with the horseradish peroxidase-conjugated anti-mouse IgG secondary antibody (Abcam, Cambridge, UK) for 1 h at room temperature. Lastly, the immuno-reactive bands were visualized using enhanced chemiluminescence reagent (Sangon Biotech Co., Ltd., Shanghai, China).
Statistics
The results were analyzed by the investigators who were blind to the treatment. All data are expressed as mean ± standard deviation (SD) from three independent experiments. Statistical analysis was carried out using GraphPad Prism software 7.0 (GraphPad Software, Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by Tukey post-hoc test was used to evaluate the statistical significance of difference. P value < 0.05 was considered to indicate a statistically significant difference.
Results
Intersected Targets of Miltirone and PD
The structure and ADME-related information of miltirone were shown in Fig. 1A. As displayed in Fig. 1B a total of 214 target genes of miltirone and 372 targets related to PD were attained from PharmMapper database and GeneCards database, respectively. The results of Venn diagram presented that 29 common targets between miltirone and PD were identified (Fig. 1B). These overlapping targets were presented in supplementary Table 1, and were input into the STRING online data platform to obtain a network of target protein interaction. Supplementary Fig. 1 showed that the interaction between targets of miltirone and PD.
KEGG Enrichment Pathway Analysis
To explore the mechanism of action of miltirone on PD, KEGG enrichment pathway analysis of the 29 common targets between miltirone and PD was performed. The information about the signaling pathway associated with these overlapping targets was obtained using the DAVID and KOBAS online analysis databases. The top ten KEGG signaling pathways were depicted in Fig. 2A and B. The results showed that the 29 overlapping targets were both significantly enriched in the PI3K/Akt pathway.
Cytotoxicity Evaluation of Miltirone and MPP+
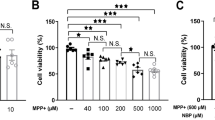
The cytotoxic effect of miltirone on SH-SY5Y cells and neuronal primary cultures was explored by using CCK-8 assay. As shown in Fig. 3A and B, miltirone at the dose of 0.25, 0.5, 1, and 2 μM did not affect cell viability in SH-SY5Y cells, and 0.25, 0.5, 1 μM miltirone showed no cytotoxicity in neuronal primary cultures. Therefore, miltirone at a concentration of 1 μM was selected to explore its effect on MPP+ -induced injury in SH-SY5Y cells and neuronal primary cultures. In addition, the impact of MPP+ on neuronal cell viability was explored. The data indicated that cell viability was impeded in a dose-dependent manner in SH-SY5Y cells and neuronal primary cultures after stimulation with MPP+ (Fig. 3C and D).
Cytotoxicity evaluation of miltirone and MPP+. A and B SH-SY5Y cells and neuronal primary cultures were exposed to various concentration of miltirone (0, 0.25, 0.5, 1, 2, 4, and 8 μM) for 24 h, followed by the detection of cell viability by CCK-8 assay. C and D SH-SY5Y cells and neuronal primary cultures were stimulated with a series of dose of MPP+ (0, 50, 100, 200, 400, and 800 μM) for 24 h, and CCK-8 assay was applied to evaluate cell viability. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group
Miltirone Attenuated ROS-Dependent Viability Reduction in MPP+-Induced PD Cell Model
CCK-8 assay was used to further investigate the protective effect of miltirone against MPP+-induced neurotoxicity in SH-SY5Y cells and neuronal primary cultures. As shown in Fig. 4A and B, MPP+-induced reduction of the viability in SH-SY5Y cells and neuronal primary cultures was significantly alleviated by treatment with miltirone. Moreover, we found that NAC, a ROS inhibitor, alleviated MPP+-induced viability decrease in SH-SY5Y cells and neuronal primary cultures. Furthermore, the anti-oxidant effect of miltirone was examined. As shown in Fig. 4C and D, MPP+ stimulation caused an elevation of intracellular ROS level, while miltirone or NAC attenuated MPP+-induced increase of ROS generation in SH-SY5Y cells and neuronal primary cultures. In addition, miltirone or NAC attenuated MPP+-induced reduction of SOD activity in SH-SY5Y cells and neuronal primary cultures (Fig. 4E and F). These data suggested that miltirone attenuated ROS-dependent viability reduction in MPP+-stimulated neuronal cells.
Effect of miltirone on cell viability and ROS generation in MPP+-induced PD cell model. SH-SY5Y cells and neuronal primary cultures were treated with 1 μM miltirone alone, 400 μM MPP+ alone, 1 μM miltirone + 400 μM MPP+, or 400 μM MPP+ + 5 mM NAC for 24 h. Cell viability (A and B), intracellular ROS level (C and D), and SOD activity (E and F) were examined by CCK-8 assay, an ROS assay kit, and an SOD assay kit, respectively. **P < 0.01, ***P < 0.001. ns not significant
Miltirone Attenuated ROS-Dependent Apoptosis in MPP+-Induced PD Cell Model
We further explored the effect of miltirone on MPP+-induced apoptosis in SH-SY5Y cells and neuronal primary cultures using TUNEL assay. Higher apoptosis percentage was observed in MPP+-treated SH-SY5Y cells and neuronal primary cultures, which was partially abolished after the addition of miltirone or NAC (Fig. 5A and B). Moreover, it was demonstrated that miltirone or NAC inhibited MPP+-induced increase of caspase-3 activity in SH-SY5Y cells and neuronal primary cultures (Fig. 5C and D). Taken together, we concluded that miltirone attenuated MPP+-induced apoptosis by suppressing ROS generation.
Influence of miltirone on apoptosis in MPP+-induced PD cell model. SH-SY5Y cells and neuronal primary cultures were treated with 1 μM miltirone alone, 400 μM MPP+ alone, 1 μM miltirone + 400 μM MPP+, or 400 μM MPP+ + 5 mM NAC for 24 h, followed by the detection of apoptosis by TUNEL (A and B) and caspase-3 activity (C and D) assays, respectively. Scale bar: 50 μm. ***P < 0.001. ns not significant
Miltirone Activated the PI3K/Akt Pathway in MPP+-Induced Cell Model of PD
The PI3K/Akt pathway is an important pathway that mediates the neuroprotection in PD [20]. Thus, we explored the involvement of PI3K/Akt signal pathway in the anti-PD effect of miltirone in the PD cell model. Western blot analysis implicated that MPP+ treatment caused a significant decrease of p-PI3K/PI3K and p-Akt/Akt in SH-SY5Y cells (Fig. 6A and B) and neuronal primary cultures (Fig. 6C and D), while those changes in MPP+-treated SH-SY5Y cells (Fig. 6A and B)and neuronal primary cultures (Fig. 6C and D) were restored after treatment with miltirone. However, LY294002, a PI3K inhibitor, overturned the promotion effect of miltirone on p-PI3K/PI3K and p-Akt/Akt expression in MPP+-treated SH-SY5Y cells (Fig. 6A and B) and neuronal primary cultures (Fig. 6C and D) These findings suggested that the PI3K/Akt pathway was activated by miltirone in the PD cell model.
Effect of miltirone on the PI3K/Akt pathway in the PD cell model. SH-SY5Y cells (A and B) and neuronal primary cultures (C and D) were exposed to 1 μM miltirone alone, 400 μM MPP+ alone, 1 μM miltirone + 400 μM MPP+, or 1 μM miltirone + 400 μM MPP+ + 10 μM LY294002 for 24 h, followed by the detection of p-PI3K, PI3K, p-Akt, and Akt expression by western blot analysis. **P < 0.01
Inhibition of the PI3K/Akt Pathway Reversed the Effect of Miltirone on MPP+-Induced Viability Reduction, ROS Generation, and Cell Apoptosis
Furthermore, our results suggested that the inhibition on MPP+-induced viability reduction (Fig. 7A and B), ROS generation (Fig. 7C and D), and SOD activity reduction (Fig. 7E and F) by miltirone treatment was reversed after the addition of LY294002. Additionally, TUNEL and caspase-3 activity assays revealed that inhibition of miltirone on MPP+-induced apoptosis (Fig. 8A and B) and increase of caspase-3 activity (Fig. 8C and D) in SH-SY5Y cells and neuronal primary cultures was partially reversed by LY294002 treatment. These results suggested that miltirone attenuated MPP+-induced viability reduction, ROS generation, and apoptosis by activating the PI3K/Akt pathway (see Fig 9).
Inactivation of the PI3K/Akt pathway abolished the effect of miltirone on MPP+-induced viability reduction and ROS generation in the PD cell model. SH-SY5Y cells and neuronal primary cultures were treated with 400 μM MPP+ alone, 1 μM miltirone + 400 μM MPP+, or 1 μM miltirone + 400 μM MPP+ + 10 μM LY294002, followed by the examination of cell viability (A and B) and ROS content (C and D), and SOD activity (E and F) using CCK-8 assay, an ROS assay kit, and an SOD assay kit, respectively. **P < 0.01, ***P < 0.001
Inactivation of the PI3K/Akt pathway abolished the effect of miltirone on MPP+-induced apoptosis in the PD cell model. SH-SY5Y cells and neuronal primary cultures were treated with 400 μM MPP+ alone, 1 μM miltirone + 400 μM MPP+, or 1 μM miltirone + 400 μM MPP+ + 10 μM LY294002, followed by the examination of apoptosis (A and B) and caspase-3 activity (C and D) using TUNEL and caspase-3 activity assays, respectively. Scale bar: 50 μm. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
The etiology and pathogenesis of PD is extremely complicated and thus has received more and more attention in recent years [21]. Accumulation of excessive ROS, mitochondrial dysfunction, apoptosis, and inflammation have been considered as the major causes of MPP+-induced neuronal injury in PD. Despite extensive preclinical investigations in in vivo models of PD, there remains a shortage of effective neuroprotective drugs that can inhibit or delay the progression of PD [22]. In our research, we demonstrated that miltirone alleviated ROS-dependent neuronal apoptosis in cell model of PD via activation of the PI3K/Akt pathway.
As a commonly used lipophilic compound presents in the root of the Chinese medicinal herb Salvia miltiorrhiza Bunge, miltirone has been reported to possess a wide range of pharmacological effects, such as renal function improvement, anti-myocardial ischemic injury, and protection of brain tissue [23]. Multiple studies reported that miltirone exhibited potent antineoplastic activity in many types of cancers and inhibited cell growth in cancers [11, 24]. A previous study proved that miltirone exerted anti-inflammation effect against inflammatory bowel disease in vitro and in vivo [25]. Additionally, miltirone exerted protective effects against oxidized low-density lipoprotein-induced oxidative stress on human endothelial cells [26]. However, there were no studies providing insight into the effect of miltirone on PD pathogenesis. In our study, a common in vitro PD model (MPP+-stimulated SH-SY5Y cells and neuronal primary cultures) was established to determine the protective effect of miltirone. The findings showed that miltirone effectively alleviated cytotoxicity and apoptosis induced by MPP+ stimulation in the PD cell model. Moreover, miltirone suppressed MPP+-induced ROS generation in the PD cell model, which might be due to its anti-oxidative effect by regulating antioxidant enzyme SOD activity. Intriguingly, application of NAC also attenuated MPP+ stimulation-induced cytotoxicity and apoptosis in the PD cell model. Published studies have demonstrated that anti-oxidant, such as NAC, could effectively alleviate MPP+-induced apoptosis [27]. More importantly, we provided the concrete evidence that NAC treatment suppressed MPP+-induced cytotoxicity and apoptosis, suggesting that miltirone inhibited ROS-dependent cytotoxicity and apoptosis in MPP+-induced cellular model of PD. Since NAC is an ROS scavenger, it had a greater ability to inhibit ROS generation than miltirone. Thus, in the current study, NAC showed higher effects on ROS-dependent cytotoxicity and apoptosis than miltirone. Whether NAC has anti-PD activity deserves further investigation.
As an emerging field of pharmacology, network pharmacological analysis of TCM was emerging as a research approach to investigate the effect of TCM on human diseases [28, 29]. To in-depth uncover the underlying intrinsic mechanism of miltirone in PD, a network pharmacology platform, including target prediction, PPI network construction, and KEGG pathway enrichment analysis were performed. Based on the Venn diagram, we obtained 29 overlapping target genes between miltirone and PD. KEGG enrichment pathway analysis using DAVID and KOBAS online analysis databases both revealed that these 29 overlapping targets were significantly enriched in the PI3K/Akt pathway. By gene annotation analysis, a total of 8 targets were associated with the PI3K/Akt pathway, and the 8 genes were presented in Supplementary Table 2. Among them, insulin like growth factor 1 (IGF1) was reported to be an upstream of the PI3K/Akt signaling in PD [30]. Hence, we hypothesized that miltirone might regulate the activation of PI3K/Akt pathway by targeting IGF1 in PD. The PI3K/Akt pathway is known as a key neuroprotective signaling pathway that played crucial roles in various biological functions [31]. Dysregulation of the PI3K/Akt pathway was demonstrated to cause the dopaminergic neuron loss and contribute to the development of PD [32, 33]. Besides, the PI3K/Akt signaling plays an essential role in protecting against oxidative stress in neuronal cells [32, 34]. For instance, decoction of Rehmanniae exerted the protective effect against MPP+-induced injury in a PD cell model through activating the PI3K/Akt pathway [35]. Activation of PI3K/Akt/mammalian target of rapamycin (mTOR) pathway contributed to metformin-induced protection against oxidative stress in PC12 cells [36]. Therefore, activation of the PI3K/Akt pathway represented a potential strategy for PD therapy [32]. In our study, the data revealed that miltirone relieved MPP+-induced inhibition of the PI3K/Akt pathway in SH-SY5Y cells and neuronal primary cultures. Moreover, the effects of miltirone on MPP+-induced ROS generation, apoptosis, and SOD activity reduction were abolished by LY294002. All these data collectively suggested that miltirone attenuated ROS-dependent apoptosis in the cell model of PD through activating the PI3K/Akt pathway. However, there are some limitations in the current study. Firstly, the neuronal primary cultures used in our study might be mixtures of various kinds of neurons, including dopaminergic neurons and others. Hence, for more reliable data, higher percent of dopaminergic neurons should be used in future study. Moreover, our study suggested that 8 µM of miltirone reduced 40–50% of cell viability in SH-SY5Y cells and neuronal primary cultures, whereas MPP+ showed no significant injury up to 100 µM in the same cells. This can be a potential detrimental effect of miltirone. The current work just investigated the in vitro function of miltirone. When it was used in animal models, the accumulation might be a concern, and the used dosages need more exploration in future.
To conclude, our research systematically expounded the neuroprotective potential of miltirone in the PD cell model. Mechanistically, our data provided the evidence that miltirone protected against ROS-dependent apoptosis in the cell model of PD partially through the PI3K/Akt pathway, offering a novel therapeutic agent for PD. Further in vivo investigations are still needed to validate our findings.
Data Availability
The data are available from the corresponding author on reasonable request.
References
Ng JSC (2018) Palliative care for Parkinson’s disease. Ann Palliat Med 7:296–303
Li G, Ma J, Cui S, He Y, Xiao Q, Liu J, Chen S (2019) Parkinson’s disease in China: a forty-year growing track of bedside work. Transl Neurodegener 8:22
Brichta L, Greengard P, Flajolet M (2013) Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci 36:543–554
Pieczenik SR, Neustadt J (2007) Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83:84–92
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106–107:17–32
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39:73–82
Stadtman ER (2004) Role of oxidant species in aging. Curr Med Chem 11:1105–1112
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110
Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ (2000) The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex i and reactive oxygen species revisited. Mol Pharmacol 58:271–278
Ma Z, Zhang M, Song Z (2009) Characterization of tanshinones with quinone reductase induction activity from Radix Salvia miltiorrhiza by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 23:2857–2866
Wang L, Hu T, Shen J, Zhang L, Li LF, Chan RL, Li MX, Wu WK, Cho CH (2016) Miltirone induced mitochondrial dysfunction and ROS-dependent apoptosis in colon cancer cells. Life Sci 151:224–234
Zhang X, Zhang P, An L, Sun N, Peng L, Tang W, Ma D, Chen J (2020) Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm Sin B 10:1397–1413
Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W, Piao G (2017) How can synergism of traditional medicines benefit from network pharmacology? Molecules 22:1135
Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L (2014) TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 6:13
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, Lai L, Pei J, Li H (2017) PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res 45:W356-w360
Rebhan M, Chalifa-Caspi V, Prilusky J, Lancet D (1998) GeneCards: a novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics 14:656–664
Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA (2003) DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol 4:P3
Ai C, Kong L (2018) CGPS: A machine learning-based approach integrating multiple gene set analysis tools for better prioritization of biologically relevant pathways. J Genet Genomics 45:489–504
Cai L, Qin X, Xu Z, Song Y, Jiang H, Wu Y, Ruan H, Chen J (2019) Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega 4:12036–12042
Jia Y, Mo SJ, Feng QQ, Zhan ML, OuYang LS, Chen JC, Ma YX, Wu JJ, Lei WL (2014) EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J Mol Neurosci 53:117–124
Abeliovich A, Gitler AD (2016) Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539:207–216
Havelund JF, Heegaard NHH, Færgeman NJK, Gramsbergen JB (2017) Biomarker research in Parkinson’s disease using metabolite profiling. Metabolites 7:42
Mostallino MC, Mascia MP, Pisu MG, Busonero F, Talani G, Biggio G (2004) Inhibition by miltirone of up-regulation of GABAA receptor α4 subunit mRNA by ethanol withdrawal in hippocampal neurons. Eur J Pharmacol 494:83–90
Zhu Z (2018) Miltirone-induced apoptosis in cisplatin-resistant lung cancer cells through upregulation of p53 signaling pathways. Oncol Lett 15:8841–8846
Wang H, Gu J, Hou X, Chen J, Yang N, Liu Y, Wang G, Du M, Qiu H, Luo Y, Jiang Z, Feng L (2017) Anti-inflammatory effect of miltirone on inflammatory bowel disease via TLR4/NF-κB/IQGAP2 signaling pathway. Biomed Pharmacother 85:531–540
Zhang L, Zhang H, Li X, Jia B, Yang Y, Zhou P, Li P, Chen J (2016) Miltirone protects human EA.hy926 endothelial cells from oxidized low-density lipoprotein-derived oxidative stress via a heme oxygenase-1 and MAPK/Nrf2 dependent pathway. Phytomedicine 23:1806–1813
Seaton TA, Cooper JM, Schapira AH (1997) Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain Res 777:110–118
Liang X, Li H, Li S (2014) A novel network pharmacology approach to analyse traditional herbal formulae: the Liu-Wei-Di-Huang pill as a case study. Mol Biosyst 10:1014–1022
Huang T, Ning Z, Hu D, Zhang M, Zhao L, Lin C, Zhong LLD, Yang Z, Xu H, Bian Z (2018) Uncovering the mechanisms of Chinese herbal medicine (MaZiRenWan) for functional constipation by focused network pharmacology approach. Front Pharmacol 9:270
Qin X, Zhang X, Li P, Wang M, Yan L, Pan P, Zhang H, Hong X, Liu M, Bao Z (2021) MicroRNA-185 activates PI3K/AKT signalling pathway to alleviate dopaminergic neuron damage via targeting IGF1 in Parkinson’s disease. J Drug Target 29:875–883
Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701
Wang G, Pan J, Chen SD (2012) Kinases and kinase signaling pathways: potential therapeutic targets in Parkinson’s disease. Prog Neurobiol 98:207–221
Timmons S, Coakley MF, Moloney AM, C ON, (2009) Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett 467:30–35
Lu S, Lu C, Han Q, Li J, Du Z, Liao L, Zhao RC (2011) Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology 279:189–195
Jiang D, Peng Y (2021) The protective effect of decoction of Rehmanniae via PI3K/Akt/mTOR pathway in MPP+-induced Parkinson’s disease model cells. J Recept Signal Transduct Res 41:74–84
Khallaghi B, Safarian F, Nasoohi S, Ahmadiani A, Dargahi L (2016) Metformin-induced protection against oxidative stress is associated with AKT/mTOR restoration in PC12 cells. Life Sci 148:286–292
Funding
This study was funded by the Health Science and Technology Project of Inner Mongolia (No. 202201406) and the Science and Technology Project of Baotou Health Committee (No. wsjkwkj003).
Author information
Authors and Affiliations
Contributions
HF and FX designed this study, conducted the experiments, collected and analyzed the results, performed the bioinformatic analysis, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, H., Xi, F. Miltirone Attenuates Reactive Oxygen Species-Dependent Neuronal Apoptosis in MPP+-Induced Cell Model of Parkinson’s Disease Through Regulating the PI3K/Akt Pathway. Neurochem Res 47, 3137–3149 (2022). https://doi.org/10.1007/s11064-022-03669-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03669-y