Abstract
The role of potassium channels provides suggestive evidence for the etiology of autism. The voltage-gated potassium channel Kv10.2 (KCNH5) is widely expressed in the brain. However, the inherent relationship between Kv10.2 and autism is still unclear. Herein, a rat valproic acid (VPA)-induced autism spectrum disorder model was established. The expression level of Kv10.2 was obviously decreased in the hippocampus of VPA rats. Kv10.2 was mainly localized in neurons. Subsequently, a recombinant lentivirus expressing Kv10.2 was used to upregulate the expression of Kv10.2 in the hippocampus of VPA-exposed rats. The results were promising as injection of the Kv10.2 lentivirus in the hippocampus relieved anxiety and stereotypical behavior, and improved the social and exploratory abilities of rats that were prenatally exposed to VPA. In addition, spectral analysis of electroencephalogram data revealed that animals exposed to VPA exhibited increased high-frequency activity compared with the control rats, and this activity recovered to a certain extent after upregulation of Kv10.2 expression by lentivirus injection. These results suggest that changes in Kv10.2 may play an important role in the etiology of autism, thus providing a promising direction for further research on autism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a highly heterogeneous neurodevelopmental disorder characterized by prevalent impairments in social interactions, deficits in verbal or non-verbal communication and repetitive or rigid behavior [1]. Genetic, environmental, neurologic and immunological factors are involved in the etiology of ASD [2]. Valproic acid (VPA), a commonly used anti-convulsant mood stabilizer, can increase the risk of ASD development in humans and rodents during critical periods of neural tube closure [3, 4]. The VPA exposure model is considered one of the best-validated models for ASD study and an excellent tool to examine the etiology, gene expression and neuronal functioning of ASD [5, 6].
Several brain regions are involved in the development of autism, including the cerebellum, amygdala, hippocampus and multiple cortical areas. The hippocampus is a crucial marginal structure that is located in the left and right hemispheres of the brain and participates in cognition, learning and emotion [7, 8]. Courchesne et al. reported that the frontal cortex was larger in autistic patients after death [9]. Autistic children exhibited changes in the shape of the hippocampus as measured by magnetic resonance imaging [10]. Bauman and Kemper reported abnormalities in the CA1 region and subiculum of the hippocampal formation in autistic patients after death that mainly included abnormally small and dense cells [7]. Furthermore, a series of changes occur in the hippocampus in the autism model, such as pyramidal cell loss [11], apoptosis and neuro-inflammatory dysfunction [12]. A reduction in dendritic branches has been reported in the CA3 area from ASD brains upon postmortem examination [13]. Lentiviral-mediated overexpression of brain-derived neurotrophic factor in the dentate gyrus of the hippocampus reversed neonatal isolation-induced anxiety- and autism-like behaviors [14]. Roman Tyzio et al. reported an abnormal GABA sequence in hippocampal CA3 pyramidal neurons of VPA rats. Bumetanil therapy rescued GABA sequence abnormalities and the autism-like features [15]. As an important member of the limbic system, the hippocampus is of great significance for research and has become a target for treatment of neurodevelopmental disorders.
The molecular mechanisms of ASD pathophysiology are still not fully understood. In recent years, potassium channels have attracted widespread attention regarding the pathophysiology of various mental disorders [16]. Potassium channels are widely present in many excitable or non-excitable cells and are involved in cellular electrical impulse and endocrine regulation. Dysfunction of potassium channels somehow participates in the development of ASD [17, 18]. The rs74582884 single nucleotide polymorphism in potassium voltage-gated channel subfamily Q member 3 (KCNQ 3) has been reported to impair the function of the Kv7.3/Kv7.5 channel complex in autistic patients [19]. In Fmr1 knockout mice, an animal model of fragile X syndrome, Kv4.2 is indirectly involved in the regulation of autism by affecting long-term potentiation [20]. KCNH5 is one of two KCNH genes (KCNH1 and KCNH5) that encodes the Kv10 family of voltage-gated potassium channels. KCNH5 is widely expressed in the central nervous system at both the RNA and protein levels. The voltage-gated potassium channel subunit Kv10.2 is mainly distributed in most neural tissues including the cortex, olfactory bulb, hippocampus and thalamus, but not in the striatum and pituitary [21, 22]. However, the physiological function and expression of Kv10.2 in the nervous system of VPA-exposed model rats remain largely unexplored.
In this study, rat fetuses exposed to VPA on day 12.5 of pregnancy were established as a rat autism model. Confocal microscopic immunofluorescence analysis, RT-PCR and western blotting were used to investigate the expression of Kv10.2 in the hippocampus. Then, lentiviral vectors were injected to produce overexpression of Kv10.2 in the hippocampus of adult rats to further confirm the effects of Kv10.2 with regard to autism. Finally, a spectral analysis of electroencephalogram (EEG) data was conducted to explore whether the pathological mechanism of ASD was related to neural circuit abnormality.
Results
Expression of Kv10.2 in the Hippocampus
Immunofluorescence staining results showed that the Kv10.2 channel was expressed in the hippocampus (CA3, CA1 and hilus, Fig. 1a left panel). To investigate the types of neural cells that expressed Kv10.2, brain tissue was double-labeled for Kv10.2 and specific makers of neurons, microglia and astrocytes. Confocal microscopic analysis revealed that Kv10.2 was co-localized with neurons (Fig. 1a), rather than astrocytes or microglia (Fig. 2). The number of Kv10.2-positive cells was significantly decreased (P < 0.001) in the hippocampus of the VPA group compared with control rats (Fig. 1a–e). The basal protein and mRNA expression levels of Kv10.2 in control and VPA rats were determined using western blotting and quantitative real-time PCR. The Kv10.2 protein expression level was significantly reduced in the VPA group compared with the control group (Fig. 1f). Accordingly, the mRNA level of Kv10.2 was also decreased in VPA-induced autism model rats (Fig. 1g).
Expression of Kv10.2 in the hippocampus of VPA-induced autism rats and the control groups. a Co-localization of Kv10.2 (red) and NeuN (green) in the hippocampus of the control group. b Co-localization of NeuN (green) and Kv10.2 (red) in the hippocampus of VPA-induced autism model rats. c–e Kv10.2- and NeuN-positive cells in the hippocampus. Quantification of the number of Kv10.2-positive cells in the CA3 (c), CA1 (d) and hilus (e) subareas of the hippocampus. NeuN was used as a neuronal cell marker. f Kv10.2 and GAPDH protein expression in the hippocampus in VPA-induced autism model rats and the control groups. g The mRNA fold change of Kv10.2. Values are expressed as the mean ± SEM. Scale bar represents 50 µm. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group. n = 5 (Color figure online)
Co-localization of Kv10.2 (red) with NeuN (green), GFAP (green) and Iba1 (green) in the hippocampus of VPA-induced autism model rats. Kv10.2 was co-localized with neurons (a) rather than co-expressed with the astrocyte marker GFAP (b) or the microglia marker Iba1 (c). d–f Kv10.2-positive cells in the hippocampus (Color figure online)
Behavior Changes Induced by Microinjection of Kv10.2 gRNA Lentivirus in the Hippocampus
After the establishment of the VPA-induced autism model in rats, postnatal growth and maturity development indicators were detected. Autistic model rats exhibited delayed neurodevelopment compared with the control group (Additional file 1: Fig. s1). These manifestations of morphological and physiological characteristics were in accordance with previous literature [23]. Subsequently, a series of behavioral experiments was performed on rats at 35 days after birth. Autistic model rats showed severe social disorders (Additional file 1: Fig. s2a, 2b), anxiety (Additional file 1: Fig. s2c–e) and stereotypical behavior (Additional file 1: Fig. s2f). All male rats of the appropriate age were used for the following behavioral and molecular experiments.
To determine whether overexpression of Kv10.2 in the hippocampus can ameliorate autism-like behavior, VPA-induced rats were infused with Kv10.2-expressing lentivirus vectors, and behavioral experiments were conducted 15 days later.
Effect on Social Interaction
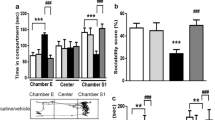
Figure 3a, b illustrates the social characteristics of rats. VPA rats administered Lv-GFP were used as the negative control group. In the first stage, social characteristics were evaluated. Significant sociability was observed in the control group, control + Lv-GFP group and control + Lv-KCNH5 group, which tended to spend more time in the chamber with the unfamiliar animal (stranger 1) than in the chamber with the object. In contrast to the control rats, VPA rats spent time in both chambers with no significant difference. VPA rats administered Lv-GFP also exhibited a lack of sociability. In contrast, VPA rats administered Lv-KCNH5 displayed increased sociability, that is, they spent significantly more time in the chamber with stranger 1 than in the chamber with the object (Fig. 3a). In the second stage, the social novelty preferences were investigated. Rats in the control group spent more time with stranger 2 than stranger 1, while VPA rats showed no significant difference in the time spent in the two chambers. However, after overexpression of Kv10.2, the social disorder of rats was improved, and normal social behavior was observed (Fig. 3b).
Effect of Kv10.2 overexpression on animal social deficits. a, b A recombinant lentivirus expressing Kv10.2 injected into VPA rats improved their social preference. All data were analyzed using the Kruskal–Wallis test and Dunn's test for post hoc analysis (mean ± SEM). c Percentage of time spent in open arms in the elevated plus maze test. d Percentage of time spent in the light box in the black and white box test. e Time spent in the center area in the open field test. f Percentage of spontaneous alternation in the Y maze. Data are expressed as the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group. #P < 0.05 versus the VPA group and the VPA + Lv-GFP group, n = 10
Impact on Anxiety
The elevated plus maze is considered an effective rodent anxiety behavior test. The results showed that the VPA rats were not inclined to enter the open arms and spent less time in the open arms than the control rats, which directly indicated the increased anxiety level of the VPA rats. The control + Lv-GFP group and the control + Lv-KCNH5 group showed normal emotions without anxiety. However, the VPA + Lv-KCNH5 rats spent a significantly increased percentage of time entering and exploring the open arms compared with the VPA rats (Fig. 3c). The same result was obtained in the black and white box test. The control rats spent a longer time in the white box than in the black box, while VPA rats spent less time in the white box than the control group, which reflected the anxious behavior of VPA rats. The time spent in the white box by the VPA + Lv-KCNH5 group was significantly greater than that of the VPA group (Fig. 3d). These results suggested that overexpression of Kv10.2 in the hippocampus can alleviate anxiety in VPA rats.
Impact on Exploratory Activity
The open field test is considered a reliable indicator of exploratory behavior. In this test, rats in the control group, control + Lv-GFP group and control + Lv-KCNH5 group spent more time in the central region, which reflected normal exploratory behavior of the animals. The VPA rats spent markedly less time in the central region than the control rats, while the VPA + Lv-KCNH5 rats spent more time in the central region than the VPA rats as depicted in Fig. 3e.
Impact on Repetitive Behavior
A Y maze device was used to investigate repetitive behavior. The spontaneous alternation rate of VPA rats was significantly lower than that of control rats, suggesting increased repetitive behavior in VPA rats. The control group, control + Lv-GFP group, control + Lv-KCNH5 group showed normal behavior. The VPA + Lv-KCNH5-administered rats exhibited a significantly increased spontaneous alternation rate compared with that of the VPA rats as shown in Fig. 3 f. This result suggested that Kv10.2 overexpression attenuates VPA-induced rat anxiety and autistic behaviors.
Evaluation of Kv10.2 Protein Levels in the Hippocampus Following Lentiviral Injection
To explore the role of hippocampal Kv10.2 in autism-like behaviors, a Kv10.2 gRNA lentiviral vector and a nontargeted lentiviral vector (2.1 × 106 TU of purified vector) were delivered into the hippocampus of VPA rats. Although the protein levels of hippocampal Kv10.2 in VPA rats were not rescued to the control level, they were remarkably upregulated in VPA rats after Kv10.2 gene delivery (Fig. 4a, b), indicating that the Kv10.2 lentivirus could effectively increase Kv10.2 expression at the translational level.
Basal expression of Kv10.2 in the hippocampus. a, b Western blot analysis revealed that Kv10.2 protein levels were significantly decreased in the VPA and VPA + Lv-GFP groups. However, the expression level of Kv10.2 was significantly increased in the VPA + Lv-KCNH5 group compared with the VPA and the VPA + Lv- GFP groups. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, #P < 0.05 versus the VPA and VPA + Lv-GFP groups, n = 7
Kv10.2 Overexpression Reversed Abnormal EEG Results
To understand the effect of Kv10.2 overexpression on the neurophysiological characteristics of ASD rats, EEG data were assessed. The recordings showed that VPA rats exhibited increased activity in the theta (4–80 Hz), alpha (8–13 Hz), beta (13–30 Hz), low gamma (30–60 Hz) and high gamma (60–100 Hz) frequency ranges, but not in the delta range (0.5–4 Hz). The frequency and amplitude of data recorded in the control rats were lower than those in the VPA group. However, injection of Kv10.2-overexpressing lentivirus into the hippocampus decreased the power of network oscillation and thus quelled the overactive state of autistic rats (Fig. 5a–e).
Injection of a recombinant lentivirus expressing Kv10.2 in the hippocampus restores brain oscillations in the VPA model. a LFPs recording from the control group, control + Lv-GFP group, control + Lv-KCNH5 group, VPA group, VPA + Lv-GFP group and Lv-KCNH5 group after band-pass filtering. b–d The 0–13 Hz, 13–30 Hz, 30–100 Hz EEG of the control group, the control group + Lv-GFP group, the control group + Lv-KCNH5 group, the VPA group, the VPA + Lv-GFP group and the Lv-KCNH5 group were recorded after band-pass filtering. e Integral power of delta (0.5 to 4 Hz), theta (4 to 8 Hz), alpha (8 to 13 Hz), beta (13 to 30 Hz), low gamma (30 to 60 Hz) and high gamma (60 to 100 Hz) band components of EEG recordings. VPA group and VPA + Lv-GFP group vs. control group: for alpha, beta, low gamma and high gamma, *P < 0.01, for theta, **P < 0.001; Lv-KCNH5 group versus VPA group and VPA + Lv-GFP group, for alpha, beta, low gamma and high gamma, *P < 0.05, for theta, **P < 0.01. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group. #P < 0.05 versus the VPA group and the VPA + Lv-GFP group. ##P < 0.01 versus the VPA group and the VPA + Lv-GFP group
Discussion
Kv10.2 is a known target for the diagnosis and treatment of tumors, such as medulloblastoma [24], renal cell carcinoma [25] and esophageal adenocarcinoma [26]. Nevertheless, few studies have revealed the relationship between Kv10.2 and VPA-induced autism. Veeramah et al. studied a Kv10.2-R327H residue mutation in a patient with an epilepsy/autism mutation [27]. On the basis of this research, it was assumed that Kv10.2 may play some role in the development of autism. In the present study, we probed the expression and distribution of Kv10.2 in the hippocampus of rats with autism-like behaviors induced by VPA as well as the changes in these behaviors after overexpression of Kv10.2 via a lentivirus. We verified that the protein and mRNA levels of Kv10.2 were significantly decreased in the hippocampus of the VPA group. In addition, we confirmed that Kv10.2 was mostly located in neurons, rather than astrocytes or microglia. Furthermore, expression of the Kv10.2 protein was significantly increased after injection of a lentivirus that mediated overexpression of Kv10.2. The behavioral experiments confirmed that the anxiety, stereotypical behaviors and social disorders were alleviated in autistic rats to some degree. In addition, EEG recordings showed that brain oscillations were also reversed to some extent. Our results suggested that the expression of Kv10.2 in hippocampal neurons contributes to the development of autism, which has not been reported previously.
VPA is a medication used to treat epilepsy and other psychiatric diseases. An increased incidence of autism is observed when children or rodents are prenatally exposed to VPA [3]. Our results showed that pups exposed to VPA had slower neurodevelopment and motor development than normal rats, which is consistent with the ASD model. Interestingly, M Nica R. Favre et al. suggested that offspring treated with VPA exhibited no significant difference in weight, and we found the same result. Schneider et al. reported that VPA exposure during pregnancy caused serious anxiety, stereotypical behavior and reduced interest in communication with rodents of the same age [28]. The similarity of these behavioral deficits to those of autistic patients highlights the validity of the rat model and the robustness of these phenotypes. A wide variety of phenotypes and pathogeneses have been studied using such VPA-induced ASD animals.
Potassium channels play an important role in the central nervous system, which participates in the regulation of activities necessary for life by controlling neuronal excitability, shaping action potential waveforms and regulating metabolism. Yang et al. identified an R327H mutation of the Kv10.2 gene by whole exome sequencing in a patient with epileptic encephalopathy accompanied by autism. The R327H mutation of Kv10.2 weakened the interaction between residue 327 and the negatively charged S1-S3 residue in the resting state but not in the activated state, which caused the channel to open more easily. This is the first convincing evidence that Kv10.2 channels play a role in human neurological diseases [29]. De Oliveira et al. showed that recovery of Kv10.2 expression in the hippocampus and prefrontal cortex of rats with transient cerebral ischemia was accompanied by a reversal of anxiety. This result suggested that Kv10.2 may be associated with cognition and anxiety [30]. In our research, expression of Kv10.2 in the hippocampus of VPA rats was significantly decreased at both the protein and mRNA levels. The VPA rats showed severe anxiety compared with the control group in the elevated plus maze and black and white box experiments, while the anxiety of the rats was effectively alleviated with recovery of Kv10.2 in the hippocampus. On the basis of these findings, we suspected that Kv10.2 may be involved in the process of anxiety in the VPA model. It is notable that anxiety is one of the typical manifestations of autistic patients [31]. In recent years, a growing recognition of the importance of potassium channels in the pathogenesis of autism has occurred. The sensitivity of autism could be significantly increased by ion channel defects [17]. In our Y maze behavioral test, the VPA rats exhibited significant stereotypical behaviors compared with the control rats. Similarly, the stereotypical behavior was ameliorated with the recovery of Kv10.2 expression. We speculated that Kv10.2 may affect stereotypical behavior by altering the sensitivity of autistic rats. Deficits in social communication are core symptoms of autism. In this research, restoring the expression of Kv10.2 alleviated social disorders to a certain extent, suggesting that Kv10.2 may play a role in the VPA autism model. Notably, restoring Kv10.2 expression in hippocampal neurons was sufficient to alleviate impaired sociability. However, prenatal VPA exposure induces complex teratogenic effects in various brain regions. These effects include changes in synaptic function, alterations of intrinsic neuronal activity and impaired expression of numerous ion channels and neurotransmitter receptors. Kv10.2 may play a role in alleviating the impairment of social behaviors through these mechanisms, which will be a direction of our follow-up research.
The pathological mechanism of neurodevelopmental disorders is generally associated with abnormalities in the neural circuit, as is autism [32]. Abnormalities in all frequency bands (delta, theta, alpha, beta, and gamma) have been reported in autistic patients [33]. Previous EEG/magnetoencephalography studies have shown that abnormal gamma oscillations reflect core features of autism with various disorders [34]. The gamma band is also thought to be related to cognition, attention and sensory processing. In this study, Kv10.2 overexpression ameliorated overactivity of phase-locked gamma oscillations in VPA rats. These results suggest that Kv10.2 expression in hippocampal neurons of autistic model rats may have a biological effect on brain function, and Kv10.2 may be involved in the occurrence of autism by affecting the balance between excitability and inhibition. However, additional animal models of autism are still needed to further verify and understand the internal function of Kv10.2 in the development of autism.
Materials and Methods
Animals
All efforts were made to minimize the number of animals used and their suffering. The animal experiments were approved in advance by the Animal Protection and Utilization Committee (Department of Laboratory Animal Science, Shanghai University) and were performed in strict accordance with the Institutional Animal Care and Use Program for Experimental Animals of Shanghai University.
Male and female Sprague-Dawley (SD) rats weighting 270–350 g were obtained from the Experimental Animal Center of the Chinese Academy of Sciences in Shanghai, China. All rats were housed individually under standard housing conditions (23 ± 2 °C; 55% ± 5% humidity) with a 12 h light-dark cycle.
Adult male and female rats were allowed to mate overnight. The day of detection of a vaginal embolus was recorded as day 0.5 of pregnancy. A single-dose intraperitoneal injection of VPA (500 mg/kg in 0.85% saline) was given to adult female rats on day 12.5 of gestation. The same dose of saline was injected at the same time in the control group. Female rats were allowed to raise the immature rats freely until weaning on the 23rd postnatal day. Subsequently, male rats were selected as experimental subjects and divided into six groups with six rats in each group and were fed separately. The behavioral assessment of rats was conducted at 6–8 weeks.
In this study, male offspring were divided into six groups (n = 10).
Group I: control group: male offspring of saline-treated females were used as the control group.
Group II: control + Lv-GFP group: Lv-GFP was delivered to the hippocampus of control rats.
Group III: control + Lv-KCNH5 group: Lv-KCNH5 was delivered to the hippocampus of control rats.
Group IV: VPA group: Pregnant female rats were administered VPA (500 mg/kg in 0.85% saline), and their male pups were used as the VPA group.
Group V: VPA + Lv-GFP group: Lv-GFP was delivered to the hippocampus of autism model rats.
Group VI: VPA + Lv-KCNH5 group: Lv-KCNH5 was delivered to the hippocampus of autism model rats.
Postnatal Growth and Maturation
Weight Gain
Weight gain was measured on postnatal days 7, 14, 21, 28, 35 and 56.
Eye Opening
Eye opening was observed on postnatal days 12, 13, 14, 15 and 16. Scoring criteria: 0 points: neither eye was open; 1 point: one eye was open; 2 points: both eyes were open.
Behavioral Determinations
Behavioral development tests were performed on pups according to the procedure described by St Omer et al. [35]. All experiments were performed on a group of 20 animals (five rats/four litters/group).
Plane Correction
The pups were placed horizontally with their abdomen upward on a table. The number of days after birth when the pups could complete a 180° rotation with all four feet landing within 3 s was observed and recorded.
Cliff Escape
The number of days until offspring showed a withdrawal trend within 15 s after being placed on the edge of a table was recorded.
Negative Geotaxis
This behavior was recorded according to the time spent turning 180° after losing grip with the paws, and the threshold was set to 30 s when the head of the offspring was placed downward on a rough wooden slope of 25°. The offspring were examined each day from postnatal day 7 to 10. Negative geotaxis reflects the development of vestibular and motor function.
Swimming Performance
An aquarium (water temperature 27–28 °C) was used for the swimming test. This behavior was observed for 5–10 s, and the scores were recorded on postnatal days 8, 10, 12 and 16. Scoring criteria: 0 points—head and nose below the water surface, 1 point—head above the water surface, nose below the water surface, 2 points—head and nose above the water surface, ear below the water surface, 3 points—head and nose above the water surface, with the water level at the middle of the ear, 4 points—the head, nose and ear were higher than the water level. At the end of each observation, the offspring were dried with a towel to prevent adverse effects caused by cold exposure.
Three-Chamber Social Test
The device consisted of two side chambers (30 (length) × 35 (width) × 35 (height) cm), a central chamber (15 (length) × 35 (width) × 35 (height) cm) and two identical transparent cylinders [31]. The three chambers were interconnected. In the adaptation phase, two empty cylinders were placed in the side chambers. The rats were placed in the central chamber, and their behavior in the three chambers was recorded for 10 min. In the first phase of the social ability test, an unfamiliar SD rat (stranger 1) was placed in the left chamber, the right side consisted of a cage, and the behavior of the rat was monitored for 10 min in the three chambers. During the social novelty test, a new unfamiliar SD rat (stranger 2) was placed in the right chamber, and the left side contained stranger 1. The communication of the test rat with stranger 1 and stranger 2 was monitored for 10 min. Ethovision was used to automatically calculate the time spent in each area.
Open Field Test
The open field test is a classic anxiety and exploratory behavioral test in rodents. A wooden open box with a length × width × height of 100 × 100 × 40 was used in this test. The walls of the box were colored black. The bottom of the box was divided into 25 equal squares. Nine small squares in the central area were designated as the central squares. The total number of times that rats crossed the box in 5 min was observed by Ethovision automatic tracking analysis to reflect the autonomous behavior of rats. The number of times that rats crossed the middle square reflected the exploratory behavior of rats in a novel environment.
Elevated Plus Maze Test
The elevated plus maze reflected the anxiety of animals based on the tendency of the animal to remain in the closed arm vs. exploring the open arms. The device consists of two open arms, two closed arms and a common central platform that is located 40 cm from the ground. The rat was placed on the platform facing the open arm. The time that the rat remained in each arm and the number of times an arm was entered were observed and recorded for 5 min.
Black and White Box
The black and white box test consisted of a white box and a black box. The rats could move freely between the two boxes. The rat to be tested was placed in the white box. The time spent in each box and the number of times the rat moved between boxes were observed and recorded for 10 min. Anxious behavior was represented by an increased percentage of time spent in the white box compared with the black box.
Y Maze
Repetitive behavior of animals is an important indicator of ASD. The Y maze apparatus (42 cm × 15 cm × 14 cm) was used to study the repetitive behavior of rats [36]. The Y maze device consisted of three identical arms. Rats were placed on the starting arm of the Y maze and allowed to randomly explore for 10 min. The spontaneous alternation rate was recorded as an indicator of repetitive behavior.
Western Blotting
Total membrane protein from the hippocampus was prepared using a ProteinExt mammalian membrane protein extraction kit (TransGen Biotech, DE301). A BCA assay kit (Beyotime, Shanghai, China) was used to quantify the protein concentration in the hippocampus. Proteins were loaded onto 6% SDS-PAGE gels and then transferred to a PVDF membrane (0.45 µm, Millipore, Billerica, USA). After incubating with QuickBlockTM Western Blocking Buffer (Beyotime Biotechnology, P0252) for 30 min, the primary antibody was added, and the membrane was incubated overnight at 4 °C. The antibodies used included rabbit anti-EAG2 (1:200, Alomone) and mouse anti-GAPDH (1:1000, TransGenBiotech). After washing with PBST, the membranes were incubated for 1.5 h with peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:10,000 Santa Cruz biotechnology USA) at room temperature. The signals were detected using an ECL chemiluminescence kit (Beyotime Biotechnology, P0018AS). Immunoblots were quantified using Image J software and normalized according to GAPDH immunoreactivity.
Immunofluorescence
The rat tissues were fixed in 4% paraformaldehyde and then post fixed in sequential buffers containing 25% and 30% sucrose. The brain tissue was serially sliced with a frozen slicer (thickness 30 µm). The primary antibodies included rabbit anti-EAG2 (Alomone Labs, 1:400), mouse anti-neuron (Abcam, 1:400), goat polyclonal antiserum to Iba1 (1:400; Abcam) and mouse polyclonal antiserum to GFAP (1:400; Abcam). The secondary antibodies included Alexa Fluor 488 anti-goat (1:500; Abcam) and Alexa Fluor 647 anti-mouse (1:500; Abcam) for neurons and GFAP and Alexa Fluor 555 anti-rabbit (1:400; Abcam) for Kv10.2. Hippocampus sections were scanned and imaged by a laser scanning confocal microscope (LSM 710; Carl Zeiss, Germany). The neuronal cell-positive areas were analyzed with Image J software. The data were assessed by an investigator who was blinded to the treatment group of the animals.
RNA Isolation, cDNA Synthesis and Real-Time PCR
Total RNA was isolated from the hippocampus using an EasyPure RNA kit (TransGen Biotech) and reverse transcribed into cDNA by cDNA Synthesis SuperMix (TransGen Biotech). All processes were in accordance with manufacturer’s instructions. Quantitative real-time PCR was performed using TransStart Top Green qPCR Mix (TransGen Biotech). The gene-specific primer sequences for Kv10.2 were forward 5′-TGTATGCCAACACCAACCG-3′ and reverse 5′-ACACTCTCTCCAGCATGGTA-3′. The gene-specific primer sequences for β-actin were forward 5′-CGCGAGTACAACCTTCTTGCAG-3′ and reverse 5′- ACTATCGGCAATGATCGGTTCC-3′.
Injections of Lentivirus Into the Hippocampus
The Kv10.2 gRNA lentivirus (Lv-KCNH5) and nontargeting lentivirus (Lv-GFP) were produced and purified by Hanbio Co. Ltd. Shanghai, China. In brief, BamH I and EcoR I were considered as insertion sites, located in the MCS (polyclonal site) region after the EF1a promoter. The inserted base sequence consisted of the CDS region sequence of Gene ID: 171146 and the 3FLAG tag. The lentiviral vector expression plasmids (pLenti-EF1A-GFP-Puro) were co-transfected into human embryonic kidney 293T cells, along with the packaging construct plasmid pSPAX2 and the envelope plasmid pMD2G, to produce the viral particles [37, 38]. Then, we delivered the Lv-KCNH5 and Lv-GFP vectors into the hippocampus. The lentiviral vectors (5 × 108 TU) were microinjected bilaterally into the CA3 region of the hippocampus at the following coordinates of the Paxinos (2004) Atlas (4.0–4.2 mm posterior to bregma, 4.1–4.3 mm lateral to the midline and 3.9–4.1 mm below the cortical surface). A volume of 3 µL was injected for 15 min using a microprocessor-controlled syringe pump (Stoelting, Wood Dale, IL). Fifteen days after infection, the animals were used for behavioral testing and protein extraction.
Electroencephalographic Recordings
The operation was performed as described in a previous report [39]. Adult rats were deeply anesthetized with isoflurane. The skin and periosteum were cleared to expose the skull; then, pre-processed microelectrodes were implanted over the hippocampus. Once the operation was completed, signal recordings were started 24 h after the rats freely recovered. EEG recordings were conducted using the OmniPlex device (Plexon, Hongkong, China) and digitized at 5 kHz using PlexControl software (Molecular Devices). The power spectrum in the hippocampus was divided into delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), low gamma (30–60 Hz) and high gamma (60–100 Hz) frequency bands. EEG recording data were analyzed using MATLAB2016a software.
Statistical Analysis
Compiled data are presented as the mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by a post hoc least significant difference test. Graphs were generated using Origin 8 and GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant in all tests.
References
Bhat S, Acharya UR, Adeli H, Bairy GM, Adeli A (2014) Autism: cause factors, early diagnosis and therapies. Rev Neurosci 25(6):841–850. https://doi.org/10.1515/revneuro-2014-0056
Neuhaus E, Beauchaine TP, Bernier R (2010) Neurobiological correlates of social functioning in autism. Clin Psychol Rev 30(6):733–748. https://doi.org/10.1016/j.cpr.2010.05.007
Roullet FI, Wollaston L, Decatanzaro D, Foster JA (2010) Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience 170(2):514–522. https://doi.org/10.1016/j.neuroscience.2010.06.069
Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK (2006) A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord 36(6):779–793. https://doi.org/10.1007/s10803-006-0117-y
Nicolini C, Fahnestock M (2018) The valproic acid-induced rodent model of autism. Exp Neurol 299 (Pt A):217–227. https://doi.org/10.1016/j.expneurol.2017.04.017
Silva GT, Le Be JV, Riachi I, Rinaldi T, Markram K, Markram H (2009) Enhanced long-term microcircuit plasticity in the valproic acid animal model of autism. Front Synaptic Neurosci 1:1. https://doi.org/10.3389/neuro.19.001.2009
Bauman M, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35:866–874. https://doi.org/10.1212/wnl.35.6.866
Chaddad A, Desrosiers C, Hassan L, Tanougast C (2017) Hippocampus and amygdala radiomic biomarkers for the study of autism spectrum disorder. BMC Neurosci 18(1):52. https://doi.org/10.1186/s12868-017-0373-0
Courchesne E, Pierce K (2005) Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci 23(2–3):153–170. https://doi.org/10.1016/j.ijdevneu.2005.01.003
Dager SR, Wang L, Friedman SD, Shaw DW, Constantino JN, Artru AA, Dawson G, Csernansky JG (2007) Shape mapping of the hippocampus in young children with autism spectrum disorder. AJNR Am J Neuroradiol 28:672–677
Sheikh AM, Li X, Wen G, Tauqeer Z, Brown WT, Malik M (2010) Cathepsin D and apoptosis related proteins are elevated in the brain of autistic subjects. Neuroscience 165(2):363–370. https://doi.org/10.1016/j.neuroscience.2009.10.035
Zhang J, Zhang JX, Zhang QL (2016) PI3K/AKT/mTOR-mediated autophagy in the development of autism spectrum disorder. Brain Res Bull 125:152–158. https://doi.org/10.1016/j.brainresbull.2016.06.007
Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, Reiss AL, Hardan AY (2014) A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuro-psychopharmacol Biol Psychiatry 48:124–128. https://doi.org/10.1016/j.pnpbp.2013.09.010
Bahi A (2017) Hippocampal BDNF overexpression or microR124a silencing reduces anxiety- and autism-like behaviors in rats. Behav Brain Res 326:281–290. https://doi.org/10.1016/j.bbr.2017.03.010
Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y (2014) Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343(6171):675–679. https://doi.org/10.1126/science.1247190
Xiong Z, Zhang K, Ren Q, Chang L, Chen J, Hashimoto K (2019) Increased expression of inwardly rectifying Kir4.1 channel in the parietal cortex from patients with major depressive disorder. J Affect Disord 245:265–269. https://doi.org/10.1016/j.jad.2018.11.016
Guglielmi L, Servettini I, Caramia M, Catacuzzeno L, Franciolini F, D'Adamo MC, Pessia M (2015) Update on the implication of potassium channels in autism: K(+) channelautism spectrum disorder. Front Cell Neurosci 9:34. https://doi.org/10.3389/fncel.2015.00034
Sun C, Zou M, Li L, Li D, Ma Y, Xia W, Wu L, Ren H (2018) Association study between inwardly rectifying potassium channels 2.1 and 4.1 and autism spectrum disorders. Life Sci 213:183–189. https://doi.org/10.1016/j.lfs.2018.10.012
Gilling M, Rasmussen HB, Calloe K, Sequeira AF, Baretto M, Oliveira G, Almeida J, Lauritsen MB, Ullmann R, Boonen SE, Brondum-Nielsen K, Kalscheuer VM, Tumer Z, Vicente AM, Schmitt N, Tommerup N (2013) Dysfunction of the heteromeric KV7.3/KV7.5 potassium channel is associated with autism spectrum disorders. Front Genet 4:54. https://doi.org/10.3389/fgene.2013.00054
Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY (2011) Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72(4):630–642. https://doi.org/10.1016/j.neuron.2011.09.033
Ludwig J, Weseloh R, Karschin C, Liu Q, Netzer R, Engeland B, Stansfeld C, Pongs O (2000) Cloning and functional expression of rat eag2, a new member of the ether-a-go-go family of potassium channels and comparison of its distribution with that of eag1. Mol Cell Neurosci 16(1):59–70. https://doi.org/10.1006/mcne.2000.0851
Saganich MJ, Machado E, Rudy B (2001) Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci 21:4609–4624
Hou Q, Wang Y, Li Y, Chen D, Yang F, Wang S (2018) A developmental study of abnormal behaviors and altered GABAergic signaling in the VPA-treated rat model of autism. Front Behav Neurosci 12:182. https://doi.org/10.3389/fnbeh.2018.00182
Huang X, He Y, Dubuc AM, Hashizume R, Zhang W, Reimand J, Yang H, Wang TA, Stehbens SJ, Younger S, Barshow S, Zhu S, Cooper MK, Peacock J, Ramaswamy V, Garzia L, Wu X, Remke M, Forester CM, Kim CC, Weiss WA, James CD, Shuman MA, Bader GD, Mueller S, Taylor MD, Jan YN, Jan LY (2015) EAG2 potassium channel with evolutionarily conserved function as a brain tumor target. Nat Neurosci 18(9):1236–1246. https://doi.org/10.1038/nn.4088
Wadhwa S, Wadhwa P, Dinda AK, Gupta NP (2009) Differential expression of potassium ion channels in human renal cell carcinoma. Int Urol Nephrol 41(2):251–257. https://doi.org/10.1007/s11255-008-9459-z
Xu E, Gu J, Hawk ET, Wang KK, Lai M, Huang M, Ajani J, Wu X (2013) Genome-wide methylation analysis shows similar patterns in Barrett's esophagus and esophageal adenocarcinoma. Carcinogenesis 34(12):2750–2756. https://doi.org/10.1093/carcin/bgt286
Veeramah KR, Johnstone L, Karafet TM, Wolf D, Sprissler R, Salogiannis J, Barth-Maron A, Greenberg ME, Stuhlmann T, Weinert S, Jentsch TJ, Pazzi M, Restifo LL, Talwar D, Erickson RP, Hammer MF (2013) Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia 54(7):1270–1281. https://doi.org/10.1111/epi.12201
Schneider T, Przewłocki. R (2011) Environmental factors in the aetiology of autism: lessons from animals prenatally exposed to valproic acid
Yang Y, Vasylyev DV, Dib-Hajj F, Veeramah KR, Hammer MF, Dib-Hajj SD, Waxman SG (2013) Multistate structural modeling and voltage-clamp analysis of epilepsy/autism mutation Kv10.2-R327H demonstrate the role of this residue in stabilizing the channel closed state. J Neurosci 33(42):16586–16593. https://doi.org/10.1523/JNEUROSCI.2307-13.2013
de Oliveira RM, Martin S, de Oliveira CL, Milani H, Schiavon AP, Joca S, Pardo LA, Stuhmer W, Del Bel EA (2012) Eag1, Eag2, and SK3 potassium channel expression in the rat hippocampus after global transient brain ischemia. J Neurosci Res 90(3):632–640. https://doi.org/10.1002/jnr.22772
Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN (2015) GABAB receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology 40(9):2228–2239. https://doi.org/10.1038/npp.2015.66
Rubenstein JL, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Brain Behav 2:255–267. https://doi.org/10.1046/j.1601-183X.2003.00037.x
Cornew L, Roberts TP, Blaskey L, Edgar JC (2012) Resting-state oscillatory activity in autism spectrum disorders. J Autism Dev Disord 42(9):1884–1894. https://doi.org/10.1007/s10803-011-1431-6
Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M (2008) Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neurosci Lett 434(2):218–223. https://doi.org/10.1016/j.neulet.2008.01.066
Omer VE, Ali SF, Holson RR, Duhart HM, Scalzo FM, Slikker W (1991) Behavioral and neurochemical effects of prenatal methylenedioxymethamphetamine (MDMA) exposure in rats. Neurotoxicol Teratol 13(1):13–20
Merali Z, Presti-Torres J, Mackay JC, Johnstone J, Du L, St-Jean A, Levesque D, Kent P, Schwartsmann G, Roesler R, Schroder N, Anisman H (2014) Long-term behavioral effects of neonatal blockade of gastrin-releasing peptide receptors in rats: similarities to autism spectrum disorders. Behav Brain Res 263:60–69. https://doi.org/10.1016/j.bbr.2014.01.008
Boyer F, Dreyer JL (2007) Alpha-synuclein in the nucleus accumbens induces changes in cocaine behaviour in rats. Eur J Neurosci 26(10):2764–2776. https://doi.org/10.1111/j.1460-9568.2007.05878.x
Chandrasekar V, Dreyer JL (2009) microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci 42(4):350–362. https://doi.org/10.1016/j.mcn.2009.08.009
Trimper JB, Galloway CR, Jones AC, Mandi K, Manns JR (2017) Gamma oscillations in rat hippocampal subregions dentate gyrus, CA3, CA1, and subiculum underlie associative memory encoding. Cell Rep 21(9):2419–2432. https://doi.org/10.1016/j.celrep.2017.10.123
Acknowledgements
This work was supported by the Shanghai Municipal Science and Technology Commission (Grant No. 16010500600).
Author information
Authors and Affiliations
Contributions
JW and SF contributed equally to this work. DD and FC conceived and designed the study. JW, YL and ML performed the experiments at Shanghai University. The first draft of the manuscript was written by JW, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All persons designated as authors qualify for authorship, and all of those who qualify for authorship are listed.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical Approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. s1
Neurological and motor hypoplasia in VPA rats (a) Body weight, (b) Eye opening category score, (c) Plane correction, (d) Cliff avoidance, (e) Negative geotaxis, (f) Swimming condition. Data are represented as the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group. n = 20. Supplementary material 1 (TIF 21450.9 kb)
Fig. s2
Fig. s2 VPA-induced rats exhibited autistic-like behavior. In the three-chamber social interaction assay, social interaction (a) and social novelty preference (b) were recorded. (c) The percentage of time that rats remained in the open arm in the elevated plus maze test was significantly decreased in the VPA group. (d) The percentage of time in the white box was decreased in the VPA group. (e) The time in the central zone in the open field was decreased in VPA-induced rats compared with control rats. (f) VPA-treated rats exhibited more stereotypical behaviors than control rats in the Y maze test. Data are represented as the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group. n = 12. Supplementary material 2 (TIF 20313.7 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Feng, S., Li, M. et al. Increased Expression of Kv10.2 in the Hippocampus Attenuates Valproic Acid-Induced Autism-Like Behaviors in Rats. Neurochem Res 44, 2796–2808 (2019). https://doi.org/10.1007/s11064-019-02903-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02903-4