Abstract
Purpose
Ether-a-go-go (EAG) or EAG-related (ERG) voltage-gated potassium ion channels are involved in tumor generation and progression. Their over- and/or misexpression has been demonstrated in various tumors, and inhibition of these channels has suppressed proliferation of various cancer cells. We investigate and compare the pattern of expression of EAG and human ERG (HERG) channels in renal cell carcinoma and “normal” renal tissue.
Method
Tissue samples, obtained at the time of radical nephrectomy from the tumor-bearing areas, and uninvolved renal tissue were preserved in 4% paraformaldehyde and cryosectioned at 20 μm. Immunohistochemical and Western blot analysis was performed on the tumor and uninvolved kidney parenchyma by incubating with polyclonal anti-HERG 1b (Alomone Lab, Israel), anti-EAG1, and anti-EAG2. Pattern of expression of EAG/HERG channels in normal renal tissue and carcinoma were noted and compared.
Results
The study was performed on 16 radical and four partial nephrectomy specimens (n = 20). All tumors in the cohort were clear cell renal carcinoma. Normal renal tissue was found to exhibit heterogeneous cytoplasmic positivity for EAG1 and focal HERG immunoreactivity (IR) in the proximal (PCT) and distal convoluted tubules (DCT). EAG2 IR was absent in the normal renal tissue. Clear cell RCC demonstrated a loss of HERG expression while diffuse overexpression of EAG1 and EAG2 was noted. Western blot analysis corroborated the immunohistochemical observations.
Conclusions
In our study both EAG1 and EAG2 potassium channels were overexpressed in clear cell renal cancer. In contrast to other adenocarcinomas, there is loss of HERG expression in clear cell RCC, which may possibly explain its chemoresistance. These ion channels may provide a potential for targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ion channels play a regulatory role in various cell functions ranging from cell excitability to regulation of cell volume and proliferation. Potassium ion channel is a large ubiquitously expressed protein family consisting of a pore-forming unit with six transmembrane-spanning segments that selectively conduct potassium (K+) ions across the cell membrane. The channel auxiliary subunits are highly diverse. These are classified on the basis of molecular biology and gene homology into Kv (voltage-dependent) [which includes ether-a-go-go (EAG) and KCNQ subfamilies], KCa (Ca2+-activated K+ channel), Kir inward rectifier, and two-pore domain K+ channels. The EAG subfamily has three distinct sequence homologues: EAG, EAG-like K+ channel (ELK), and EAG-related gene (ERG).
While Kv channels control electrical activity in excitable cells [1], these channels are also expressed in nonexcitable cells, where they are involved in cell differentiation and growth by influencing intracellular calcium (Ca+) concentration [2]. Activation of K+ channels is considered necessary for progression of cells through the G1 phase of cell cycle. In fact inhibition of these channels has been shown to inhibit cell proliferation in both normal activated lymphocytes and various cancer cell lines [3]. Involvement of ion channels in cytoskeleton reshaping and cell–cell interaction also suggests a role in regulation of processes such as cell migration and invasive growth [4]. Overexpression of Kv channels is one of the key emerging factors involved in transformation of a normal cell into a neoplastic cell. This study explores the expression of various Kv channels—EAG [EAG1 (Kv 10.1) and EAG2 (Kv 10.2)] and H Kv 11.1 (human EAG-related voltage-gated potassium ion channels, HERG)—in human renal cell carcinoma (RCC) and compares their expression with that in “normal” renal tissue.
Patients and methods
Patients
Tissue samples were obtained from patients (n = 20) with renal tumors undergoing surgery, according to the protocol approved by the institute research committee, with prior informed consent from the patients allowing tissue usage for research purpose. Tissue was collected at the time of radical nephrectomy (n = 16) or partial nephrectomy (n = 4) from tumor-bearing areas as well as uninvolved “normal” renal tissue (collected from a site well away from the tumor margin in radical nephrectomy specimens, and from the peritumoral renal tissue in cases of partial nephrectomy). These were preserved in 4% paraformaldehyde for immunohistochemistry or snap-frozen in liquid nitrogen to perform Western blot.
Haemotoxylin and eosin staining
From each of the collected samples a representative slice was cut, embedded in paraffin, and subjected to histopathological evaluation to confirm type and grade of tumor, and to check for microscopic tumor-free status of the “normal” renal tissue.
Immunohistochemistry
The fixed tumor and control kidney tissue was cryoprotected in 15% and 30% sucrose, sectioned at 20 μm thickness on a cryostat (Reichert Jung), and mounted on gelatinized slides. Immunohistochemical analysis was performed on the tumor and uninvolved kidney parenchyma by incubating with polyclonal antibodies (Alomone Lab, Israel). Anti-HERG 1b (anti-hKv1.11), anti-EAG1, and anti-EAG2 were used at a dilution of 1:1,000, 1:100, and 1:100, respectively. Antigen–antibody complexes were visualized with 3′3-diaminobenzidine tetrahydrochloride (DAB)-nickel sulfate (0.06%).
Western blotting
Proteins were extracted from tumor-bearing tissue and uninvolved renal tissue (in radical nephrectomy specimens only) using rapid immuno precipitation assay (RIPA) buffer and estimated by Bradford method. Eighty micrograms of protein from each sample was loaded and separated on an 8% polyacrylamide gel at 90 V. The proteins were transferred, and immunoblotting was done using polyclonal antibodies anti-HERG 1b (Alomone Lab, Israel) (1:100), anti-EAG1 (Alomone Lab, Israel) (1:100), and anti-EAG2 (Alomone Lab, Israel) (1:100). Antigen–antibody complexes were visualized with DAB-nickel sulfate.
Quantification and statistical analysis
Densitometric analysis of the Western blots was performed on the coded specimens blind to the nature of the tissue type. The intensity of the bands in the immunoblots was analyzed using the software (Quantity 1) of the gel documentation system (Bio-Rad, USA). The data was entered into Microsoft EXCEL 2000 and analyzed with the SPSS statistical software package (version 15). Descriptive statistics, including mean and median, were initially calculated. The expression of Kv channel proteins in control and tumor tissue was analyzed using the Mann–Whitney test. Analysis was considered significant at P < 0.05.
Results
Histologically, the “normal” renal tissue showed well-preserved morphology of the renal glomeruli, proximal (PCT), and distal convoluted tubules (DCT) (Fig. 1a). The entire cohort of tumor specimens was identified to be of the clear cell variety of RCC (Fig. 1b). Two of these were eosinophilic variants of clear cell RCC. Four patients had T1a disease, 13 had T1b, and 1 each had T2, T3N1, and T4N1 disease. Eleven patients were classified as nuclear grade I; grade II was noted in seven; and grade III in two cases.
EAG1 expression
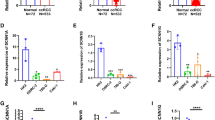
In normal renal tissue, EAG1 showed heterogeneous juxtamembranous cytoplasmic positive immunoreactivity (IR) in the proximal and few distal tubule cells. Patchy heterogeneous expression was consistently noted in all the studied samples of “normal” renal tissue. The tumor tissue, on the other hand, showed overexpression of EAG1 in the cytoplasm of the clear cells and the surrounding stroma (Fig. 2a, b). In the Western blots, the band for EAG1 was seen in both the normal and tumor tissue at 130 kD (Fig. 2c). The enhanced expression of EAG1 (P = 0.028) in the tumor compared with the control was statistically confirmed (Fig. 2c). Interestingly, higher-stage tumors demonstrated more intense EAG1 expression than did lower-stage tumors (especially in those who underwent partial nephrectomy).
Photomicrograph of EAG1 IR, showing (a) juxtamembrane positivity for EAG1 in a few PCTs and DCTs in control renal tissue and (b) overexpression of EAG1 in neoplastic cell (white arrow) and stroma (white diamond arrow) of RCC (5×). (c) Western blots showing EAG1 expression at 130 kD. The densitometric analysis graph shows significantly increased levels of EAG1 in RCC
EAG2 expression
This molecule did not show any IR in the normal kidney tissue, while a significant intense signal was present in the cytoplasm of the clear cell tumor tissue (Fig. 3a, b). The immunoblots also showed absence of an IR band in the normal lane, while a band was noted in the tumor samples at 112 kD (P = 0.02) (Fig. 3c).
Photomicrographs of EAG2 IR show (a) absence of staining in PCTs and DCTs in control renal tissue (10×) and (b) enhanced expression of EAG2 in clear cell cytoplasm (white arrow) of RCC (5×). The section has been counterstained with haemotoxylin. (c) Immunoblots show EAG2 band at 112 kD only in RCC. The densitometric analysis graph shows significantly increased levels of EAG2 in RCC
HERG expression
Focal HERG immunopositivity was noted in membrane and cytoplasm of some of the tubules of both proximal and distal types in the normal renal tissue (Fig. 4a). In the tumor specimens, notably a loss of HERG expression was seen in the neoplastic cells, while the surrounding stromal tissue showed an intense HERG IR (Fig. 4b, c). The Western blots demonstrated a weak IR band at 155 kD in the normal renal tissue lane and a very faint band in the tumor tissue lane (Fig. 4 d).
Photomicrographs of HERG show (a) cytoplasmic positivity for HERG in a few focal PCTs and DCTs in the control renal tissue (5×). (b) Note the loss of expression of HERG in neoplastic cell (arrow) of RCC (10×). (c) IR in the stroma (white arrow) (5×). (d) Immunoblots show a HERG band at 155 kD in both control and tumor cases. The band in the RCC is probably due to stromal positivity. The densitometric analysis graph shows an insignificant decrease in HERG expression
Discussion
Due to their restricted distribution in normal tissues and upregulated expression in cancer cells, voltage-gated K+ channels have generated immense interest as a promising tool for developing specific cancer therapeutic options. EAG1 is expressed normally in the adult brain with little or no expression in other organs (liver, lungs, heart, and kidney) [5]; however, it has been demonstrated in several cell lines of human neoplasms, such as neuroblastoma [5, 6], melanoma [7], breast [5], and cervical carcinoma [8], and in various human cancers including RCC [9]. EAG1 is seen to be essential for the proliferation of cancerous cells, and is required for the maintenance of growth. Even more interesting is its relationship to cellular transformation; cells transfected with EAG1 are able to grow in the absence of serum, lose contact inhibition, and induce aggressive tumors when implanted into immune-depressed mice [5]. Overexpression of EAG channels enhances aggressive growth of tumors [5].
We found overexpression of EAG1 in RCC as demonstrated in histopathological sections and immunoblots (P = 0.028). Hemmerlein et al. studied nine cases of RCC and noted a consistently similar overexpression of EAG1 in the tumor tissue [9]. EAG1 is unique in its restricted expression outside the central nervous system (CNS) [10], and its overexpression in various cancers including RCC qualifies it as a suitable candidate for further study as a target for immunotherapeutic intervention. Additionally, most normal cells expressing EAG1 (predominantly in the brain) are either protected by the blood–brain barrier or represent terminally differentiated cells, thereby allowing more aggressive therapeutic intervention [9].
Like EAG1, EAG2 is also present predominantly in the nervous system and a number of other tissues, including the skeletal muscle [11], heart, placenta, lung, and liver. In our study, the normal renal tissue was devoid of EAG2 expression while it was significantly overexpressed in RCC. Its presence in the tumor makes it a potential diagnostic marker as well as a membrane therapeutic target for clear cell carcinoma of the kidney.
HERG has also been found to be selectively upregulated in a variety of human cancers such as cancers of the colon [12], prostate [13], and uterus [14], while its expression is absent in normal tissue or its cell line counterparts. Bianchi et al. demonstrated the presence of a HERG gene which encodes a K+ channel that plays a pathophysiological role in the regulatory mechanisms of neoplastic cell survival by possibly keeping the resting potential within the depolarized values required for unlimited tumor growth and hence affording a selective advantage in an ischemic environment [15]. Selective pharmacologic blockage of HERG channels in primary leukemic cells significantly reduces cell proliferation [16]. Also it has been shown that v-src (a constitutively active form of protein tyrosine kinase src) could phosphorylate the HERG channel and increases current passage. The aberrant function of the ras-src signaling pathway causes neoplastic transformation of the cell [17]. Further, the HERG protein has also been shown to interact with tumor necrosis receptor factor type 1 (TNFR1) in tumor cell membrane lines and TNFR1 can mediate both cell proliferation and apoptosis [18].
In our study, we noted heterogeneous expression of HERG in the PCT and DCT of “normal” renal parenchyma. This expression was downregulated in neoplastic cells. However, the tumor tissue immunoblot demonstrated a HERG band, albeit of very low intensity (statistically not significant). Although on immunohistochemistry the neoplastic cells themselves were negative for HERG expression, the stromal tissue in the tumor demonstrated marked expression, especially in high-grade tumors, thereby explaining the presence of the HERG band.
Downregulation of HERG in RCC is in contrast to findings noted in other adenocarcinomas (uterine, breast, lung, prostate) in which overexpression of HERG was noted. Interestingly, Chen et al. demonstrated that higher HERG-expressing cancer cell lines positively correlated to chemosensitivity to paclitaxel, vincristine, and hydroxy-camptothecin [19]. In their study, human colonic carcinoma cells were highly HERG expressive in comparison with human lung adenocarcinoma cells and were more sensitive to chemotherapy. On transfecting the lung adenocarcinoma cells with HERG, these cell lines also became sensitive to chemotherapy. RCC is a chemoresistant tumor; whether this loss of HERG expressivity is an indirect marker for the same needs further evaluation.
Conclusion
Our study shows that, in addition to EAG1, EAG2 potassium channels are also overexpressed in clear cell renal cancer and may provide a potential for targeted therapy.
In contrast to other adenocarcinomas, there is loss of HERG expression in clear cell RCC that may possibly explain its chemoresistance and warrants further investigation.
References
Yellen G (2002) The voltage-gated potassium channels and their relatives. Nature 419:35–42. doi:10.1038/nature00978
Deutsch C, Chen L (1993) Heterologous expression of specific K+ channels in T lymphocytes: functional consequences for volume regulation. Proc Natl Acad Sci USA 90:10036–10040. doi:10.1073/pnas.90.21.10036
Wonderlin WF, Strobl JS (1996) Potassium channels, proliferation and G1 progression. J Membr Biol 154(2):91–107. doi:10.1007/s002329900135
Schwab A (2001) Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol 280(5):F739–F747
Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S et al (1999) Oncogenic potential of EAG K(+) channels. EMBO J 18:5540–5547. doi:10.1093/emboj/18.20.5540
Meyer R, Heinemann SH (1998) Characterization of an eag-like potassium channel in human neuroblastoma cells. J Physiol 508(Pt 1):49–56
Gavrilova-Ruch O, Schonherr K, Gessner G, Schonherr R, Klapperstuck T, Wohlrab W et al (2002) Effects of imipramine on ion channels and proliferation of IGR1 melanoma cells. J Membr Biol 188:137–149. doi:10.1007/s00232-001-0181-3
Farias LM, Ocana DB, Diaz L, Larrea F, Avila-Chavez E, Cadena A et al (2004) Ether a go-go potassium channels as human cervical cancer markers. Cancer Res 64(19):6996–7001. doi:10.1158/0008-5472.CAN-04-1204
Hemmerlein B, Weseloh RM, de Queiroz FM, Knötgen H, Sánchez A, Rubio ME et al (2006) Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer 5:41. doi:10.1186/1476-4598-5-41
Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S et al (1999) Oncogenic potential of EAG K(+) channels. EMBO J 18:5540–5547. doi:10.1093/emboj/18.20.5540
Ju M, Wray D (2002) Molecular identification and characterization of human EAG2 potassium channel. FEBS Lett 524(1–3):204–210. doi:10.1016/S0014-5793(02)03055-7
Abdul M, Hoosein N (2002) Voltage-gated potassium ion channels in colon cancer. Oncol Rep 9(5):961–964
Abdul M, Hoosein N (2002) Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett 186(1):99–105. doi:10.1016/S0304-3835(02)00348-8
Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L et al (2000) HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer 83(12):1722–1729. doi:10.1054/bjoc.2000.1497
Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P et al (1998) Herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res 58(4):815–822
Pillozzi S, Brizzi MF, Balzi M, Crociano O, Cherubini A, Guasti L et al (2002) HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate proliferation of normal and leukemic hematopoietic progenitors. Leukemia 16(9):1791–1798. doi:10.1038/sj.leu.2402572
Cayabyab FS, Schlichter LC (2002) Regulation of an ERG. J Biol Chem 277(16):13673–13681. doi:10.1074/jbc.M108211200
Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B et al (2002) HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res 62(17):4843–4848
Chen S, Jiang M, Zhen Y (2005) HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother Pharmacol 56:212–220. doi:10.1007/s00280-004-0960-5
Acknowledgments
The authors would like to thank Prof. Shashi Wadhwa, Dr. TC Nag, and Ms. Sraboni Chaudhary for their invaluable assistance in this study. This work was done while Dr. Pankaj Wadhwa was a Senior Research Associate of CSIR in Department of Urology at AIIMS, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wadhwa, S., Wadhwa, P., Dinda, A.K. et al. Differential expression of potassium ion channels in human renal cell carcinoma. Int Urol Nephrol 41, 251–257 (2009). https://doi.org/10.1007/s11255-008-9459-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-008-9459-z