Abstract
Evidence suggests that the dopamine receptor rate-limiting enzyme, tyrosine hydroxylase (TH), and the glutamate receptor, N-methyl-d-aspartate receptor 2B (NR2B), contribute to morphine dependence. Previous studies show that chronic exposure to morphine changes the expression of opioid receptors. In this study, we focus on the effects of sinomenine on morphine-dependent mice and its related neural mechanisms. Conditioned place preference (CPP) mouse model was established using morphine (9 mg/kg, s.c.), and their expression levels of TH and NR2B were observed by immunohistochemistry. Moreover, their mu opioid receptor (MOR) and delta opioid receptor (DOR) contents were assessed using quantitative reverse transcription polymerase chain reaction. Results showed that high sinomenine dose (80 mg/kg) effectively attenuated the behavior of CPP mice and reversed increased expression levels of TH and NR2B induced by morphine. Moreover, compared with the morphine group, sinomenine up-regulated the content of MOR to a normal level but did not significantly affect the DOR expression. In summary, these data indicate that sinomenine can inhibit morphine dependence by increasing the expression levels of TH, NR2B, and MOR in the mouse brain; however, DOR may not contribute to this effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphine is an opioid widely used for clinical pain management, however, its misuse might result in addiction [1]. The current main treatment for opioid addiction is agonist substitution therapy, which uses long-duration opioid agonists, such as methadone or buprenorphine. However, this treatment is associated with several problems, such as limited number of patients and community acceptance. Moreover, methadone itself has addictive effects. Furthermore, most patients relapse after treatment termination [2]. Withdrawal from repeated exposure to abused drugs results in a series of negative emotions, such as anhedonia, dysphoria, and anxiety, which contributes to the compulsive maintenance of drug intake and relapse to drug use [3]. Morphine-dependent rats exhibit somatic signs, withdrawal such as wet dog shakes, teeth chattering, piloerection, sniffing, irritability, and episodes of writhing after opiate [4]. Despite the numerous studies devoted to morphine dependence, the cellular and molecular mechanisms of chronic opioid exposure leading to intracellular changes remain poorly understood [5, 6]. This study aims to elucidate the accurate mechanism responsible for opioid addiction to develop effective compounds.
Studies have shown that opioid addiction and withdrawal are related to the regulation of dopamine, glutamate, and opioid receptors. Morphine can combine with mu opioid receptors (MORs), delta opioid receptors (DORs), and kappa opioid receptors (KORs) to produce pharmacological effects. MOR and DOR can mediate the rewarding effects of opioid systems to induce the conditioned place preference (CPP) behavior in animals [7,8,9]. MORs play a critical role in morphine-dependent incidences via the protein kinase C alpha or other pathways. DOR subtype participates in morphine addiction by the formation of MOR–DOR dimers, whereas the activation of KOR inhibits the MOR–DOR-mediated rewarding effects of morphine [10]. Tyrosine hydroxylase (TH), a rate-limiting enzyme that occurs in dopamine biosynthesis, can modulate neuronal activities in the brain [11]. N-Methyl-d-aspartate (NMDA) receptor 2B (NR2B) is a subunit of the NMDA receptor (an ionotropic glutamate receptor) and plays a dominant role in CPP development and psychostimulant abuse [12]. In addition, previous experiments have shown that chronic morphine exposure can significantly up-regulate NR2B and TH expression in opioid addiction-related brain areas of mice [4, 10, 13]. Therefore, studying the changes on opioid, glutamate, and dopamine receptors in opioid-addicted animal brains can elucidate the mechanism of opioid addiction to provide direction and basis for researching opioid withdrawal drugs.
Sinomenine is an active compound from the plant Sinomenium acutum, known as Fang-ji or Qing-teng in Chinese. Sinomenine exerts a variety of pharmacological effects, including anti-inflammatory, antiangiogenic, anti-arrhythmic, and immunosuppressive properties [14]. It has attracted considerable interest due to its potential effects on treating drug dependence. Its chemical structure is similar to that of morphine (Fig. 1); however, sinomenine causes neither psychological nor physical drug dependence and could attenuate morphine-induced tolerance and morphine withdrawal symptoms, which may associate with its agonist-like action to regulate MOR or other neural systems in the brain [15,16,17]. Our previous animal experimental research has confirmed that alcohol extracts from sinomenine can inhibit the release of neurotransmitters and regulate the concentration of intracellular calcium to alleviate withdrawal contractile response of morphine-dependent ex vivo ileum from guinea pigs and withdrawal syndromes of morphine-dependent mice [18]. Moreover, we also found that sinomenine can effectively inhibit naloxone-precipitated withdrawal response in morphine-dependent animal models in vivo and in vitro [19, 20]. By establishing the CPP mouse model, we suggested that sinomenine can inhibit the acquisition of place preference induced by morphine [21]. All studies indicated that sinomenine may exert certain effects on drug dependence treatment.
In this study, we aimed to investigate the inhibitory effects of sinomenine on morphine-induced CPP in mice and elucidate the possible molecular mechanisms of sinomenine detoxification by measuring the expression levels of TH, NR2B, MOR, and DOR in mouse brains.
Materials and Methods
Animals
Kunming mice weighing 20–25 g were provided by the Experimental Animal Center of Southern Medical University (Guangzhou, China). Prior to testing, the animals were habituated to laboratory conditions (temperature: 20 ± 2 °C, humidity: 55 ± 5%, and 12/12 h dark-light cycle: lights on from 7:00 a.m. to 7:00 p.m.) for 1 week. All mice had free access to food and water. Animal care and procedures strictly followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Experimental Animal Ethics Committee of Southern Medical University.
Drugs and Reagents
Morphine hydrochloride (No. 710303) was purchased from the medication supply station of the Chinese People’s Liberation Army General Logistics Department, China. Sinomenine (No. 20000528, purity 99.7%) was bought from Hunan Zhengqing Co., Ltd., China. Methadone (No. 020111) was obtained from the Central Pharmaceutical Co., Ltd., Tianjin, China. Anti-NMDAR2B Antibody (AB1557P) and Anti-Tyrosine Hydroxylase Antibody (AB152) were bought from Millipore, USA. Protein assay reagents A (500-0113, Bio-Rad) and B (500-0114, Bio-Rad), APS (A3678), Sigma extraction reagent (71009-3, Novagen), protein inhibitor (539134, Calbiochem), and all other chemicals and reagents were of standard quality for commercially available biochemicals.
Conditioned Place Preference Procedure
The CPP apparatus consisted of two equal-sized compartments (15 × 15 × 15 cm): one with a white box and the other with a black box, joined by a sliding board. For testing, the sliding door was raised above the floor to allow the mice free access to both sides of the box [22]. The time the mice spent on the box was calculated. The activity routes of the mice in the box were video recorded and analyzed using the Noldus EthoVision XT 8.5 software (Noldus, Wageningen, Netherlands).
Experimental Design
All mice were tested before the CPP experiment. In brief, the sliding board in the CPP apparatus was removed, which allowed the mice to move freely. The mice were placed in the middle of two parts, and then the time the mice stayed in each side was recorded within 15 min. The result showed a significant difference between the time of mice spent on the white compartment (595.20 ± 38.37 s, n = 60) and the time of mice spent on the black compartment (304.80 ± 38.37 s, n = 60) (**P < 0.01), which suggested that almost all mice preferred the black box. Therefore, we chose the white compartment as the medicine compartment. The mice that showed significant preference for one side of the apparatus were removed. Fifty qualified mice were selected after the CPP test to assess the effect of sinomenine on the morphine-induced CPP mice. These mice were randomly divided into five groups: (1) control group, (2) morphine model group (9 mg/kg), (3) morphine with low dose of sinomenine group (40 mg/kg), (4) morphine with high dose of sinomenine group (80 mg/kg), and (5) morphine with methadone group (15 mg/kg).
The CPP test consisted of three phases and was conducted within 8 consecutive days. For the pre-conditioning phase (days 1–3), the mice were placed under the door, which was left open to allow free access to the entire box for 5 min daily. During the conditioning phase (days 4–7), the door was closed to separate the two boxes and mice underwent two conditioning sessions. On day 4, the first session was performed in the morning. All mice received morphine (9 mg/kg, s.c.) except for the control group mice, which were treated with the same volume of physiological saline and then immediately confined to the white compartment for 1 h. After an interval of 8 h, the second session of the day began. All mice were treated with saline (0.3 ml, s.c.) and immediately confined to the black compartment for 1 h. In the following days (day 5–7), 30 min before the start of the first session in the morning, the mice in the control group were injected with saline (10 ml/kg, i.p.), those in the sinomenine group were treated with different doses of sinomenine (40 and 80 mg/kg, i.p.), and those in the methadone group were injected with methadone (15 mg/kg, s.c.); then, the same schedule as day 4 was followed. Twenty-four hours after the last morphine-paired conditioning trial (day 8), the duration that the mice spent in the white compartment was calculated within 5 min, and the activity routes of the mice were video recorded and analyzed using the Noldus EthoVision XT 8.5 software (Noldus, Wageningen, Netherlands).
Immunohistochemistry for TH and NR2B Expression Levels
The mice were killed through cervical dislocation after the CPP test. The brains were fixed with 4% paraformaldehyde in phosphate buffer saline (PBS, pH 7.4) at 4 °C for 24 h. They were sectioned using a vibratome at 3 µm, deparaffinized with xylene, and dehydrated in decreasing concentrations of alcohol. After blocking the endogenous peroxidase activity in 3% H2O2 in PBS for 30 min, the slices were boiled for 10 min under pressure in citrate buffer for antigen retrieval. Nonspecific binding was blocked with 5% bovine serum albumin in PBS for 15 min. The tissues were incubated with primary antibodies for anti-TH and anti-NR2B (1:200 dilution; Millipore, Billerica, MA, USA) in PBS containing 5% bovine serum albumin overnight at 4 °C. Brain slices were then washed thrice with PBS and incubated with horseradish peroxidase-labeled goat anti-rabbit IgG as the secondary antibody for 1 h at room temperature. After rinsing, the sections were immersed in DAB/H2O2 for a reasonable time, stained with hematoxylin for 80 s, and then sealed with neutral gum. Tissue images were captured using a light microscope. The positive expression levels of TH and NR2B in the hippocampuses of the mouse brains were defined as the appearance of brown particles in the cell nuclei. Image-Pro Plus 6.0 image analysis software was used to measure the integrated optical density of the positive cells, and the mean value of the group was noted as the relative contents of TH and NR2B [22].
Quantitative Reverse Transcription Polymerase Chain Reaction for MOR and DOR Expression Levels
Total RNA from mouse brains was extracted using the RNeasy kit (Takara Biotechnology, Dalian, China). Reverse transcription and complementary DNA (cDNA) amplification were performed according to the manufacturer’s instructions (Takara Biotechnology, Dalian, China). The primer sequences (Table 1) were designed and synthesized by Invitrogen Inc. (Shanghai, China). Relative quantitative real-time PCR was used to assess the mRNA levels of these genes (ABI 7500 real-time PCR detection system), in which β-actin was used as an internal control and was measured in each sample. The mixture was prepared by combining 10 µl of SYBR Green Mix (2X), 1 µl of cDNA template, 1 µl of primer pair mix (5 pmol/ml each primer), and 8 µl of double distilled water. The total volume of each combined reaction mixture was 20 µl. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) amplification was conducted under the following conditions: cDNA synthesis at 50 °C for 2 min, RT inactivation at 95 °C for 2 min, 40 cycles of denaturation at 95 °C for 15 s, and annealing at 60 °C for 1 min. The dissociation reactions were performed on the qPCR system at 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. All reactions were run in triplicate and independently repeated at least thrice.
Statistical Analysis
Data are presented as mean ± SD. The statistical analysis was performed with SPSS software (version 21.0). Differences were tested for significance by one-way analysis of variance (ANOVA), followed by a post-hoc test with Bonferroni correction when appropriate. A value of P < 0.05 was considered to be statistically significant.
Results
Effects of Sinomenine on the Time of Mice in the Morphine-Induced Compartment
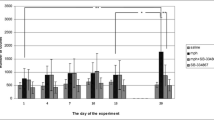
The effects of sinomenine on the time spent by the morphine-induced CPP mice are shown in Fig. 2. Compared with the control group, morphine dramatically increased the time spent by mice in the white compartment (**P < 0.01). Compared with the morphine model group, the high dose of sinomenine (80 mg/kg) and methadone (15 mg/kg) significantly reduced the time spent by mice in the white compartment (**P < 0.01), and the values had no significant difference with those in the control group (P > 0.05). However, the low dose of sinomenine (40 mg/kg) showed no significant difference on the values (P > 0.05).
Activity Routes of Mice in CPP
As shown in Fig. 3, the activity routes of the morphine model group were significantly different from those of the control group. Compared with the morphine model group, the low (40 mg/kg) and high (80 mg/kg) doses of sinomenine significantly reduced the activity routes. Similar results can be observed in the methadone group.
Effects of Sinomenine on TH Expression
Immunohistochemistry results showed that the morphine and low dose of sinomenine groups (40 mg/kg) have a significantly increased number of TH-positive cells and significantly higher TH expression than the control group (**P < 0.01); by contrast, the values in the sinomenine (80 mg/kg) and methadone (15 mg/kg) groups showed no significant difference compared with that in the control group (P > 0.05). Compared with the morphine model group, high dose of sinomenine (80 mg/kg) and methadone (15 mg/kg) can significantly down-regulate the expression of TH (**P < 0.01). Moreover, no significant difference was observed in TH expression between the sinomenine (40 mg/kg) and morphine model groups (P > 0.05) (Fig. 4; Table 2).
Effects of Sinomenine on NR2B Expression
Immunohistochemistry results showed that the morphine group had a significantly increased number of NR2B-positive cells and significantly higher NR2B expression than the control group (**P < 0.01); however, the values in the sinomenine (80 mg/kg) and methadone (15 mg/kg) groups showed no significant difference compared with that in the control group (P > 0.05). Compared with the morphine model group, the high dose of sinomenine (80 mg/kg) and methadone (15 mg/kg) significantly down-regulated NR2B expression (**P < 0.01); however, no significant difference in NR2B expression was observed between the sinomenine (40 mg/kg) and morphine model groups (P > 0.05) (Fig. 5; Table 3).
Effects of Sinomenine on MOR and DOR Expression Levels
qRT-PCR was performed to determine the mRNA expression of MOR and DOR after the behavioral test extinction CPP testing. As shown in Table 4, the expression levels of MOR were significantly down-regulated in the morphine model and sinomenine (40 mg/kg) groups compared with those in the control group (**P < 0.01); however, the high dose of sinomenine (80 mg/kg) and methadone (15 mg/kg) reversed and significantly up-regulated the MOR expression (**P < 0.01). Compared with the morphine model group, MOR expression was significantly increased in the brains of mice in the high dose of sinomenine (80 mg/kg) and methadone groups (**P < 0.01). However, the low dose of sinomenine (40 mg/kg) did not significantly change the levels of MOR.
Compared with the control group, the expression levels of DOR were significantly down-regulated in the morphine model and sinomenine (40 mg/kg) groups (**P < 0.01). The low (40 mg/kg) and high doses of sinomenine (80 mg/kg) significantly lowered the DOR expression levels (**P < 0.01). However, the values in the methadone (15 mg/kg) group were higher than those in the control group (**P < 0.01). In addition, compared with the morphine model group, DOR expression in mice brains were significantly higher in the methadone group (**P < 0.01) but remained unchanged in the high and low dose of sinomenine groups (P > 0.05).
Discussion
CPP is a classical experimental model for evaluating drug dependence [23]. The conditioned mice exhibited a preference for the environment that was previously paired with morphine in a drug-free condition [24]. When the morphine administration was repeatedly associated with a distinct circumstance, the circumstance served as a cue and induced positive subjective feelings even in the absence of the drug. In this study, the mouse model in the morphine-induced CPP was used to further investigate the anti-addictive effect of sinomenine and its underlying mechanisms by focusing on the neurotransmitters and their drug addiction-related receptors [25]. Methadone is a MOR agonist. Several studies indicated that blocking the NMDA receptor can mediate the pharmacological effects of methadone, attenuate morphine tolerance and dependence, and reduce the expression of CPP induced by morphine [26, 27]; moreover, methadone maintenance treatment is an effective intervention for treating heroin dependence and is widely used as an opioid replacement therapy. Research suggests that a good maintaining effect was obtained for patients treated with 60 mg/day or a higher dose of methadone. According to the formula of dose conversion relationship between mice and humans, our previous toxicology measurement found that in mice, treatment with methadone (15 mg/kg) was safe, and no side effects were observed. Thus, methadone (15 mg/kg) was selected as the positive drug in this experiment [28,29,30]. In a previous study, we used morphine at a dose of 9 mg/kg to induce stable CPP with a reasonably low mortality [21]. The current results showed that morphine significantly increases the time spent and the activity routes of mice in the morphine-paired group compared with those of the control group, indicating successful morphine-dependent mouse CPP models. Methadone and high doses of sinomenine dramatically reversed the morphine-induced CPP effect in mice, which was similar to previous reports that sinomenine can suppress the acquisition of morphine-induced CPP in mice [21].
TH and NR2B were the primary substances in the current research [22]. TH plays a key role in drug addiction in humans and animals. Its main function is to catalyze the conversion of l-tyrosine into l-dopa [31]. NMDA receptors are involved in morphine tolerance and dependence; however, little is known about the roles of individual NMDA receptor subtypes [32]. For example, NR2B has a lower desensitization capacity and requires a longer recovery time from desensitization than the NR2A subunits [33, 34]. The reduced NR2B expression during development is marked by a concomitant change in the NMDA receptor function [35]. The functional properties of NR2B renders it an attractive target in studying the mechanisms behind the experience-dependent changes in behavioral responses [32]. Therefore, we selected the morphine dependence-associated substances, TH and NR2B, as the biochemical indicators in detecting the morphine-dependent formation in the mouse brains to evaluate the morphine-associated neurobiological mechanisms.
Immunohistochemistry results showed that morphine-positive cells were significantly increased in the brains of the model group, indicating that morphine can increase the density of positive neurons in the mouse brains. The increases in TH- and NR2B-positive neurons have certain connections with CPP, and these might be among the neurobiological mechanisms involved in the changes in animal behavior. Compared with the morphine model group, the methadone can significantly inhibit the expression levels of TH and NR2B in mouse brain induced by morphine. The low and high doses of sinomenine dramatically reduced the expression levels of TH and NR2B, indicating that sinomenine has inhibitory effects on the CPP in mice induced by morphine. Therefore, sinomenine may down-regulate the expression levels of TH and NR2B.
Numerous studies have revealed that the endogenous opioid system plays a key role in regulating mood and reward and is central in modulating addictive behavior [36]. Some in vitro studies demonstrated that the down-regulation of opioid receptors follow the tolerance induced by chronic agonist exposure [37, 38]. Moreover, chronic morphine exposure will change DOR function, and aberrant DOR activity may contribute to the behavioral disorder caused by repeated MOR activation [39]. Shippenberg TS et al. found that the DOR antagonist naltrindole inhibited morphine-induced CPP [40]. Chefer et al. also reported that morphine tolerance and reward decreased in DOR gene knockout rats [41]. The present study used qRT-PCR to examine the alterations in the mRNA expression levels of MOR and DOR in the brains of morphine CPP mice. We found that the mRNA levels of MOR and DOR decreased in the morphine model group, whereas sinomenine significantly reversed this down-regulation and up-regulated the MOR mRNA expression levels to a certain extent compared with those in the control group. However, the mRNA level of DOR may not be involved in this process. The increased mRNA level of MOR indicated that the anti-morphine dependence of sinomenine is associated with the ameliorated content of MOR but not the mRNA level of DOR in mouse brains.
In summary, our research showed that sinomenine reverses the expression of morphine-induced reward effect, as mediated by the regulation of TH, NR2B, and MOR expression levels in the mouse brains. Therefore, sinomenine can be developed to treat or prevent morphine abuse. Further study is needed to clarify the possible mechanisms of the effect of sinomenine in morphine dependence to provide new directions for studying the mechanisms underlying drug addiction.
References
Li W, Zhang CL, Qiu ZG (2017) Differential expression of endocannabinoid system-related genes in the dorsal hippocampus following expression and reinstatement of morphine conditioned place preference in mice. Neurosci Lett 643:38–44
Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ (2010) A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev 30:155–166
Li Y, Zheng X, Xu N, Zhang Y, Liu Z, Bai Y (2017) The consummatory and motivational behaviors for natural rewards following long-term withdrawal from morphine: no anhedonia but persistent maladaptive behaviors for high-value rewards. Psychopharmacology 234(8):1277–1292
Garcia-Perez D, Lopez-Bellido R, Rodriguez RE, Laorden ML, Nunez C, Milanes MV (2015) Dysregulation of dopaminergic regulatory mechanisms in the mesolimbic pathway induced by morphine and morphine withdrawal. Brain Struct Funct 220:1901–1919
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Nestler EJ, Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278:58–63
Sante AB, Nobre MJ, Brandao ML (2000) Place aversion induced by blockade of mu or activation of kappa opioid receptors in the dorsal periaqueductal gray matter. Behav Pharmacol 11:583–589
Noble F, Szucs M, Kieffer B, Roques BP (2000) Overexpression of dynamin is induced by chronic stimulation of mu- but not delta-opioid receptors: relationships with mu-related morphine dependence. Mol Pharmacol 58:159–166
Ma J, Yuan X, Qu H, Zhang J, Wang D, Sun X, Zheng Q (2015) The role of reactive oxygen species in morphine addiction of SH-SY5Y cells. Life Sci 124:128–135
Guo M, Cao D, Zhu S, Fu G, Wu Q, Liang J, Cao M (2015) Chronic exposure to morphine decreases the expression of EAAT3 via opioid receptors in hippocampal neurons. Brain Res 1628:40–49
Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P (2010) Two tyrosine hydroxylase genes in vertebrates new dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci 43:394–402
Cadet JL, Jayanthi S, McCoy MT, Ladenheim B, Saint-Preux F, Lehrmann E, De S, Becker KG, Brannock C (2013) Genome-wide profiling identifies a subset of methamphetamine (METH)-induced genes associated with METH-induced increased H4K5Ac binding in the rat striatum. BMC Genom 14:545
Tsai RY, Chou KY, Shen CH, Chien CC, Tsai WY, Huang YN, Tao PL, Lin YS, Wong CS (2012) Resveratrol regulates N-methyl-d-aspartate receptor expression and suppresses neuroinflammation in morphine-tolerant rats. Anesth Analg 115:944–952
Yamasaki H (1976) Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med Okayama 30:1–20
Wang MH, Chang CK, Cheng JH, Wu HT, Li YX, Cheng JT (2008) Activation of opioid mu-receptor by sinomenine in cell and mice. Neurosci Lett 443:209–212
Liu Z, Zheng JF, Yang LQ, Yi L, Hu B (2007) Effects of sinomenine on NO/nNOS system in cerebellum and spinal cord of morphine-dependent and withdrawal mice. Sheng Li Xue Bao 59:285–292
Liu Z, Zheng JF, Hu B (2007) The effect of sinomenine on nNOS activity of brain tissues in morphine dependent and withdrawal mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi 23:297–299
Zhang GM, Mo ZX, Wang CY (2009) Study on the detoxification of alcohol extracts from orientvine and its effective component on withdrawal syndromes of morphine. Zhong Yao Cai 32:1414–1418
Mo ZX, Xu DD, Wang CY (2004) Effect of Caulis Sinomenii and sinomenine on naloxone-precipitated withdrawal response in morphine-dependent models in vitro and in vivo. Chin J Clin Rehabil 8:7879–7881
Wang CY, Mo ZX, Tu HH (2003) Effect of sinomenine on morphine dependence in isolated guinea pig ileum. Di Yi Jun Yi Da Xue Xue Bao 23:329–331
Mo ZX, An SL, Zhou JY (2006) Effects of Caulis Sinomenii and sinomenine on morphine-induced place preference and brain histamine level in mice. Nan Fang Yi Ke Da Xue Xue Bao 26:1709–1713
Li J, Liu W, Peng Q, Jiang M, Luo C, Guo Y, Liu Y, Fang M, Mo Z (2014) Effect of rhynchophylline on conditioned place preference on expression of NR2B in methamphetamine-dependent mice. Biochem Biophys Res Commun 452:695–700
Zhu C, Liu W, Luo C, Liu Y, Li C, Fang M, Lin Y, Ou J, Chen M, Zhu D, Yung KK, Mo Z (2017) Inhibiting effects of rhynchophylline on methamphetamine-dependent zebrafish are related with the expression of tyrosine hydroxylase (TH). Fitoterapia 117:47–51
Zhou JY, Mo ZX, Zhou SW (2010) Effect of rhynchophylline on central neurotransmitter levels in amphetamine-induced conditioned place preference rat brain. Fitoterapia 81:844–848
Jiang M, Chen Y, Li C, Peng Q, Fang M, Liu W, Kang Q, Lin Y, Yung KK, Mo Z (2016) Inhibiting effects of rhynchophylline on zebrafish methamphetamine dependence are associated with amelioration of neurotransmitters content and down-regulation of TH and NR2B expression. Prog Neuropsychopharmacol Biol Psychiatry 68:31–43
Matsui A, Williams JT (2010) Activation of micro-opioid receptors and block of Kir3 potassium channels and NMDA receptor conductance by l- and d-methadone in rat locus coeruleus. Br J Pharmacol 161:1403–1413
Siahposht-Khachaki A, Fatahi Z, Haghparast A (2016) Reduction of the morphine maintenance by blockade of the NMDA receptors during extinction period in conditioned place preference paradigm of rats. Basic Clin Neurosci 7:341–350
Vigezzi P, Guglielmino L, Marzorati P, Silenzio R, De Chiara M, Corrado F, Cocchi L, Cozzolino E (2006) Multimodal drug addiction treatment: a field comparison of methadone and buprenorphine among heroin- and cocaine-dependent patients. J Subst Abuse Treat 31:3–7
Craig RJ (1980) Effectiveness of low-dose methadone maintenance for the treatment of inner city heroin addicts. Int J Addict 15:701–710
Freedman RR, Czertko G (1981) A comparison of thrice weekly LAAM and daily methadone in employed heroin addicts. Drug Alcohol Depend 8:215–222
Gibb JW, Kogan FJ (1979) Influence of dopamine synthesis on methamphetamine-induced changes in striatal and adrenal tyrosine hydroxylase activity. Naunyn Schmiedebergs Arch Pharmacol 310:185–187
Ko SW, Wu LJ, Shum F, Quan J, Zhuo M (2008) Cingulate NMDA NR2B receptors contribute to morphine-induced analgesic tolerance. Mol Brain 1:2
Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR (1998) Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol 79:555–566
Loftis JM, Janowsky A (2003) The N-methyl-d-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther 97:55–85
Dumas TC (2005) Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol 76:189–211
Yoo JH, Kitchen I, Bailey A (2012) The endogenous opioid system in cocaine addiction: what lessons have opioid peptide and receptor knockout mice taught us? Br J Pharmacol 166:1993–2014
Dang VC, Christie MJ (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165:1704–1716
Harrison RS, Ruiz-Gomez G, Hill TA, Chow SY, Shepherd NE, Lohman RJ, Abbenante G, Hoang HN, Fairlie DP (2010) Novel helix-constrained nociceptin derivatives are potent agonists and antagonists of ERK phosphorylation and thermal analgesia in mice. J Med Chem 53:8400–8408
Wu Q, Xia S, Lin J, Cao D, Chen W, Liu L, Fu Y, Liang J, Cao M (2013) Effects of the altered activity of delta-opioid receptor on the expression of glutamate transporter type 3 induced by chronic exposure to morphine. J Neurol Sci 335:174–181
Shippenberg TS, Chefer VI, Thompson AC (2009) Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry 65:169–174
Chefer VI, Shippenberg TS (2009) Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology 34:887–898
Acknowledgements
This work was supported by Fund Projects: the National Natural Science Foundation of China (No. 81229003, 81673628); the Guangzhou Major Science and Technology Project (No. 201300000050).
Author information
Authors and Affiliations
Contributions
Designed the study: MF, JKL, DQZ, ZXM. Coordinated the study and finalized the manuscript: MF, DQZ. Read and approved the manuscript: CL. Performed the experiments: JKL, CZ, MLF, MF, DQZ. Analyzed the data: CZ. Wrote the paper: MF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fang, M., Li, J., Zhu, D. et al. Effect of Sinomenine on the Morphine-Dependence and Related Neural Mechanisms in Mice. Neurochem Res 42, 3587–3596 (2017). https://doi.org/10.1007/s11064-017-2407-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2407-5