Abstract
Postpartum depression is a mood disorder with a distinct neurobiological and behavioural profile occurring during and after the postpartum period. Dopamine pathways in the limbic regions of the brain such as the nucleus accumbens (NAc) have been shown to be involved in the etiology of depressive disorders. Selective activation of the dopamine D1–D2 receptor heteromer has been demonsrated to cause depressive- and anxiogenic-like behaviours in rats. The maternal separation model involving three hour daily maternal separation (MS) from pups on PPD 2–15 on anxiety-, depression- and anhedonia-like behaviors in the dams was investigated, together with plasma corticosterone, oxytocin and D1–D2 heteromer expression in the NAc core and shell in non-MS and MS dams. Depression, anxiety and anhedonia-like behaviours were measured using the forced swim test, elevated plus maze and sucrose preference test, respectively. In comparison to non-MS controls, MS dams displayed slightly higher depressive and anxiety-like behaviours with no difference in anhedonia-like behaviours. The MS dams displayed significantly increased care of pups after their retrieval with higher bouts of nursing, lower latency to nurse, lower bouts of out nest behaviour and decreased self-care. There was no significant alteration in D1–D2 heteromer expression in NAc core and shell between mothers of either group (MS, non-MS) or between postpartum rats and nonpregnant female rats, remaining higher than in male rats. This data provides evidence for the maternal separation model in producing a postpartum depression-like profile, but with maternal resilience able to modify behaviours to counteract any potential deleterious consequences to the pups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parturition and the postpartum period is a time when many physiological changes occur in brain and is often associated with feelings of joy, yet 10 to 15% of new mothers experience postpartum depression (PPD) within the first 3 months after delivery [1]. The etiology of PPD is not yet elucidated but risk factors such as hypo-responsiveness of the hypothalamic pituitary adrenal (HPA) axis, hormone withdrawal theories, genetic factors, changes in immune system mediated inflammatory states or psychological and other clinical factors have been proposed [2,3,4]. Strong to moderate predictive elements of PPD include previous depression, antenatal depression and anxiety, stressful life events, marital dissatisfaction, poor social support, unwanted pregnancy, low self-esteem, negative emotionality, obstetric, biological and hormonal factors [3, 5, 6]. Current treatments for PPD focus on psychotherapeutic interventions and pharmacological agents such as antidepressants or a combination of the two [7,8,9].
PPD is often a result of extended “postpartum blues”, occurring two weeks after delivery consisting of mood changes and sleep disturbances. The symptomatology of PPD makes it difficult to distinguish from other depressive episodes, though is usually identified by loss of interest in the infant [10]. Without efficacious treatments, PPD can result in impaired maternal caretaking behaviour, negative cognitive and health consequences to the mother and child, and stress on the family [3]. As mentioned, there are genetic and environmental components of PPD, though environmental stressors are much better understood [11]. Rodent models are used to evaluate the etiology of PPD, by employing a stress paradigm or biological intervention such as hormone withdrawal or corticosterone treatment [11]. The maternal separation model implements psychological and psychosocial stress to both dam and pup by disrupting interactions during the critical pre-weaning period when mother–pup contact is essential for the social, behavioural and physiological development of the pups [12,13,14,15,16]. Pups that experienced early-life stress have impaired HPA axis activity, increased anxiety, helplessness, anhedonia and reduced immunity [17,18,19,20]. Though this model is largely used with a focus on pups, the dams are also affected in similar and significant ways, displaying signs of reduced maternal care, alterations of neurochemical parameters and accompanied by higher levels of corticosterone secondary to modulation of the activity of the HPA axis [18, 21, 22]. This model has been shown to have sufficient construct and face validity and has been shown to produce depression-like behaviours in many studies despite some contradictory results [22]. In addition, the stressor is social rather than physical which more closely resembles human depression which typically involves loss of social relationships [11, 22]. PPD has many of the same behavioural and neurobiological characteristics as major depressive disorder (MDD), though there are key differences in some brain regions, with propensity to develop co-morbid anxiety and bipolar disorder and a decreased level of maternal care and parenting [23, 23,24,25].
Dopamine has been studied with respect to its relationship to MDD and has also been found to metabolize abnormally in PPD [26, 27]. It is involved in cognitive functions like learning, memory, as well as reward and affect [28, 29]. The mesocorticolimbic dopaminergic projection from the ventral tegmental area to the nucleus accumbens (NAc) and prefrontal cortex is a widely studied reward pathway and evidence has pointed that alterations in these neurons play some role in depression [30,31,32]. The dopamine D1–D2 receptor heteromer is a complex formed between D1 and D2 receptors which in the striatum, is expressed in a discrete subpopulation of medium spiny neurons present in relatively higher density in the NAc compared to the caudate putamen [33, 34]. The D1–D2 complex induces intracellular calcium release through a Gq/11-dependent pathway by a mechanism involving phospholipase C, inositol triphosphate receptors leading to Calcium/calmodulin-dependent protein kinase type II subunit alpha (CaMKIIα) activation and an increase in brain-derived neurotrophic factor (BDNF) production as well as an increased GABAergic tone of neurons in the NAc, both of which are implicated in depressive-like symptoms [31, 34]. Disrupting the heteromer has been shown to have antidepressant and anxiolytic-like effects in rodents whereas stimulation of the receptor heteromer had pro-depressive and anxiogenic effects [31].
Considering the studies on the D1–D2 heteromer, it is clear it may have a considerable physiological role in normal mood function and its dysfunction may potentially be linked to neuropsychiatric diseases such as depression, including in the postpartum state. We employed a maternal separation (MS) paradigm to model postpartum depression in rodents using a social stressor. Maternal behaviour was recorded to measure the effects of the MS paradigm on maternal care in the postpartum period, partially indicative of PPD [35]. Other measurements assessed symptoms associated with PPD, performed after pup weaning. This included sucrose preference test to measure anhedonia, elevated plus maze test to measure anxiety-like behaviour and forced swim test to determine depressive-like behaviour such as helplessness. Biochemical data was collected from serum and brain samples of the dams and the expression of the D1–D2 heteromer in striatum was evaluated.
Materials and Methods
Animals
Female timed-pregnant Sprague Dawley rats were obtained from Charles River, 15 days pregnant and maintained on a 12 hr light/dark cycle (lights on at 07:00) in a 25 °C colony room at 50% humidity. They were singly housed in polyethylene cages with environmental enrichment provided by corn bedding, a red plastic shelter, a plastic bone toy and nesting material. Rats were handled regularly for less than 2 min a day and allowed to habituate to the testing room for 45 min until 3 days prior to parturition. This was done to remove the stresses of transport, exposure to the testing room and the experimenter. Standard laboratory chow and water were available ad libitum. Animals were housed and tested in compliance with the guidelines described in the Guide to the Care and the Use of Experimental Animals (Canadian Council on Animal Care), and protocols were approved by the Animal Care Ethics Committee of the University of Toronto.
Maternal Separation
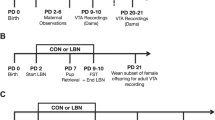
The day of delivery was appointed as postpartum day (PPD) 0. Litters were culled to four females and four males per dam. Dams were left undisturbed after this in the colony room till PPD 2. Dams were randomly assigned to a maternal separation group (n = 8) and a control group (n = 8). Maternal separation (MS) consisted of separating MS group dams from their pups for 3 h daily (09:30 to 12:30) from PPD 2–15, while dams in control group (n = 4) were exposed to animal rearing facility (ARF) conditions which comprised of weekly cage changes from PPD 5 onwards between 12:00 and 13:00. Following the MS protocol, dams were placed in a separate clean cage for temporary holding and pups individually picked up and placed in a container filled with ~ 2 cm of home cage bedding and nesting kept at 32 ± 0.5 °C (PPD 2–5), 30 ± 0 0.5 °C (PPD 6–14) and 28 ± 0.5 °C (PPD 15) in a humid incubator. The dams were returned to the home cage in the colony room for the duration of the separation. For reunion, the dams were placed in a clean holding container and pups were placed individually back in the original nest location in the original cage and the dam placed immediately after. From PPD 15–21, both groups were raised in ARF conditions with weekly cage changes. Cage changes were performed by removing dams and placing them in a clean holding container. Then some soiled bedding and nesting from the home cage was added to the new cage, while making the best effort to maintain nest location. Pups were then transferred to the new cage, followed immediately by the dam. Pups were weaned at PPD 21 and placed in group housing, separate from the dams to be used in another project.
Maternal Behavior
The pup retrieval test was conducted to measure maternal behaviour. The test was conducted three times on PPD 3 and 9 for 10 min between 11:30 and 13:30. Two 5 cm high, 0.5 cm thick clear plexiglass dividers were used to create four quadrants in the cage for pup retrieval scoring and to prevent pups from crawling back to the nest. On test days, the dam was removed from the cage and placed in a holding container for less than 2 min. During this time, the litter was weighed and placed on the opposite quadrant of the original nest location. The dam was returned in the original nest location and maternal behaviour was recorded with a video camera in the testing room. For MS group dams, pup weighing, and relocating were done immediately before the separation period ended in the testing room. The following behaviours were scored every 15 s for the 30 min testing period: pup retrieval [scored as duration] (defined as dam picking up pup with mouth and transporting across one quadrant), latency to first pup contact [scored as duration] (bringing nose close to pup, sniffing pup), number of pups retrieved in the first 10 min, nursing latency, pup grooming and licking (dam brings nose near pup to sniff or licks pups with tongue), arched back nursing (dam positioned herself over the pups and arching her back allowing reach of mammary region), self-grooming (4 phases of self-grooming), out nest (when the dam was not in her nest) and rearing (dam standing up on its hind legs). Pup retrieval test ended after 10 min or when all the pups were returned. Any pups not retrieved by the dam after 10 min were returned to the nest by the observer.
Sucrose Preference Test
The two-bottle sucrose preference test is a measure of anhedonia and was conducted at PPD 22. The rats were taken to the testing room and were allowed to habituate to the new wire cage with no access to chow or water prior to the test for 2 h. Bottles were pre-weighed, one bottle had tap water and the other had 2% sucrose solution. The bottles were placed counterbalanced on the left and right sides at 12:00 and after 2 h, the bottles were re-weighed. Sucrose preference was calculated by sucrose consumed/total consumed *100%. Sucrose/water bottle weights were measured with a balance.
Elevated Plus Maze
The elevated plus maze (EPM) tests anxiety-like behaviour and was conducted in the test room on PPD 22 from 12:30 to 13:30. The maze is a plus shaped apparatus made of black plexiglass, resting 50 cm above the floor supported by four plastic legs. The platform has two 10 cm wide open arms and two 10 cm wide closed arms with 30 cm high black plexiglass walls on either side. The rat was placed facing the same open arm away from the observer. The time spent, and the number of entries into open and closed arms were scored for 5 min. An entry is described as the rat having all four paws within the arm. After testing was complete, the rat was placed back into its cage and the maze cleaned with pre-empt and dried prior to the next test. A video camera was placed above the maze on the ceiling and the analysis was done through the recording. If a rat fell off the open arm of the maze, the rat is quickly put back on the maze and testing continued, however the data was excluded from the final analysis. Timing and number of arm entries in the EPM were recorded using a Noldus video camera.
Forced Swim Test
The forced swim test (FST) is a measure of depressive-like behaviour characterized by immobility in an inescapable container filled with water. Rats are placed in a black plastic cylindrical container of height 50 cm, and diameter 30 cm. Water is filled to 30 cm and kept at room temperature of 25 ± 1 °C, ensuring the rats cannot stand with their hind legs on the bottom while having their nose above water. Rats were exposed to water for 15 min between 12:30 and 14:30 on PPD 26, to remove the acute stress of immersion in water on the test day. The next day on PPD 27, the test was repeated for 5 min. Behaviours recorded were immobility (no movement except that necessary to keep head above water), swimming (movement of limbs in addition to that necessary to keep head above water) and climbing (scratching against the wall of the container in an effort to escape). Each rat was dried with a towel (one towel for control and one for MS rats) and placed under a heating lamp for 5 min after the test to prevent hypothermia. Water was replaced after each rat. Behaviour was recorded with a video camera placed above and recordings analyzed. Behaviours during FST were recorded using a Noldus video camera.
Evaluation of Corticosterone and Oxytocin Levels
Rats (non-MS: n = 4, MS: n = 4) were anaesthetized on PPD 27 between 12:00 and 15:00 by hexafluoride gas, confirmed by nonreactivity to the toe pinch test. Intravenous perfusion with 4% paraformaldehyde was done through a right atrial incision and 1–2 mL blood was collected into ice chilled 2 mL EDTA-coated centrifuge tubes. Within an hour of collection, tubes were centrifuged (5 000 rpm, 4 °C, 10 min), plasma isolated and stored at −70 °C until used. Plasma corticosterone and oxytocin were measured using ELISA (Invitrogen) following the manufacturer’s protocol. The remaining 8 rats from both groups were placed in a CO2 chamber until loss of consciousness then decapitated. Trunk blood was collected in EDTA-coated centrifuge tubes and the brain was harvested for further analysis.
Estimation of the D1–D2 Heteromer Expression Using Proximity Ligation Assay (PLA)
After perfusion, the brain was removed and post-fixed in 4% paraformaldehyde for 2 h at 4 °C, then placed in 10% sucrose overnight. The brains were then transferred to a 30% sucrose solution for 24 h at 4 °C. The brains were flash-frozen and stored at −70 °C until use. Brain were sliced from bregma 0.2 to 2.2 (16 µm sections) with a cryostat to obtain sections with nucleus accumbens. Sections adhered to glass microscope slides were stored at −70 °C until used. PLA probes were created by conjugating a rat anti-D1R antibody (Sigma, D2944) with a PLUS oligonucleotide (Duolink®In Situ Probemaker PLUS DUO92009, Sigma-Olink) and a rabbit anti-D2R (Millipore, AB5084P) antibody with a MINUS oligonucleotide (Duolink® In Situ Probemaker MINUS DUO92010, Sigma-Olink) with PLA experiments performed as described previously (Hasbi et al.). Briefly, rat brain slices were incubated for 1 h at 37 °C with blocking solution in a pre-heated humidity chamber, followed by incubation with the above PLA probes (final concentration of 60 μg/mL) and wash with buffer A (DUO82047, Sigma-Olink). Duolink II in situ PLA detection kit (DUO92008, Sigma-Olink) detected positive PLA signals with cell nuclei labeled with DAPI. Cells were visualized using confocal Fluoview Olympus microscope (FV 1000) with 60 × /1.2 NA objective and Z-stacks recorded to verify signals were localized around cell bodies. Analysis of images was performed through Imagetool (Duolink®). Negative control assays were carried out in the absence of one of the two PLA probes or both, or in the absence of the ligase and/or polymerase to ensure PLA specificity. No PLA signal was observed in these conditions.
Statistics
All values are reported as mean ± S.E.M. Statistical differences were calculated using the Student t test. Correlations were calculated using the Pearson’s correlation test, linear regression was performed to evaluate line of best fit, and p values calculated significance of correlation using GraphPad Prism 8. p values of less than 0.05 were considered statistically significant.
Results
Effects of Maternal Separation on Maternal Behavior
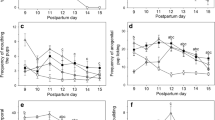
On PPD 3 and 9, non-MS and MS dams were observed and maternal behavior was recorded in order to assess the amount of pup-directed vs self-directed behaviours. Five different behaviours were measured along with three different latencies to a specific behaviour. On PPD 3, there was no significant difference in the number of bouts of any behaviour including nursing, pup grooming, out nest and rearing except for self grooming, where MS dams had significantly fewer bouts (Fig. 1a) (non-MS 24.63 ± 5.74, MS 7 ± 1.98, p = 0.016, t-test). In addition, MS dams showed a trend to more bouts of nursing (non-MS 38.38 ± 12.39, MS 57.13 ± 13.83) and lower bouts of out nest (non-MS 55 ± 11.80, MS 29.25 ± 9.51) behaviours. Nursing latency was significantly lower for MS dams on PPD 3 (Fig. 1b) (non-MS 405.71 ± 95.63 s, MS 135.71 ± 52.12 s, p = 0.022), with no difference in pup retrieval or pup grooming latency. These behavioral results were further supported on PPD 9, as MS dams had a significantly higher number of bouts of nursing (non-MS 67.33 ± 16.309, MS 112.6 ± 3.140, p = 0.007) and a lower number of bouts of out nest behaviour (Fig. 1c) (non-MS 37.17 ± 17.03, MS 6.00 ± 2.88, p = 0.012, t-test). There were no significant differences observed (p > 0.05, t-test) for behavioural latencies on PPD 9 (Fig. 1d).
Maternal behaviors on PPD 3 and PPD 9 in control dams or subjected to pup separation. Number of bouts of a given behaviour measured in 15 s intervals over a 30 min testing window are shown. a Maternal behaviours on PPD 3 and b latencies on PPD 3. c Maternal behaviours on PPD 9 and d latencies on PPD 9. Results of n = 8 for non-MS and MS groups are expressed as mean ± S.E.M. *p = 0.0223, **p = 0.0069
Effects of Maternal Separation on Litter Weight
Pup litters were weighed to investigate any link between the amount of milk each litter was receiving and maternal separation. There were no statistically significant differences between the two groups on any of the days (Table 1).
Effects of Maternal Separation on Sucrose Consumption
On PPD 22, dams were left in home cages with no food or water for two hours, followed by a two bottle choice test (water and 2% sucrose) for two hours, to measure their tendency to consume food reward. The sucrose preference test (Fig. 2a) did not reveal any statistically significant differences (p > 0.05, t-test) between the two groups (Non-MS 92.24 ± 0.77%, MS 87.23 ± 4.24%).
Sucrose preference and Anxiety-like behaviour. Sucrose preference test (a) was assessed using a two-bottle choice paradigm in control dams or those with pups subjected to maternal separation. Sucrose preference was expressed as a percentage of 2% sucrose consumed to total liquid consumed. Results are expressed as mean ± S.E.M. The individual responses are shown as well. Anxiety-like behaviour (b–d) was assessed using the elevated plus maze (EPM) in control dams or those subjected to maternal separation. b Time (seconds) spent in open arms of the EPM. c Latency (seconds) to enter closed arms. d Frequency of entries in the open arms. Results are expressed as mean ± S.E.M. *p = 0.046245
Effects of Maternal Separation on Anxiety-like Behavior
Dams were tested in the elevated plus maze to investigate anxiety-like behaviours in a novel environment. Rats have an unconditioned fear of heights/open spaces and a tendency to prefer enclosed dark places [36]. There were no differences found between groups in either the time spent in open arms (Fig. 2b) (Non-MS 68.70 ± 17.74 s, MS 62.95 ± 11.69 s, p > 0.05, t-test) or the frequency of entries in the open arms (Fig. 2d) (Non-MS 17.43 ± 3.39, MS 15.25 ± 1.48, p > 0.05, t-test). However, the latency time to enter the closed arms was significantly lower in the MS group (Fig. 2c) (Non-MS 39.03 ± 11.61 s, MS 8.42 ± 5.04 s, t = 2.213, *p = 0.045).
Effects of Maternal Separation on Depressive-like Behavior
The FST was used to investigate depressive-like behaviour based on climbing, swimming and immobile behaviours. Behaviour times were recorded over the 5 min duration of the test and converted to a score. A mobility score of three was given to climbing behaviour, two for swimming behaviour and one for immobility. The immobility score was calculated as the inverse of the mobility score. The MS dams had a tendency towards a higher immobility score than non-MS dams except at the fourth minute where both groups had the same score. Conducting a t-test for each half-minute interval while assuming every sample comes from a population with the same standard deviation reveals the MS dams had significantly higher immobility (Fig. 3a) at 3.5 min (t = 2.557, *p = 0.012) and 4.5 min (t = 2.209, *p = 0.029) as well as close to significance at 1.5 min (t = 1.976, ns. p = 0.050) compared to Non-MS. In addition, taking the first 3.5 min into account, the MS dams had on average a higher immobility score (t = 2.487, *p = 0.014). The latency to immobility was not significant (Fig. 3b). Similarly, the total time spent immobile did not reach significance (Fig. 3c) (Non-MS 60.63 ± 8.73 s, MS: 90.00 ± 14.14 s, p = 0.099, t-test).
Forced swim test. Forced swim test (FST) was used to assess depressive-like behaviour in control dams or those with pups subjected to maternal separation. a Immobility score for every half minute. b Latency to first immobile behaviour. c Total time spent immobile over the 5 min test. Results are expressed as mean ± S.E.M. *p < 0.029
Effects of Maternal Separation on Plasma Corticosterone and Oxytocin
Upon sacrifice, plasma samples from trunk blood were obtained. The total corticosterone (free and bound) and oxytocin levels were measured. Non-MS rats displayed higher levels of plasma corticosterone compared to MS rats (Fig. 4a), but there was no significant difference due to high variability (non-MS 109.97 ± 47.31 ng/mL, MS 49.73 ± 16.40 ng/mL, p > 0.05, t-test).
Similarly, the MS dams had lower circulating oxytocin compared to non-MS (Fig. 4b), but no significant difference was observed due to the high variability in non-MS group (non-MS 355.40 ± 108.13 pg/mL, MS 223.71 ± 50.91 pg/mL, p > 0.05, t-test).
Effects of Maternal Separation on D1–D2 Heteromer in the Nucleus Accumbens
The nucleus accumbens core and shell of dams were investigated using in situ PLA to detect D1-D2 heteromers. Representative images for D1-D2 heteromer PLA from the nucleus accumbens (NAc) of MS and non-MS groups are shown in Fig. 5g and Fig. 5h. The analysis of PLA data showed that the percent of cells expressing D1–D2 heteromer in the whole nucleus accumbens was similar in both groups (Fig. 5a) (non-MS 30.39 ± 2.97%, MS 31.11 ± 4.79%, p > 0.05, t-test). Also, subregion analysis showed that the percent of cells expressing the heteromer in the NAc core alone (Fig. 5b) (non-MS 31.74 ± 3.36%, MS 28.69 ± 4.34%, p > 0.05, t-test) and NAc shell alone (Fig. 5c) (non-MS 28.88 ± 3.22%, MS 28.14 ± 4.38%, p > 0.05, t-test) were similar. The PLA signal density count per cell that expressed D1–D2 heteromer was calculated and no significant difference was found in both sub compartments of the NAc or in the NAc overall (Fig. 5d, e, f) (non-MS 1.64 ± 0.18, MS 1.96 ± 0.26, p > 0.05, t-test). Previous data from our laboratory on D1–D2 expression levels in nonpregnant female rats were compared to the pregnant (MS + non-MS) rats in this study (Supplementary Figure 1a) and we found no significance between these two groups (p > 0.05, t-test). In addition, we found no significant difference between this group and each of the two pregnant groups separately (p > 0.05, t-test), probably due to lower number of nonpregnant female rats analyzed.
D1–D2 heteromer quantification in the nucleus accumbens of dams. Dopamine D1–D2 heteromer expression was quantified using proximity ligation assay (PLA). Data shows a the percent of positive cells in NAc expressing D1–D2 heteromer, b the percent of positive cells in NAc core expressing D1–D2 heteromer, and c the percent of positive cells in NAc shell expressing D1–D2 heteromer. The relative number of heteromer per cell (number of PLA signal) was also quantified. Data shows d the signal count per D1–D2 expressing cell in the NAc, e the signal count per D1–D2 expressing cell in the NAc core, and f the signal count per D1–D2 expressing cell in the NAc shell. Results are expressed as mean ± S.E.M of approximately 2300 cell bodies on average counted in each NAc
Correlation of Behaviors Irrespective of Separation Group
All parameters measured were correlated against every other parameter irrespective of maternal separation status to reveal any patterns in the data relating to stress responses and D1–D2 heteromer expression. Sucrose preference had a significant negative correlation with plasma corticosterone (Fig. 6a) (R2 = 0.3911, p = 0.022). It was hypothesized that behaviours that indicate higher levels of anxiety and depression would be correlated with D1–D2 heteromer expression in the NAc. However, the correlations between EPM closed arm latency and FST performance at 3.5 min with PLA signals per cell were not significant though an r value of −0.3940 and + 0.4996 suggests weak negative correlations exist. (Fig. 6b, c; R2 = 0.1522, R2 = 0.2496, p > 0.05). There was no significant correlation between higher anxiogenic behaviour on the EPM and plasma corticosterone levels (Fig. 6d) (R2 = 0.0513, p > 0.05).
Correlation between multiple parameters regardless of maternal separation status. Correlation between a plasma corticosterone and sucrose preference, b PLA signals per cell and EPM closed arm latency, c PLA signals per cell and FST immobility score @ 3.5 min, and d EPM closed arm latency and corticosterone. The only significant correlation was between corticosterone and sucrose preference (p = 0.022)
Discussion
It has been shown that the application of stress during the postpartum period in rats may result in a decreased level of maternal care and reduced attention towards pups [18, 22]. These results may suggest a depressive-like state similar to human PPD where subjects have been observed to be disengaged with their child and interact in a less involved, less affectionate manner in physical, vocal and visual terms [35]. In the present study, we examined the effects of the previously studied maternal separation paradigm with a focus on the D1–D2 heteromer on Sprague Dawley rats [12, 21, 31, 37] to simulate postpartum depression through the application of a social stressor, maternal separation. Maternal separation presents a social stress, which is often associated with human postpartum depression [11]. To keep consistent with testing protocols from previous studies [12, 18, 21, 37], the light–dark cycle was inverted. The dams had no previous pregnancies to exclude any previous life stressors that could act as confounding variables. The control group was raised in regular animal rearing facility (ARF) conditions to create an environment with minimal to no social stress and only exposed to handling for cage changes and recording pup weight [18, 22].
Our results show in contrast that maternal behaviour in the Sprague Dawley rat dams subjected to daily maternal separation, there was evidence of greater engagement with their litters right after periods of daily 3 h long maternal separation. The MS group dams in the present study seem to engage in more pup-directed behaviour and less in self-directed behaviour. The MS group dams were also significantly quicker to nurse their pups upon reunion of all pups to the nest and seem to be quicker at pup retrieval. At first these results were unexpected as it is opposite to what other studies have shown [18, 22, 38, 39]. However, these results are in good agreement with some studies in rats and mice, which have shown that pup-directed maternal behaviours such as arched-back nursing and pup grooming is increased upon reunion after a period of maternal separation [40, 41], and increases over the duration of the stress protocol [40]. These results can be explained by the dam making up for the separation period by increasing her care and access, responding to the ultrasonic vocalization of the pups [42], demonstrated in the present paradigm with the higher frequency of nursing, lower nursing latency and lower frequency of being out of the nest or time spent in self-grooming. The increased nursing observed may also explain the MS pups’ tendency to increased weight over the experiment period as the pups had more access to milk, knowing that the only form of nutrition they obtain is the mother’s milk during the testing period. Though the total body weight of the two groups of pups were not significantly different, and most likely similar in the long term, as seen in previous studies [41].
The MS dams did not display significant stress-induced depression-like behaviours like anhedonia but did display certain other features of depressive-like, and anxiogenic behaviour. The sucrose test provides a measure of reward-related behaviour, and in stress-induced models of depression, the stressed rats consume less sucrose solution than their unstressed counterparts [43, 44]. However, we did not observe any statistical differences in sucrose preference between the MS and non-MS dam groups, in agreement with other reports [45, 46], suggesting that the maternal separation paradigm did not induce overt anhedonia in these rats. However, in a test of anxiety-like behaviour, the elevated plus maze, our results showed that MS dams had significantly lower latency to enter the closed arms than the non-MS group. This indicates that the dams subjected to maternal separation preferred the closed arms, were less exploratory with a tendency to avoid open spaces/height, which is indicative of an unconditioned fear. Although there was no meaningful difference in the ratio of time spent in open vs closed arms, the shorter latency to go into the safer appearing area of the maze (closed arms) by the MS dams could represent an indication of increased anxiety-like behaviour [36].
Higher immobility in an inescapable situation is interpreted as learned helplessness/depressive-like behaviour. The gold standard for such a test is considered to be the FST. The MS dams showed a significantly higher immobility score during two time periods in the test. Although, the overall total immobility time during the 5 min of the test in the MS dams was not significantly different from non-MS dams, the MS dams participated less in active stress-coping strategies (swimming/climbing) than non-MS dams. Our results show mild depressive-like behaviours in MS dams and are in agreement with those of other groups who have used similar forms of stress [18, 22, 46, 47].
During the postpartum period, there are many hormonal adaptations, such as an increased level of oxytocin which correlates with increased maternal behaviour [44, 48, 49] and oxytocin release is increased from the pituitary during breastfeeding for milk letdown. Reduced basal oxytocin levels are a predictor of postpartum depression and coincide with increased depressive symptoms [44]. In contrast, we did not observe any significant difference in plasma oxytocin levels between the groups, likely due to a high variability among the non-MS group. HPA-axis dysregulation is often reported in depressed humans and reports in rats modeling postpartum depression usually cite higher basal levels of circulating corticosteroid hormones persisting even 4 months after parturition [21]. It is known that during pregnancy and after parturition, there are higher basal levels of circulating corticosterone compared to virgin female rats [50]. Elevated corticosterone levels are associated with HPA dysfunction and chronic injections of corticosterone in rats after parturition can recapitulate depressive-like symptoms in the FST and decreased maternal care [47]. Unexpectedly, we did not see any difference in levels of total plasma corticosterone between the two groups, again likely due to a high variability among the non-MS group. To better understand these results, in a follow up experiment additional plasma samples in a time course schedule could be collected during the postpartum period.
The NAc functions as the limbic-motor interface, connecting learned associations of rewarding stimuli to behaviour directed towards that stimuli [51]. Research is ongoing on the physiological and functional implications of the subset of medium spiny neurons in the mesolimbic dopaminergic pathways that express the D1–D2 heteromer [52]. It has been shown that activation of the D1–D2 heteromer resulted in depressive-like and anxiogenic-like behaviour measured in the FST and EPM in male rats; conversely, a selective antagonist that disrupted the complex abolished these effects [31]. In addition, there are lines of evidence that suggest a role for the heteromer in regulating brain reward in the NAc that impacts drug addiction [33, 53]. This is indicative of a potential role of D1–D2 heteromer in the pathophysiology of diseases that affect reward and anxiety pathways like depression. Our study is novel in that it is the first of its kind to study the heteromer in parturient rats in a model of postpartum depression. We demonstrate that the parturient dams seemed to have no change in overall D1–D2 heteromer expression in NAc compared to nonpregnant female rats. We have shown that adult female rats have a higher density of D1–D2 heteromer and heteromer expressing neurons in NAc compared to adult male rats [54] and the present study shows that female rats maintain the density of heteromer expressing neurons unchanged during pregnancy and the postpartum period. The NAc core and shell subregions function in separate ways to regulate active and tonic inhibition of natural reward mechanisms [37, 55]. We found no main effect of maternal separation on D1–D2 heteromer expression or signal count per cell.
The increased depressive-like behaviour being mild, rather than debilitating such as after much greater stressors, could have resulted in the observed increased care of pups by MS dams in this study after their retrieval with higher bouts of nursing, lower latency to nurse, lower bouts of out nest behaviour and decreased self-care.
To account for high inter individual variability seen in EPM and hormonal tests, a multiple correlation test was run for each parameter against every other parameter measured for each rat individually, regardless of maternal separation status, which showed just one significant correlation between sucrose preference and corticosterone. Higher anxiety-like behaviour is also found to be co-morbid with high corticosterone levels in rodent models of depression [18, 21, 56]. Some conflicting data exist on the anxiogenic effects of maternal separation determined by the EPM [55, 57] which could explain why we found no correlation. Interestingly, we found a weak negative correlation between EPM closed arm latency and D1–D2 heteromer expression and a weak positive correlation between immbolity score on the FST and D1–D2 heteromer expression. It is important to note statistical significance was not reached on these tests, but these data show further potential in implicating heteromer expression to manifestation of depression-like behaviour.
In summary, the maternal separation model produced mild depression-like and anxiety-like findings in dams subjected to maternal separation with no effect on anhedonic behaviour. Maternal separation increased maternal care of the pups, possibly due to the MS dams compensating for the absence of care immediately following the separation with marked reduction in self-grooming. Neuroendocrine results did not show any significant effect on HPA axis activation. This study shows that D1–D2 heteromer expression in either NAc core or NAc shell was not found to be statistically different between MS dams and non-MS dams. Also, only weak correlations were observed between the depressive and anxiety-like behaviour and the expression of heteromer in the NAc, probably due to the relatively mild depressive and anxiety-like effects of the maternal separation model on the dams. Thus, heteromer expression in NAc may be differentially regulated in a model of postpartum depression compared to stress-induced depression-like behaviour in nonpregnant female rats or with more significant stressors. Activation of D1–D2 heteromer in male rats induces anxiogenic-like and depression-like behavior. In the present study, MS dams showed only mild anxiogenic-like and depression-like behaviors. These moderate changes in behavior after maternal separation were accompanied by a tendency to higher D1–D2 expression in MS group compared to non-MS group. In future studies, it would be of interest to explore the functional differences in heteromer expression in NAc subregions with more significant stressors and during gestation. More research is needed to elucidate the etiology of postpartum depression in relation to dopaminergic pathways that involve the D1–D2 heteromer.
Data Availability
All data and supportive data are presented. The datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel SC (2015) Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol 11(1):99–137. https://doi.org/10.1146/annurev-clinpsy-101414-020426
Skalkidou A, Hellgren C, Comasco E, Sylvén S, Poromaa IS (2012) Biological aspects of postpartum depression. Women’s Heal 8(6):659–672. https://doi.org/10.2217/WHE.12.55
O’Hara MW, McCabe JE (2013) Postpartum depression: current status and future directions. Annu Rev Clin Psychol 9(1):379–407. https://doi.org/10.1146/annurev-clinpsy-050212-185612
Robertson E, Grace S, Wallington T, Stewart DE (2004) Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 26(4):289–295. https://doi.org/10.1016/J.GENHOSPPSYCH.2004.02.006
Leigh B, Milgrom J (2008) Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry 8(1):24. https://doi.org/10.1186/1471-244X-8-24
Putnam K (2015) Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiat 2(1):59–67. https://doi.org/10.1016/S2215-0366(14)00055-8
Field T (2010) Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev 33(1):1–6. https://doi.org/10.1016/J.INFBEH.2009.10.005
Bloch M, Meiboom H, Lorberblatt M, Bluvstein I, Aharonov I, Schreiber S (2012) The effect of sertraline add-on to brief dynamic psychotherapy for the treatment of postpartum depression. J Clin Psychiatry 73(02):235–241. https://doi.org/10.4088/JCP.11m07117
Miller LJ (2002) Postpartum depression. JAMA 287(6):762. https://doi.org/10.1001/jama.287.6.762
Perani CV, Slattery DA (2014) Using animal models to study post-partum psychiatric disorders. Br J Pharmacol 171(20):4539–4555. https://doi.org/10.1111/bph.12640
Ming L, Shinn-Yi C (2016) Modeling postpartum depression in rats: theoretic and methodological issues. Zool Res 37(4):229. https://doi.org/10.13918/J.ISSN.2095-8137.2016.4.229
Weiss IC, Domeney AM, Moreau J-L, Russig H, Feldon J (2001) Dissociation between the effects of pre-weaning and/or post-weaning social isolation on prepulse inhibition and latent inhibition in adult sprague-dawley rats. Behav Brain Res 121(1–2):207–218. https://doi.org/10.1016/S0166-4328(01)00166-8
Pedersen CA, Prange AJ, Sharma S, Francis D, Plotsky PM, Meaney MJ (1979) Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A 76(12):6661–6665. https://doi.org/10.1073/pnas.76.12.6661
Kojima S, Stewart RA, Demas GE, Alberts JR (2012) Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J Neuroendocrinol 24(5):831–840. https://doi.org/10.1111/j.1365-2826.2012.02280.x
Meaney M, Aitken D, van Berkel C, Bhatnagar S, Sapolsky R (1988) Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 239(4841):766–768. https://doi.org/10.1126/science.3340858
Levine S (2001) Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol Behav 73(3):255–260. https://doi.org/10.1016/S0031-9384(01)00496-6
Duman CH (2010) Models of depression. Vitam Horm 82:1–21. https://doi.org/10.1016/S0083-6729(10)82001-1
Aguggia JP, Suárez MM, Rivarola MA (2013) Early maternal separation: neurobehavioral consequences in mother rats. Behav Brain Res 248:25–31. https://doi.org/10.1016/J.BBR.2013.03.040
Omahony SM, Marchesi JR, Scully P et al (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2008.06.026
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res 18(3):195–200. https://doi.org/10.1016/0169-328X(93)90189-V
Maniam J, Morris MJ (2010) Long-term postpartum anxiety and depression-like behavior in mother rats subjected to maternal separation are ameliorated by palatable high fat diet. Behav Brain Res 208(1):72–79. https://doi.org/10.1016/J.BBR.2009.11.005
Boccia ML, Razzoli M, Prasad Vadlamudi S, Trumbull W, Caleffie C, Pedersen CA (2007) Repeated long separations from pups produce depression-like behavior in rat mothers. Psychoneuroendocrinology 32(1):65–71. https://doi.org/10.1016/J.PSYNEUEN.2006.10.004
Wisner KL, Chambers C, Sit DKY (2006) Postpartum depression. JAMA 296(21):2616. https://doi.org/10.1001/jama.296.21.2616
Fiorelli M, Aceti F, Marini I et al (2015) Magnetic resonance imaging studies of postpartum depression: an overview. Behav Neurol 2015:1–7. https://doi.org/10.1155/2015/913843
Sharma V, Khan M (2010) Identification of bipolar disorder in women with postpartum depression. Bipolar Disord 12(3):335–340. https://doi.org/10.1111/j.1399-5618.2010.00809.x
Wu J, Xiao H, Sun H, Zou L, Zhu L-Q (2012) Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol 45(3):605–620. https://doi.org/10.1007/s12035-012-8278-5
Duan C, Cosgrove J, Deligiannidis KM (2017) Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep 19(10):70. https://doi.org/10.1007/s11920-017-0824-4
Pei L, Li S, Wang M et al (2010) Uncoupling the dopamine D1–D2 receptor complex exerts antidepressant-like effects. Nat Med 16(12):1393–1395. https://doi.org/10.1038/nm.2263
Beaulieu J-M, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63(1):182–217. https://doi.org/10.1124/pr.110.002642
Haim A, Sherer M, Leuner B (2014) Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur J Neurosci 40(12):3766–3773. https://doi.org/10.1111/ejn.12752
Shen MYF, Perreault ML, Bambico FR et al (2015) Rapid anti-depressant and anxiolytic actions following dopamine D1–D2 receptor heteromer inactivation. Eur Neuropsychopharmacol 25(12):2437–2448. https://doi.org/10.1016/J.EURONEURO.2015.09.004
Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14(9):609–625. https://doi.org/10.1038/nrn3381
Hasbi A, O’Dowd BF, George SR (2011) Dopamine D1–D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain 4:26. https://doi.org/10.1186/1756-6606-4-26
Hasbi A, Fan T, Alijaniaram M et al (2009) Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A 106(50):21377–21382. https://doi.org/10.1073/pnas.0903676106
Brummelte S, Galea LAM (2016) Postpartum depression: etiology, treatment and consequences for maternal care. Horm Behav 77:153–166. https://doi.org/10.1016/J.YHBEH.2015.08.008
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328. https://doi.org/10.1038/nprot.2007.44
Hasbi A, Perreault ML, Shen MYF et al (2017) Activation of dopamine D1–D2 receptor complex attenuates cocaine reward and reinstatement of cocaine-seeking through inhibition of DARPP-32, ERK, and ΔFosB. Front Pharmacol 8:924. https://doi.org/10.3389/fphar.2017.00924
Fernandez JW, Grizzell JA, Philpot RM, Wecker L (2014) Postpartum depression in rats: differences in swim test immobility, sucrose preference and nurturing behaviors. Behav Brain Res 272:75–82. https://doi.org/10.1016/j.bbr.2014.06.041
Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG (2003) Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav 43(5):561–567. https://doi.org/10.1016/S0018-506X(03)00063-1
Orso R, Wearick-Silva LE, Creutzberg KC et al (2018) Maternal behavior of the mouse dam toward pups: implications for maternal separation model of early life stress. Stress 21(1):19–27. https://doi.org/10.1080/10253890.2017.1389883
Bailoo JD, Jordan RL, Garza XJ, Tyler AN (2014) Brief and long periods of maternal separation affect maternal behavior and offspring behavioral development in C57BL/6 mice. Dev Psychobiol 56(4):674–685. https://doi.org/10.1002/dev.21135
Yin X, Chen L, Xia Y et al (2016) Maternal deprivation influences pup ultrasonic vocalizations of C57BL/6J mice. PLoS ONE 11(8):e0160409. https://doi.org/10.1371/journal.pone.0160409
Navarre BM, Laggart JD, Craft RM (2010) Anhedonia in postpartum rats. Physiol Behav 99(1):59–66. https://doi.org/10.1016/j.physbeh.2009.10.011
Wang T, Shi C, Li X et al (2018) Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviors in the postpartum depression rat model. Behav Brain Res 336:236–243. https://doi.org/10.1016/J.BBR.2017.09.012
Shalev U, Kafkafi N (2002) Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 73(1):115–122. https://www.ncbi.nlm.nih.gov/pubmed/12076730 Accessed 1 May 2019
Genty J, Tetsi Nomigni M, Anton F, Hanesch U (2018) The combination of postnatal maternal separation and social stress in young adulthood does not lead to enhanced inflammatory pain sensitivity and depression-related behavior in rats. PLoS ONE 13(8):e0202599. https://doi.org/10.1371/journal.pone.0202599
Workman JL, Brummelte S, Galea LAM (2013) Postpartum corticosterone administration reduces dendritic complexity and increases the density of mushroom spines of hippocampal CA3 arbours in dams. J Neuroendocrinol 25(2):119–130. https://doi.org/10.1111/j.1365-2826.2012.02380.x
Li M, Chou SY (2016) Modeling postpartum depression in rats: theoretic and methodological issues. Dong wu xue yan jiu. Zool Res 37(4):229–236. https://doi.org/10.13918/j.issn.2095-8137.2016.4.229
Strathearn L, Iyengar U, Fonagy P, Kim S (2012) Maternal oxytocin response during mother-infant interaction: associations with adult temperament. Horm Behav 61(3):429–435. https://doi.org/10.1016/j.yhbeh.2012.01.014
Kalyani M, Callahan P, Janik JM, Shi H (2017) Effects of pup separation on stress response in postpartum female rats. Int J Mol Sci. https://doi.org/10.3390/ijms18071370
Ito R, Hayen A (2011) Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci 31(16):6001–6007. https://doi.org/10.1523/JNEUROSCI.6588-10.2011
Perreault ML, Hasbi A, O’Dowd BF, George SR (2011) The dopamine d1–d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat 5:31. https://doi.org/10.3389/fnana.2011.00031
Yen M, Shen F 2019 The Role of the Dopamine D1-D2 Receptor Heteromer in Brain Reward Function: Relevance to Drug Addiction and Depression. https://tspace.library.utoronto.ca/bitstream/1807/82526/1/Shen_Yen_F_201511_PhD_thesis.pdf Accessed 1 May 2019
Hasbi A, Nguyen T, Rahal H et al (2020) Sex difference in dopamine D1–D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol Sex Differ. https://doi.org/10.1186/s13293-020-00285-9
Kwak HR, Lee JW, Kwon K-J et al (2009) Maternal social separation of adolescent rats induces hyperactivity and anxiolytic behavior. Korean J Physiol Pharmacol 13(2):79–83. https://doi.org/10.4196/kjpp.2009.13.2.79
El Tanbouly N, El Sayed AM, Ali ZY et al (2017) Antidepressant-like effect of selected egyptian cultivars of flaxseed oil on a rodent model of postpartum depression. Evid Based Complement Alternat Med 2017:6405789. https://doi.org/10.1155/2017/6405789
Savignac HM, Dinan TG, Cryan JF (2011) Resistance to early-life stress in mice: effects of genetic background and stress duration. Front Behav Neurosci 5:13. https://doi.org/10.3389/fnbeh.2011.00013
Funding
This project was funded by grants from NIDA (DA042178) and CIHR (PJT-148633) for SRG.
Author information
Authors and Affiliations
Contributions
AH and SRG: proposed the project. MN, AH, and SRG: designed the experiments. MN, AH, MS, MM: performed experiments and analysis. MN, AH and SRG: interpreted the results. MN, AH and SRG: wrote the manuscript. MN, AH and SRG: proofread the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All Authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
All procedures in this study were carried out in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993). The protocol was approved by the University of Toronto Animal Use Protocol Committee.
Consent for publication
All authors read and approved the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noori, M., Hasbi, A., Sivasubramanian, M. et al. Maternal Separation Model of Postpartum Depression: Potential Role for Nucleus Accumbens Dopamine D1–D2 Receptor Heteromer. Neurochem Res 45, 2978–2990 (2020). https://doi.org/10.1007/s11064-020-03145-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03145-5