Abstract
Pramipexole (PPX) is a common drug for the treatment of Parkinson’s disease. However, the mechanism allows PPX in the progression of Parkinson’s disease remains largely unknown. This study aimed to investigate the role of PPX in 1-Methyl-4-phenylpyridinium (MPP+)-treated neuroblastoma cells and explore the interaction between PPX and miR-494-3p/brain derived neurotrophic factor (BDNF) axis. SK-N-SH and CHP 212 cells challenged by MPP+ were used as cellular model of Parkinson’s disease and incubated with PPX. The expression levels of miR-494-3p and BDNF were measured by quantitative real-time polymerase chain reaction or western blot. Neurotoxicity was investigated by cell apoptosis, inflammatory response and oxidative stress. The target association between miR-494-3p and BDNF was confirmed by luciferase reporter and RNA immunoprecipitation assays. miR-494-3p expression was increased and BDNF level was decreased in MPP+-treated SK-N-SH and CHP 212 cells, which were reversed by introduction of PPX. Pramipexole attenuated cell apoptosis, inflammatory response and oxidative stress in MPP+-treated SK-N-SH and CHP 212 cells. Knockdown of miR-494-3p also suppressed neurotoxicity induced by MPP+ in SK-N-SH and CHP 212 cells. BDNF was validated as a target of miR-494-3p and its silence abated the suppressive effect of miR-494-3p on MPP+-induced neurotoxicity. Moreover, addition of miR-494-3p and silence of BDNF mitigated the effect of PPX on MPP+-induced neurotoxicity. PPX inhibited MPP+-induced neurotoxicity in SK-N-SH and CHP 212 cells by decreasing miR-494-3p and increasing BDNF, indicating the potential therapeutic effect of PPX on Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease is one of the common neurodegenerative diseases involving multiple pathways, such as mitochondrial dysfunction, oxidative stress, neuroinflammation and cell death [1]. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a potent toxin and metabolizes to 1-Methyl-4-phenylpyridinium (MPP+) in glia, which could lead to the neurotoxicity, contributing to the development of Parkinson’s disease [2, 3]. Although great advance has been gained on understanding the pathogenesis and treatment of Parkinson’s disease [4], the outcomes of patients remain unsatisfactory. Hence, it is urgent to explore novel strategies for the neuroprotection and neurorestoration in Parkinson’s disease [5].
Pramipexole (PPX) is a selective dopamine D2 receptor agonist with dopaminergic activity and neuroprotective actions by acting as an antioxidant to decrease motor deficits [6]. Moreover, PPX has high bioavailability of > 90% with little metabolism, and the max daily dose of PPX should not exceed 4.5 mg in the treatment of Parkinson’s disease [7]. Previous studies suggest that PPX has been used as a common neuroprotective drug in the treatment of Parkinson’s disease [8,9,10]. However, the mechanism elucidating the pharmacological role of PPX in Parkinson’s disease remains elusive.
Previous study suggests that dysregulation of microRNA (miRNA) is mediated by PPX in Parkinson’s disease [11]. miRNAs have been revealed to play essential roles in the development, diagnosis and treatment of Parkinson’s disease [12, 13]. Increasing evidences suggest that miR-494-3p could act as an oncogene or tumor suppressor in multiple cancers, including prostate cancer, glioblastoma, oral squamous carcinoma and endometrial cancer [14,15,16,17]. Especially, miR-494-3p exhibits the promoting role in the development of Parkinson’s disease [18, 19]. Furthermore, brain derived neurotrophic factor (BDNF) has been reported as a promising therapeutic option for the neurodegeneration in brain disorders, which plays as an important target for the diagnosis and treatment of neurodegenerative disorders, including Parkinson’s disease [20,21,22]. More importantly, the database of starBase online predicts the existence of complementary sequences between miR-494-3p and BDNF, indicating that BDNF might be a target of miR-494-3p. We hypothesized that miR-494-3p and BDNF might be associated with PPX-mediated inhibition in Parkinson’s disease development.
In this study, we established Parkinson’s disease model in vitro using SK-N-SH and CHP 212 cells challenged by MPP+. We measured the effect of PPX on expression levels of miR-494-3p and BDNF in MPP+-treated cells and explored its effect on cell apoptosis, inflammatory response and oxidative stress. Moreover, we confirmed the target association between miR-494-3p and BDNF and explored the interaction between PPX and miR-494-3p/BDNF axis.
Materials and Methods
Cell Culture, Treatment and Transfection
The human neuroblastoma cell lines SK-N-SH and CHP 212 cells were purchased from BeNa Culture Collection (Beijing, China) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher Scientific, Wilmington, DE, USA) with 10% fetal bovine serum at 37 °C in a humidified incubator with 5% CO2. To induce neurotoxicity, SK-N-SH and CHP 212 cells were incubated with 0.5, 1 and 2 mM MPP+ (Sigma, St. Louis, MO, USA) for 24 h. To investigate the effect of PPX on neurotoxicity, cells were treated with 10, 50 and 100 μM PPX (Sigma) for 24 h.
Small interfering RNA (siRNA) against BDNF (si-BDNF) (5′-UUUUGCUAUCCAUGGUAAGGG-3′), siRNA negative control (scramble) (5′-UCUUCCGAACGUGUCACGUTT-3′), miR-494-3p mimic (miR-494-3p) (5′-UGAAACAUACACGGGAAACCUC-3′), miRNA negative control (NC) (5′-UUCUCCGAACGUGUCACGUTT-3′), miR-494-3p inhibitor (anti-miR-494-3p) (5′-GAGGUUUCCCGUGUAUGUUUCA-3′) and inhibitor negative control (anti-NC) (5′-CAGUACUUUUGUGUAGUACAA-3′) were generated by GenePharm (Shanghai, China). For cell transfection, SK-N-SH and CHP 212 cells (1 × 105) in 6-well plates were transfected with 40 nM oligonucleotides for 24 h using Lipofectamine 2000 (Thermo Fisher Scientific).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR assay was performed to measure the RNA levels of miR-494-3p and BDNF. After washing with phosphate buffer saline (PBS), cells were incubated in Trizol reagent (Thermo Fisher Scientific) for the isolation of total RNA following the manufacturer’s instructions. The complementary DNA (cDNA) was generated from mRNA or miRNA by using specific reverse transcription kit (Thermo Fisher Scientific) and then subjected to qRT-PCR response on Bio-Rad CFX96 Real-time PCR Systems (Bio-Rad, Hercules, CA, USA) with SYBR mix (TaKaRa, Dalian, China) and specific primers. GAPDH and U6 were used as controls for BDNF and miR-494-3p, respectively. The primer sequences were listed as follows: BDNF (Forward, 5′-CTACGAGACCAAGTGCAATCC-3′; Reverse, 5′-AATCGCCAGCCAATTCTCTTT-3′); GAPDH (Forward, 5′-ATCACTGCCACCCAGAAGAC-3′; Reverse, 5′-TTTCTAGACGGCAGGTCAGG-3′); miR-494-3p (Forward, 5′-GAAACATACACGGGAAACC-3′; Reverse, 5′-GTGCAGGGTCCGAGGT-3′); U6 (Forward, 5′-CTCGCTTCGGCAGCACA-3′; Reverse, 5′-AACGCTTCACGAATTTGCGT-3′). The relative expression levels of miR-494-3p and BDNF mRNA were determined by 2−ΔΔCt method [23].
Flow Cytometry
Flow cytometry was used to determine cell apoptosis through using Annexin V-FITC apoptosis detection kit (Beyotime, Shanghai, China). In brief, SK-N-SH and CHP 212 cells (5 × 105/well) in 24-well plates were subjected to 1 mM MPP+ or combined with 100 μM PPX for 24 h. Every group was prepared in triplicates. Subsequently, cells were resuspended in binding buffer after washing with PBS, followed by staining in the dark with Annexin V-FITC and PI for 10 min. Stained cells were analyzed through a flow cytometer (BD, San Jose, CA, USA) and cells with Annexin V-FITC positive and PI positive or negative were regarded as apoptotic cells.
Western Blot
After washing with PBS, SK-N-SH and CHP 212 cells were lysed in Radio-Immunoprecipitation Assay (RIPA) buffer (Beyotime) on ice and the protein contents were determined by bicinchoninic acid (BCA) protein assays kit (Thermo Fisher Scientific). Proteins (20 μg) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and each sample was run in triplicate, followed by transferring onto nitrocellulose membranes (Millipore, Billerica, MA, USA). Subsequently, the membranes were blocked using 5% skim milk and then incubated at 4 °C overnight with primary antibodies against Cleaved caspase 3 (ab2302, 1:300 dilution, Abcam, Cambridge, MA, USA), B cell lymphoma-2 (Bcl-2) (ab196495, 1:1000 dilution, Abcam), Bcl-2-associated X protein (Bax) (ab199677, 1:1000 dilution, Abcam), BDNF (ab220679, 1:300 dilution, Abcam) or GAPDH (ab70699, 1:5000 dilution, Abcam). GAPDH was used as a control. After an incubation of corresponding secondary antibody, protein signals were visualized using enhanced chemiluminescence reagent (Beyotime).
MTT
SK-N-SH and CHP 212 cells (1 × 104/well) were placed into 96-well plates in triplicates overnight and then incubated with 1 mM MPP+ or combined with 100 μM PPX for 24 h. Subsequently, the medium was replaced with fresh DMEM medium containing 0.5 mg/mL MTT solution (Solarbio, Beijing, China) and cells were cultured for 4 h. Then medium was removed and 150 μL dimethyl sulfoxide (DMSO) (Sigma) was added to each well. The absorbance of each well at 490 nm was determined through a microplate reader (Bio-Rad) and cell viability was normalized to the matched control group.
Enzyme Linked Immunosorbent Assay (ELISA)
The secretion levels of inflammatory cytokines were determined by using specific ELISA kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, SK-N-SH and CHP 212 cells (5 × 105/well) in 24-well plates in triplicates were subjected to 1 mM MPP+ or combined with 100 μM PPX for 24 h, followed by detection of concentrations of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) in cell culture supernatant.
Lactate Dehydrogenase (LDH) Release and Superoxide Dismutase (SOD) Detection
LDH is a soluble cytosolic enzyme present in most eukaryotic cells, released into the culture medium upon cell death due to the damage of plasma membrane. Cell apoptosis was also investigated by LDH release in culture medium. The oxidative stress was evaluated by SOD activity. After the treatment of 1 mM MPP+ or combined with 100 μM PPX for 24 h, SK-N-SH and CHP 212 cells were lysed for SOD activity analysis using SOD assay kit (Beyotime) and culture medium was collected for LDH level assay using LDH cytotoxicity assay kit (Beyotime) according to the manufacturer’s instructions [24, 25]. The relative levels of LDH and SOD were normalized to the indicated control group.
Luciferase Reporter Assay and RNA Immunoprecipitation (RIP)
The potential complementary sequences of miR-494-3p and BDNF were predicted by starBase. The wild-type (wt) 3′ untranslated regions (UTR) sequences of BDNF containing binding sites of miR-494-3p were cloned into pGL3-Report vector (Promega, Madison, WI, USA) and generated corresponding luciferase reporter vectors BDNF-wt. The mutant (mut) luciferase reporter vectors BDNF-mut were produced by mutating the predicted seed sites of miR-494-3p using Quick Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA). For luciferase reporter assay, SK-N-SH and CHP 212 cells were co-transfected with the constructed vectors BDNF-wt or BDNF-mut and miR-494-3p mimic or NC. After transfection for 48 h, cells were harvested for the detection of luciferase activity using dual-luciferase reporter assay system (Promega).
For RIP assay, SK-N-SH and CHP 212 cells transfected with miR-494-3p mimic or NC were lysed and used for enrichment response with Magna RIP kit (Millipore) and Ago2 antibody according to the manufacturer’s instructions. IgG was used as control. The enrichment level of BDNF in each group was detected by qRT-PCR.
Statistical Analysis
Statistical analysis was performed by GraphPad Prism 7 software (GraphPad Inc., La Jolla, CA, USA). The difference was analyzed by paired Student’s t-test for two groups and one-way ANOVA with Tukey post hoc test for three or more groups. The data were presented as mean ± standard deviation (S.D.) from three independent experiments and the significant difference was considered at P value less than 0.05.
Results
PPX Down-Regulates miR-494-3p Expression and Up-Regulates BDNF level in MPP+-Treated Cells
To explore the role of PPX in MPP+-induced injury, the related miRNAs and mRNA were analyzed. In MPP+-treated SK-N-SH and CHP 212 cells, the expression of miR-494-3p was progressively elevated in a concentration dependent manner (Fig. 1a and Supplementary Fig. 1A). However, the treatment of PPX significantly decreased the level of miR-494-3p in the two cell lines challenged by MPP+ (Fig. 1b and Supplementary Fig. 1B). Moreover, the mRNA level of BDNF in SK-N-SH and CHP 212 cells was markedly reduced by the exposure of different concentrations of MPP+ (Fig. 1c and Supplementary Fig. 1C), while it was progressively up-regulated by the introduction of PPX (Fig. 1d and Supplementary Fig. 1D). These results suggested that abnormally expressed miR-494-3p and BDNF might be associated with the development of Parkinson’s disease.
PPX decreases miR-494-3p expression and increases BDNF level in MPP+-treated SK-N-SH cells. a qRT-PCR assay was performed to detect the expression of miR-494-3p after treatment of different concentrations (0, 0.5, 1 and 2 mM) of MPP+ for 24 h. b The level of miR-494-3p was measured in 1 mM MPP+-treated SK-N-SH cells after exposure of different concentrations (0, 10, 50 and 100 μM) of PPX for 24 h. c The expression of BDNF mRNA was examined after treatment of different concentrations (0, 0.5, 1 and 2 mM) of MPP+ for 24 h. d The level of BDNF mRNA was measured in 1 mM MPP+-treated SK-N-SH cells after exposure of different concentrations (0, 10, 50 and 100 μM) of PPX for 24 h. *P < 0.05 compared with control (non-treated with MPP+) group for A and C or MPP+ (non-treated with PPX) group for B and D
PPX Suppresses MPP+-Induced Neurotoxicity
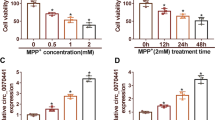
To investigate the effect of PPX on MPP+-induced toxicity, SK-N-SH and CHP 212 cells were stimulated with PPX for 24 h before the treatment of MPP+. As a result, PPX could protect cell viability from MPP+ in SK-N-SH and CHP 212 cells, but it did not affect the viability on normal condition (Supplementary Fig. 2A and B). Hence, this study just investigated the role of PPX in MPP+-treated cell lines. As shown in Fig. 2a and Supplementary Fig. 3A, flow cytometry analysis displayed that treatment of 1 mM MPP+ induced obvious apoptosis production in SK-N-SH and CHP 212 cells, which was evidently attenuated by introduction of 100 μM PPX. Furthermore, the levels of proteins that were involved in cell apoptosis were detected and results showed that the protein levels of Bax and Cleaved caspase 3 were notably increased and Bcl-2 level was decreased by treatment of MPP+ in SK-N-SH and CHP 212 cells, while these events were weakened via PPX (Fig. 2b and Supplementary Fig. 3B). Meanwhile, the viability of SK-N-SH and CHP 212 cells was remarkably inhibited by MPP+, which was restored by introduction of PPX (Fig. 2c and Supplementary Fig. 3C). In addition, the levels of TNF-α and IL-1β in the cultured supernatant were significantly enhanced in MPP+-treated SK-N-SH and CHP 212 cells compared with those in control group, which were mitigated by challenge of PPX (Fig. 2d, e and Supplementary Fig. 3D and E). Besides, treatment of MPP+ led to obviously increased LDH level and reduced SOD level in SK-N-SH and CHP 212 cells, whereas this effect was abated by PPX (Fig. 2f, g and Supplementary Fig. 3F and G). These data indicated that PPX decreased cell apoptosis, inflammatory response and oxidative stress caused by MPP+ in SK-N-SH and CHP 212 cells.
PPX inhibits neurotoxicity in MPP+-treated SK-N-SH cells. Cell apoptosis (a), apoptotic related protein levels (b), cell viability (c), TNF-α (d), IL-1β (e), LDH level (f) and SOD level (g) were determined after treatment of 1 mM MPP+ and 100 μM PPX for 24 h. *P < 0.05 compared with control (non-treated with MPP+) group or MPP+ (non-treated with PPX) group
Knockdown of miR-494-3p Inhibits MPP+-Induced Neurotoxicity
To evaluate the role of miR-494-3p in MPP+-induced toxicity, SK-N-SH and CHP 212 cells were transfected with anti-miR-494-3p or anti-NC and then treated with MPP+ for 24 h. As shown in Fig. 3a and Supplementary Fig. 4A, the abundance of miR-494-3p up-regulated by MPP+ was effectively decreased in SK-N-SH and CHP 212 cells transfected with anti-miR-494-3p compared with that in anti-NC group. Moreover, by detecting apoptotic rate and apoptosis-related protein levels, results presented that knockdown of miR-494-3p decreased the apoptosis of SK-N-SH and CHP 212 cells induced by MPP+ (Fig. 3b, c and Supplementary Fig. 4B and C). Meanwhile, deficiency of miR-494-3p reversed MPP+-induced viability suppression in SK-N-SH and CHP 212 cells (Fig. 3d and Supplementary Fig. 4D). Additionally, down-regulation of miR-494-3p alleviated the secretion levels of TNF-α and IL-1β triggered by MPP+ in SK-N-SH and CHP 212 cells (Fig. 3e, f and Supplementary Fig. 4E and F). Besides, MPP+-induced the elevated LDH and reduced SOD in SK-N-SH and CHP 212 cells were relieved by exhaustion of miR-494-3p (Fig. 3g, h and Supplementary Fig. 4G and H). These findings showed that miR-494-3p knockdown inhibited cell apoptosis, inflammatory response and oxidative stress caused by MPP+ in SK-N-SH and CHP 212 cells.
Knockdown of miR-494-3p suppresses neurotoxicity in MPP+-treated SK-N-SH cells. miR-494-3p level (a), cell apoptosis (b), apoptotic related protein levels (c), cell viability (d), TNF-α (e), IL-1β (f), LDH level (g) and SOD level (h) were examined in SK-N-SH cells transfected with anti-miR-494-3p or anti-NC after treatment of 1 mM MPP+ for 24 h. *P < 0.05 compared with control (non-treated with MPP+) group or MPP++anti-NC group
BDNF is a Target of miR-494-3p in SK-N-SH and CHP 212 Cells
starBase predicted that there are potential complementary sequences between miR-494-3p and BDNF (Fig. 4a). To validate the target association between them, the luciferase reporter vector BDNF-wt and BDNF-mut containing wild-type or mutant binding sites of miR-494-3p were generated. As shown in Fig. 4b and Supplementary Fig. 5A, luciferase activity in the BDNF-wt group was markedly decreased by more than 70% in both cell lines due to miR-494-3p overexpression. Moreover, Ago2 RIP assay showed that the enrichment level of BDNF in SK-N-SH and CHP 212 cells transfected with miR-494-3p was obviously increased in comparison to that in NC group (Fig. 4c and Supplementary Fig. 5B). In addition, the protein level of BDNF in SK-N-SH and CHP 212 cells was negatively regulated by miR-494-3p when compared with the matched control groups (Fig. 4d and Supplementary Fig. 5C). These results suggested that BDNF acted as a target of miR-494-3p in SK-N-SH and CHP 212 cells.
BDNF is a target of miR-494-3p in SK-N-SH cells. a The binding sites of miR-494-3p and BDNF were predicted by starBase. b and c Luciferase reporter and RIP assays were carried out to validate the association between miR-494-3p and BDNF. d The effect of miR-494-3p on BDNF protein level was measured. *P < 0.05 compared with NC group or anti-NC group
Silence of BDNF Reverses the Effect of miR-494-3p Knockdown on MPP+-Induced Neurotoxicity
To explore whether BDNF is required for miR-494-3p-mediated neurotoxicity, SK-N-SH and CHP 212 cells were transfected with anti-NC, anti-miR-494-3p, anti-miR-494-3p and scramble or si-BDNF and then treated with MPP+ for 24 h. In MPP+-treated SK-N-SH and CHP 212 cells, the protein level of BDNF up-regulated by miR-494-3p knockdown was significantly decreased by interference of BDNF (Fig. 5a and Supplementary Fig. 6A). Furthermore, the suppressive effect of miR-494-3p knockdown on cell apoptosis was counteracted by silence of BDNF in SK-N-SH and CHP 212 cells challenged by MPP+ (Fig. 5b, c and Supplementary Fig. 6B and C). Additionally, knockdown of miR-494-3p-induced viability restoration was markedly abrogated by silence of BDNF in MPP+-treated SK-N-SH and CHP 212 cells (Fig. 5d and Supplementary Fig. 6D). What’s more, the secretion levels of TNF-α and IL-1β repressed by miR-494-3p deficiency were evidently up-regulated by BDNF interference in SK-N-SH and CHP 212 cells treated by MPP+ (Fig. 5e, f and Supplementary Fig. 6E and F). Moreover, the regulatory effect of miR-494-3p knockdown on levels of LDH and SOD was reversed by BDNF inhibition in the two cell lines after treatment of MPP+ (Fig. 5g, h and Supplementary Fig. 6G and H). These data revealed that miR-494-3p targeted BDNF to regulate neurotoxicity induced by MPP+ in SK-N-SH and CHP 212 cells.
Interference of BDNF reverses the effect of miR-494-3p knockdown on neurotoxicity in MPP+-treated SK-N-SH cells. BDNF protein level (a), cell apoptosis (b), apoptotic related protein levels (c), cell viability (d), TNF-α (e), IL-1β (f), LDH level (g) and SOD level (h) were analyzed in SK-N-SH cells transfected with anti-NC, anti-miR-494-3p, anti-miR-494-3p and scramble or si-BDNF after treatment of 1 mM MPP+ for 24 h. *P < 0.05 compared with MPP+ + anti-NC group or MPP+ + anti-miR-494-3p + scramble group
miR-494-3p overexpression or BDNF knockdown attenuates the effect of PPX on neurotoxicity in MPP+-treated SK-N-SH cells. Cell apoptosis (a), apoptotic related protein levels (b), cell viability (c), TNF-α (d), IL-1β (e), LDH level (f) and SOD level (g) were detected in SK-N-SH cells transfected with NC, miR-494-3p, scramble or si-BDNF after treatment of 1 mM MPP+ and 100 μM PPX for 24 h. *P < 0.05 compared with matched control group
miR-494-3p Overexpression or BDNF Knockdown Attenuates the Effect of PPX on MPP+-Induced Neurotoxicity
In order to further explore whether PPX-induced inhibition of neurotoxicity was mediated by miR-494-3p and BDNF, SK-N-SH and CHP 212 cells were transfected with NC, miR-494-3p, scramble or si-BDNF, followed by treatment of PPX and MPP+ for 24 h. As displayed in Fig. 6a, b and Supplementary Fig. 7A and B, the apoptosis inhibition mediated by PPX was significantly abolished via miR-494-3p overexpression or BDNF knockdown compared with their corresponding controls in MPP+-treated SK-N-SH and CHP 212 cells. Moreover, addition of miR-494-3p or silence of BDNF attenuated the cell viability induced by PPX in MPP+-treated SK-N-SH and CHP 212 cells (Fig. 6c and Supplementary Fig. 7C). In addition, PPX-mediated inhibitive effect on inflammatory cytokine secretions was weakened by miR-494-3p overexpression or BDNF inhibition in the two cell lines challenged by MPP+ (Fig. 6d, e and Supplementary Fig. 7D and E). Besides, reduction of LDH level and increase of SOD level induced by PPX in MPP+-treated SK-N-SH and CHP 212 cells were obviously abrogated by miR-494-3p addition or BDNF deficiency (Fig. 6f, g and Supplementary Fig. 7F and G). These data indicated that PPX inhibited neurotoxicity induced by MPP+ in SK-N-SH and CHP 212 cells by regulating miR-494-3p and BDNF.
Discussion
Previous studies demonstrated the neuroprotective role of PPX in Parkinson’s disease [26, 27]. However, the mechanism underlying PPX in the development of Parkinson’s disease remains to be further explored. In the present study, we using MPP+-induced cell model of Parkinson’s disease investigated the suppressive effect of PPX on cell apoptosis, inflammatory response and oxidative stress. Moreover, we were the first to disclose that the mechanism mediated by PPX was associated with miR-494-3p and BDNF.
Previous study displayed that patients received PPX extended-release formulation 0.375–4.5 mg once daily and immediate-release formulation 0.125–1.5 mg three times daily [6]. According to the data of in vitro study, 0–1000 μM PPX showed little effect on oxidative stress in normal cells, but 100 and 1000 μM PPX could inhibit the oxidative stress induced by MPP+ [28]. Moreover, this study showed that 100 μM PPX could affect the viability on normal but protected the cell viability from MPP+. Meanwhile, 100 μM PPX led to significant change on miR-494-3p expression. Hence, cells treated by 100 μM PPX were used for the further study, which was also in agreement with previous work [11]. Programmed cell death such as apoptosis is a key cause of dopaminergic neurons loss in Parkinson’s disease [29]. Bcl-2 family is associated with regulation of apoptosis in diseases [30], in which Bcl-2 protein plays an anti-apoptotic role [31] and Bax protein is an important regulator of intrinsic apoptosis [32]. Moreover, caspase proteins are involved in apoptosis and caspase 3 activation mediates apoptosis in Parkinson’s disease [33, 34]. Furthermore, LDH release indicates the damage of cell membrane integrity induced by apoptosis. By detecting cell viability, apoptotic rate, related protein levels and LDH level, we confirmed that MPP+ induced cell apoptosis in our cell lines. In addition, neuroinflammation contributes to neurodegeneration in Parkinson’s disease [35, 36]. Through measuring inflammatory cytokines (TNF-α and IL-1β) levels, we found that inflammatory response was triggered by MPP+ in our cell lines. Besides, oxidative stress is also a common feature of neurodegenerative diseases, including Parkinson’s disease [37] and SOD is a key anti-oxidant enzyme in oxidative stress modulation [38]. Our results showed that MPP+ led to oxidative stress by decreasing SOD activity. These data revealed that treatment of MPP+ resulted in neurotoxicity in our cell lines. Moreover, to investigate the function of PPX on MPP+-induced injury, the cells were pre-treated with PPX before the exposure of MPP+. The results showed that PPX attenuated MPP+-induced neurotoxicity, uncovering the therapeutic role of PPX in Parkinson’s disease by acting as an anti-inflammatory response and anti-oxidative agent, which is also in agreement with former works [39, 40]. However, the underlying mechanism by which PPX participates in the inhibition of neurotoxicity remains poorly understood.
Previous study suggested that PPX could induce the dysregulation of noncoding RNA, including miRNA [11]. This study displayed that miR-494-3p expression was up-regulated and BDNF was down-regulated in MPP+-induced cells, which was reversed by PPX, indicating that miR-494-3p and BDNF might be associated with regulation of PPX on MPP+-induced neurotoxicity. Loss-of-function experiments showed that miR-494-3p knockdown inhibited neurotoxicity induced by MPP+ in our cell lines, indicating the promoting role of miR-494-3p in neurodegeneration, which is consistent with previous studies [18, 19]. Moreover, overexpression of miR-494-3p reversed the effect of PPX on neurotoxicity, suggesting that the therapeutic effect of PPX on Parkinson’s disease development might be associated with miR-494-3p. In addition, the function of miRNAs is realized by targeting mRNAs. This study validated BDNF as a functional target of miR-494-3p by luciferase reporter and RIP assays, which has been reported as potential therapeutic target in neurodegenerative diseases including Parkinson’s disease [41, 42]. By performing rescue experiments, silence of BDNF abated the suppressive role of miR-494-3p knockdown or PPX in neurotoxicity induced by MPP+, indicating that PPX inhibited the neurotoxicity via miR-494-3p/BDNF axis. The animal model is responsible for better understanding the pathogenesis of Parkinson’s disease [43]. However, the in vivo data were absent in the present work, which is expected to be explored in future study. Besides, PPX is a selective dopamine D2 receptor agonist to involve in the treatment of Parkinson’s disease. However, the high concentration (100 μM) of PPX might open the possibility that PPX is acting through an D2-independent mechanism, indicating that the neuroprotective effects of PPX described in this study may be mediated by a D2-independent mechanism, which may involve direct interrelations between MMP+ and PPX. This should be explored in future.
In conclusion, this study investigated the neuroprotective role of PPX in MPP+-induced neurotoxicity via inhibiting cell apoptosis, inflammatory response and oxidative stress, possibly by decreasing miR-494-3p and increasing BDNF, elucidating a novel mechanism for understanding the pharmacological role of PPX in Parkinson’s disease.
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3:17013. https://doi.org/10.1038/nrdp.2017.13
Hare DJ, Adlard PA, Doble PA, Finkelstein DI (2013) Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Metallomics 5:91–109. https://doi.org/10.1039/c2mt20164j
Gonzalez-Polo RA, Soler G, Fuentes JM (2004) MPP+: mechanism for its toxicity in cerebellar granule cells. Mol Neurobiol 30:253–264. https://doi.org/10.1385/MN:30:3:253
Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci 12:612. https://doi.org/10.3389/fnins.2018.00612
Francardo V, Schmitz Y, Sulzer D, Cenci MA (2017) Neuroprotection and neurorestoration as experimental therapeutics for Parkinson’s disease. Exp Neurol 298:137–147. https://doi.org/10.1016/j.expneurol.2017.10.001
Frampton JE (2014) Pramipexole extended-release: a review of its use in patients with Parkinson’s disease. Drugs 74:2175–2190. https://doi.org/10.1007/s40265-014-0322-5
Silindir M, Ozer AY (2014) The benefits of pramipexole selection in the treatment of Parkinson’s disease. Neurol Sci 35:1505–1511. https://doi.org/10.1007/s10072-014-1891-5
Favier M, Duran T, Carcenac C, Drui G, Savasta M, Carnicella S (2014) Pramipexole reverses Parkinson’s disease-related motivational deficits in rats. Mov Disord 29:912–920. https://doi.org/10.1002/mds.25837
Wang Y, Sun SG, Zhu SQ, Liu CF, Liu YM, Di Q, Shang HF, Ren Y, Xiang W, Chen SD (2017) Analysis of pramipexole dose-response relationships in Parkinson’s disease. Drug Des Devel Ther 11:83–89. https://doi.org/10.2147/DDDT.S112723
Motyl J, Przykaza L, Boguszewski PM, Kosson P, Strosznajder JB (2018) Pramipexole and Fingolimod exert neuroprotection in a mouse model of Parkinson’s disease by activation of sphingosine kinase 1 and Akt kinase. Neuropharmacology 135:139–150. https://doi.org/10.1016/j.neuropharm.2018.02.023
Sang Q, Liu X, Wang L, Qi L, Sun W, Wang W, Sun Y, Zhang H (2018) CircSNCA downregulation by pramipexole treatment mediates cell apoptosis and autophagy in Parkinson’s disease by targeting miR-7. Aging (Albany NY) 10:1281–1293. https://doi.org/10.18632/aging.101466
Ridolfi B, Abdel-Haq H (2017) Neurodegenerative disorders treatment: the MicroRNA role. Curr Gene Ther 17:327–363. https://doi.org/10.2174/1566523218666180119120726
Leggio L, Vivarelli S, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Marchetti B, Iraci N (2017) microRNAs in Parkinson’s disease: from pathogenesis to novel diagnostic and therapeutic approaches. Int J Mol Sci 18:E2698. https://doi.org/10.3390/ijms18122698
Shen PF, Chen XQ, Liao YC, Chen N, Zhou Q, Wei Q, Li X, Wang J, Zeng H (2014) MicroRNA-494-3p targets CXCR4 to suppress the proliferation, invasion, and migration of prostate cancer. Prostate 74:756–767. https://doi.org/10.1002/pros.22795
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH, Chen GL, Xie XS, Li B, Wei YX, Huang YL, Zhou YX, Du ZW (2015) miR-494-3p regulates cellular proliferation, invasion, migration, and apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell Mol Neurobiol 35:679–687. https://doi.org/10.1007/s10571-015-0163-0
Weng JH, Yu CC, Lee YC, Lin CW, Chang WW, Kuo YL (2016) miR-494-3p induces cellular senescence and enhances radiosensitivity in human oral squamous carcinoma cells. Int J Mol Sci 17:E1092. https://doi.org/10.3390/ijms17071092
Zhu L, Wang X, Wang T, Zhu W, Zhou X (2019) miR4943p promotes the progression of endometrial cancer by regulating the PTEN/PI3 K/AKT pathway. Mol Med Rep 19:581–588. https://doi.org/10.3892/mmr.2018.9649
Xiong R, Wang Z, Zhao Z, Li H, Chen W, Zhang B, Wang L, Wu L, Li W, Ding J, Chen S (2014) MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol Aging 35:705–714. https://doi.org/10.1016/j.neurobiolaging.2013.09.027
Geng L, Zhang T, Liu W, Chen Y (2018) miR-494-3p modulates the progression of in vitro and in vivo Parkinson’s disease models by targeting SIRT3. Neurosci Lett 675:23–30. https://doi.org/10.1016/j.neulet.2018.03.037
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175. https://doi.org/10.1016/j.pharmthera.2013.01.004
Shen T, You Y, Joseph C, Mirzaei M, Klistorner A, Graham SL, Gupta V (2018) BDNF polymorphism: a review of its diagnostic and clinical relevance in neurodegenerative disorders. Aging Dis 9:523–536. https://doi.org/10.14336/AD.2017.0717
Lima Giacobbo B, Doorduin J, Klein HC, Dierckx R, Bromberg E, de Vries EFJ (2019) Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56:3295–3312. https://doi.org/10.1007/s12035-018-1283-6
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Yao J, Zou Z, Wang X, Ji X, Yang J (2016) Pinoresinol diglucoside alleviates oxLDL-induced dysfunction in human umbilical vein endothelial cells. Evid Based Complement Alternat Med 2016:3124519. https://doi.org/10.1155/2016/3124519
Bounda GA, Zhou W, Wang DD, Yu F (2015) Rhein elicits in vitro cytotoxicity in primary human liver HL-7702 cells by inducing apoptosis through mitochondria-mediated pathway. Evid Based Complement Alternat Med 2015:329831. https://doi.org/10.1155/2015/329831
Contin M, Lopane G, Mohamed S, Calandra-Buonaura G, Capellari S, De Massis P, Nassetti S, Perrone A, Riva R, Sambati L, Scaglione C, Cortelli P (2019) Clinical pharmacokinetics of pramipexole, ropinirole and rotigotine in patients with Parkinson’s disease. Parkinsonism Relat Disord 61:111–117. https://doi.org/10.1016/j.parkreldis.2018.11.007
Zhao H, Ning Y, Cooper J, Refoios Camejo R, Ni X, Yi B, Parks D (2019) Indirect comparison of ropinirole and pramipexole as levodopa adjunctive therapy in advanced Parkinson’s disease: a systematic review and network meta-analysis. Adv Ther 36:1252–1265. https://doi.org/10.1007/s12325-019-00938-1
Cassarino DS, Fall CP, Smith TS, Bennett JP (1998) Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. J Neurochem 71:295–301. https://doi.org/10.1046/j.1471-4159.1998.71010295.x
Venderova K, Park DS (2012) Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009365. https://doi.org/10.1101/cshperspect.a009365
Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20:175–193. https://doi.org/10.1038/s41580-018-0089-8
Siddiqui WA, Ahad A, Ahsan H (2015) The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol 89:289–317. https://doi.org/10.1007/s00204-014-1448-7
Pena-Blanco A, Garcia-Saez AJ (2018) Bax, Bak and beyond—mitochondrial performance in apoptosis. FEBS J 285:416–431. https://doi.org/10.1111/febs.14186
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–290. https://doi.org/10.1146/annurev.cellbio.15.1.269
Tatton WG, Chalmers-Redman R, Brown D, Tatton N (2003) Apoptosis in Parkinson’s disease: signals for neuronal degradation. Ann Neurol 53(Suppl 3):S61–70. https://doi.org/10.1002/ana.10489discussion S70-62
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. https://doi.org/10.1126/science.aag2590
More SV, Kumar H, Kim IS, Song SY, Choi DK (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm 2013:952375. https://doi.org/10.1155/2013/952375
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19. https://doi.org/10.1016/j.pneurobio.2016.07.005
Bresciani G, da Cruz IB, Gonzalez-Gallego J (2015) Manganese superoxide dismutase and oxidative stress modulation. Adv Clin Chem 68:87–130. https://doi.org/10.1016/bs.acc.2014.11.001
Sadeghi H, Parishani M, Akbartabar Touri M, Ghavamzadeh M, Jafari Barmak M, Zarezade V, Delaviz H, Sadeghi H (2017) Pramipexole reduces inflammation in the experimental animal models of inflammation. Immunopharmacol Immunotoxicol 39:80–86. https://doi.org/10.1080/08923973.2017.1284230
Wang Y, Yu X, Zhang P, Ma Y, Wang L, Xu H, Sui D (2018) Neuroprotective effects of pramipexole transdermal patch in the MPTP-induced mouse model of Parkinson’s disease. J Pharmacol Sci 138:31–37. https://doi.org/10.1016/j.jphs.2018.08.008
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5:311–322. https://doi.org/10.1038/nrneurol.2009.54
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10:209–219. https://doi.org/10.1038/nrd3366
Dawson TM, Golde TE, Lagier-Tourenne C (2018) Animal models of neurodegenerative diseases. Nat Neurosci 21:1370–1379. https://doi.org/10.1038/s41593-018-0236-8
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, C., Zhu, J., Yuan, J. et al. Pramipexole Inhibits MPP+-Induced Neurotoxicity by miR-494-3p/BDNF. Neurochem Res 45, 268–277 (2020). https://doi.org/10.1007/s11064-019-02910-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02910-5