Abstract
Long-term metformin treatment reduces the risk of stroke. However, the effective administration pattern and indications of metformin on acute cerebral ischemia are unclear. To investigate the neuroprotective treatment duration and dosage of metformin on focal ischemia mice and the association of neuroprotection with 5′-adenosine monophosphate-activated protein kinase (AMPK) regulations, male C57BL/6 mice were subjected to permanent or transient middle cerebral artery occlusion (MCAO) and metformin of 3, 10 and 30 mg/kg was intraperitoneally injected 1, 3 or 7 days prior to MCAO, or at the onset, or 1, 3 or 6 h after reperfusion, respectively. Infarct volumes, neurological deficit score, cell apoptosis, both total and phosphorylated AMPK expressions were assessed. Results showed that prolonged pretreatment to 7 days of metformin (10 mg/kg) significantly ameliorated brain infarct, neurological scores and cell apoptosis in permanent MCAO mice. Shorter (3 days or 1 day) or without pretreatment of metformin was not effective, suggesting a pretreatment time window. In transient MCAO mice, metformin showed no neuroprotection even with pretreatment. The expressions of total and phosphorylated AMPK were sharply decreased with effective metformin pretreatments in ischemic brains. Our data provided the first evidence that in acute ischemic injury, a 7-days pretreatment duration of 10 mg/kg metformin is necessary for its neuroprotection, and metformin may not be beneficial in the cases of blood reperfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia is one of the leading causes of mortality and disability worldwide with limited therapeutic approaches. Extensive studies have been carried out to find effective drugs against cerebral ischemia during the last decades, yet unfortunately little achievement has been obtained in this field.
Emerging evidences suggest that metformin, a hypoglycemic, is related to reduce the risk of stroke in diabetic patients [1]. Animal studies demonstrate that long-term treatment of metformin promotes neuronal recovery after ischemia [2, 3]. In the molecular level, activation of adenosine monophosphate-activated protein kinase (AMPK) is proposed to associate with its neuroprotection [2, 4–6]. However, the effects of metformin on acute injury after ischemia remain controversial. The acute phase outcomes measured by experimental animals are critical in evaluating one potential neuroprotectant prior to clinical trials according to the recommendations from Stroke Therapy Academic Industry Roundtable (STAIR) [7]. As revealed by middle cerebral artery occlusion (MCAO) models on mice, metformin reduces cerebral infarct volumes 1 day after both transient and permanent MCAO (tMCAO, pMCAO thereafter) [2, 5]. On the contrary, however, metformin shows no neuroprotection against acute tMCAO injury but promote long-term neuronal recovery [4, 8]. Enigmatically, metformin even aggravates brain infarct size in diabetic rats as well as intact mice subject to tMCAO [9, 10]. The deleterious effects may be attributable to AMPK activation by metformin, which has been clearly shown to aggravate ischemic brain injury [9, 11, 12]. Therefore, the potential therapeutic effects of metformin for ischemic stroke need careful consideration [3, 9].
In the present investigation, we aimed to identify the effective treatment pattern, duration and dosage of metformin on acute cerebral ischemia and further address the involvement of AMPK regulations in the neuroprotection.
Materials and Methods
Animals
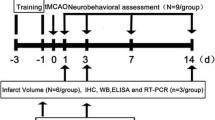
Male C57BL/6 mice weighing 22–25 g were used. All experiments were approved by and conducted in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize any pain or discomfort, and the minimum number of animals was used.
Metformin and Compound C Treatments
Metformin hydrochloride (Selleckchem, USA) was dissolved in saline and given in a dose volume of 0.2 ml/20 g body weight by intraperitoneal (i.p.) injection. For pretreatment, mice were pretreated with metformin (3, 10 or 30 mg/kg/day, i.p.) for 1, 3 or 7 days prior to stroke. For the treatment after ischemia, metformin was administrated at the onset or at 1, 3 or 6 h after reperfusion, respectively. Compound C (Selleckchem, USA) was dissolved in saline and administered (20 mg/kg/day, i.p.) concomitant with metformin administration. The control group received an equal volume of saline.
Permanent and Transient MCAO Mouse Models
Focal cerebral ischemia was induced by right MCAO as described previously [13]. Mice were anesthetized by intraperitoneal injection of choral hydrate (350 mg/kg). Cerebral blood flow was determined by laser Doppler flowmetry (Moor Instruments, Devon, UK). For permanent MCAO (pMCAO), a 6-0 nylon monofilament suture was gently inserted 10 mm into the internal carotid to occlude the origin of the MCA. Animals with <80 % reduction in CBF in the MCA territory were excluded. For transient MCAO (tMCAO), the suture was withdrawn smoothly to allow blood flow restoration after 60 min of occlusion. Body temperature was maintained at 37 °C by a heatlamp (FHC, Bowdoinham, USA) during surgery and within 2 h after the onset of reperfusion. Sham-operated mice received the same procedure except for the suture insertion process.
Infarct Analysis
At 24 h after surgery, mice were anesthetized and decapitated. Coronal brain slices at 2-mm intervals were stained with 2,3,5-triphenyltetrazolium hydrochloride (TTC; 0.25 %; Sigma-Aldrich, USA) at 37 °C for 30 min, and then fixed with formalin (4 %). The extents of the normal and infarct areas were analyzed with Image-Pro Plus 7.0 (Media Cybernetics, Bethesda, MD, USA) and determined by the indirect method, which corrected for edema. The percentage of the corrected infarct volume was calculated by dividing the infarct volume by the total contralateral hemispheric volume.
Neurological Deficit Scores
Neurological deficit scores were evaluated at 24 h after surgery as follows: 0, no deficit; 1, forelimb weakness and turning to the ipsilateral side when held by tail; 2, circling to the contralateral side; 3, unable to bear weight on affected side; and 4, no spontaneous motor activity.
Western Blot Analysis
Western blots were done as described previously [14]. The right brain cortex tissues were collected before occlusion or at 12 h after occlusion or reperfusion and were homogenized using RIPA buffer. Samples with an equal amount of protein were separated on 10–12 % SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane, which was then blocked with 5 % BSA powder in Tris-buffered saline containing 0.1 % Tween 20 at room temperature for 1 h. Primary antibodies against cleaved Caspase-3 (1:1000, CST, USA), Bax (1:1000, CST, USA), Bcl-2 (1:1000, CST, USA), pAMPK (1:500, CST, USA), AMPK (1:1000, CST, USA) and GAPDH (1:3000, KangChen, China) were added for overnight incubation at 4 °C. After washing, the membranes were incubated with rabbit or mouse IgG-HRP-linked secondary antibody (1:3000, KangChen, China) for 2 h at room temperature. ECL detection kit (Thermo Fisher, USA) was used for signal detection. The relative optical density was obtained by comparing the measured values with the mean values from the control group by Image-Pro Plus 7.0.
Statistical Analysis
All data were collected and analyzed in a blinded manner. Data were presented as mean ± SEM. For comparison between two groups, statistical significance was determined through a Student t test. For comparison among multiple groups, statistical significance was evaluated by One-way ANOVA (with Tukey post hoc test, when appropriate) except the neurological deficit scores, which was determined by Mann–Whitney U test. p < 0.05 was considered statistically significant.
Results
Metformin Pre-treatment Protected Against Permanent Cerebral ischemia
Mice were pretreated with indicated dosages of metformin (Met) for 7, 3, or 1 day before pMCAO. For 7-days pretreatment, only 10 mg/kg/day Met significantly decreased the brain infarct volumes (Met 22.93 ± 7.95 % vs. Saline 56.95 ± 5.24 %, p < 0.01). This neuroprotection was reduced for 3-days pretreatment (Met 31.70 ± 5.26 % vs. Saline 56.95 ± 5.24 %, p < 0.05) and was not observed for 1 day pretreatment. We further found that 3 or 30 mg/kg/day Met showed no neuroprotective effects regardless of the pretreatment duration (Fig. 1a, b).We also assayed 100 mg/kg/day Met with aforementioned pretreatment durations, which also showed no protection (data not shown). However, Met treatment at the ischemia onset was unavailing. These results suggested that pretreatment was required for the neuroprotection of Met against acute permanent ischemia injury. The dosage of Met pretreatment also seems critical for its neuroprotection, which was reflected in the neurological deficient scores as well (NDS, Met 10 mg/kg/day Pre-7 day 1.50 ± 0.29 vs. Saline 2.78 ± 0.15, p < 0.01; Met 10 mg/kg/day Pre-3 day 1.83 ± 0.31 vs. Saline 2.78 ± 0.15, p < 0.05) (Fig. 1c).
Metformin pretreatment protected against permanent cerebral ischemia. Metformin 3, 10 or 30 mg/kg/day (Met 3, 10 or 30) were administrated from 1, 3 or 7 days prior to ischemia (Pre-1, 3, 7 days) continuously or at the ischemia onset (Isc-0 h). Mice were sacrificed 24 h after pMCAO and infarct volumes were determined by TTC staining. a The representative TTC-stained brain slices from each group were shown. b The infarct volumes of each group were analyzed by one-way ANOVA with Tukey post-hoc test. c NDS was determined as previously described and was analyzed by Mann–Whitney U test. n = 7/group. *p < 0.05 and **p < 0.01 vs. saline; Data were expressed as Mean ± SEM
Blood restore after ischemia leads to brain reperfusion injury. To identify the neuroprotection of Met on reperfusion injury, transient MCAO (tMCAO) model was employed. Results showed that neither Met pretreatment nor treatment at the onset of reperfusion reduced the corrected infarct volumes and NDS (Fig. 2a–c). Besides, we found that delayed Met treatments at 3 h after reperfusion even aggregated the ischemic injury as revealed by enlarged infarct volumes (Met 59.88 ± 9.48 % vs. Saline 23.33 ± 10.06 %, p < 0.05) and increased NDS (Met 3.67 ± 0.33 vs. Saline 2.33 ± 0.33, p < 0.05) (Fig. 2d–f). These results indicated that Met may not be beneficial in the stroke cases with reperfusion.
Metformin cannot rescue tMCAO-induced brain injury. a Metformin 3, 10 or 30 mg/kg/day (Met 3, 10 or 30) were treated from 1, 3 or 7 days prior to ischemia (Pre-1, 3, 7 days) continuously or at the reperfusion onset (Rep-0 h). Mice were sacrificed 24 h after tMCAO and the representative TTC-stained brain slices from each group were shown. b Infarct volumes and c NDS were measured as mentioned previously. d Metformin 10 mg/kg (Met 10) was treated at 1, 3 or 6 h after reperfusion and mice were sacrificed 24 h after tMCAO. The representative TTC-stained brain slices from each group were shown. e Infarct volumes and f NDS were measured as mentioned previously. n = 7/group. The infarct volumes were analyzed by one-way ANOVA with Tukey post-hoc test and the NDS by Mann–Whitney U test. *p < 0.05 vs. saline; Data were expressed as Mean ± SEM
Metformin Pretreatment Inhibited pMCAO-Induced Cell Apoptosis
Apoptosis is responsible for cell demise in ischemic brains. To further confirm the beneficial effect of Met in pMCAO model, apoptosis-related proteins were determined at 12 h after ischemia. Met 10 mg/kg/day Pre-7 days administration significantly reversed MCAO-induced reduction of the Bcl-2/Bax ratio (p < 0.001) and down regulated the expression of cleaved Caspase-3 (p < 0.001) following pMCAO, while these effects were not seen in other dosages (Fig. 3a, b). Then we investigated whether different duration of Met treatment affected apoptosis in ischemic brains. As expected, the protection of Met 10 mg/kg/day on apoptosis was reproduced with 7 days pretreatment and Met 10 mg/kg/day Pre-3 days treatment also increased the Bcl-2/Bax ratio (p < 0.001) in a less extent (Fig. 3c, d).
Metformin pretreatment prevented pMCAO-induced cell apoptosis. Right brain cortex tissues were collectedat12 h after pMCAO. a, b Metformin 3, 10 or 30 mg/kg/day (Met 3, 10 or 30) were administrated 7 days prior to stroke (Pre-7 days), and the expressions of Bcl-2, Bax, cleaved Caspase-3 were detected as markers of cell apoptosis, GAPDH was used as loading control. c, d Metformin 10 mg/kg/day (Met 10) was administrated from 7, 3 or 1 day before ischemia (Pre-7, 3 or 1 day) or at the stroke onset (Isc-0 h), respectively. The expressions of Bcl-2, Bax, cleaved Caspase-3 and GAPDH were detected. n = 4/group. ### p < 0.001 vs. sham, ***p < 0.001 vs. saline (One-way ANOVA with Tukey post-hoc test); Data were presented as Mean ± SEM
Metformin Pretreatment Reduced the Expressions of pAMPK and Total AMPK After Ischemia
Metformin is an AMPK activator, but the roles of AMPK in cerebral ischemia with metformin treatment were not clear. The western blot analysis showed increased AMPK activation following pMCAO, which peaked at 12 h and started to decline after 24 h (Online Resource Fig. S1). So the mice were sacrificed at 12 h after pMCAO for further pAMPK detection. In intact brains, 30 but not 3 or 10 mg/kg/day Met pretreatment for 7 d increased pAMPK level. Unexpectedly, in ischemic brains, Met 10 mg/kg/day, but not 3 or 30 mg/kg/day, pretreatment reduced rather than further increased pMCAO-induced pAMPK. Interestingly, the total AMPK level sharply decreased as well in ischemic brains pretreated with Met 10 mg/kg, and in a less extent with 3 or 30 mg/kg pretreatment (Fig. 4a, b). We also determined the mRNA levels of both AMPK α1 and α2 subunits by real-time PCR, the results showed no significant reduction (Online Resource Fig. S2), implying the post-translational regulations of the total AMPK. This reduction of total AMPK cannot be found in other organs from the same mice, suggesting a specific action (Online Resource Fig. S3). When mice was treated with shorter duration (1 day or 3 days) of Met 10 mg/kg/day, the reduction of pAMPK and total AMPK in ischemic brains was not that remarkable comparing to 7 days pretreatment (Fig. 4c, d).
Metformin pretreatment reduced the expression of pAMPK and total AMPK after ischemia. Right brain cortex samples were harvested at 12 h after MCAO. a, b Metformin 3, 10 or 30 mg/kg/day (Met 3, 10 or 30) was treated 7 days before pMCAO. The expressions of AMPK and pAMPK were determined by western blot. c, d Metformin 10 mg/kg/day (Met 10) was treated 1, 3 or 7 days prior to stroke (Pre-1, 3 or 7 days), respectively. The expressions of AMPK and pAMPK were determined by western blot. n = 4/group. The columns represented the semi-quantified optidensity of the bands normalized by GAPDH, the control bands were defined as 1.00. * and # p < 0.05; ** and ## p < 0.01; *** and ### p < 0.001 vs. indicated groups
Compound C Showed Additional Neuroprotective Effects to Metformin
To further confirm that metformin-reinforced pAMPK reduction was associated with its neuroprotection, we conducted an AMPK inhibitor, Compound C (CC). The results showed that Pre-7 days CC 20 mg/kg (CC 20) co-treatment with Met 10 mg/kg did not reverse, but synergistically enhanced its neuroprotection (Met 10 22.32 ± 4.90 % vs. Met 10 + CC 20 8.29 ± 2.22 %, p < 0.05; CC 20 20.28 ± 3.99 % vs. Met 10 + CC 20 8.29 ± 2.22 %, p < 0.05) (Fig. 5a, b). NDS presented similar results (Met 10 1.6 ± 0.24 vs. Met 10 + CC 20 0.83 ± 0.17, p < 0.05; CC 20 1.6 ± 0.24 vs. Met 10 + CC 20 0.83 ± 0.17, p < 0.05) (Fig. 5c). In addition, we found that the combinational treatment with CC and Met further reduced both the total and phosphorylated AMPK levels (Fig. 5e, f). These results suggested that further AMPK inactivation may be responsible for the additional neuroprotection of CC.
Compound C showed additional neuroprotective effects to metformin. Mice were treated with metformin 10 mg/kg (Met 10) or Compound C 20 mg/kg (CC 20) or co-treatment (Met 10 + CC 20) 7 days before stroke continuously. CC was injected prior to Met each time. At 24 h after pMCAO, infarct volumes were detected by TTC staining. a The representative TTC-stained brain slices from each group were shown. b The infarct volumes of each group were analyzed by one-way ANOVA with Tukey post-hoc test. c NDS was determined as previously described and was analyzed by Mann–Whitney U test. n = 7/group. d Representative results of pAMPK and AMPK by western blot. e The columns represent semi-quantitative analysis of pAMPK and AMPK bands normalized by GAPDH. The values of control were defined as 1.0. n = 4/group *p < 0.05 and **p < 0.01; data were expressed as Mean ± SEM
Discussion
Although a few evidences have showed that prestroke metformin treatment protects against brain injuries, the administration duration, dosage, and indications for metformin are largely unclear. To identify the pretreatment duration, metformin was administrated at indicated time points before stroke onset. We found that 10 mg/kg/day metformin significantly counteracted ischemic injury in a time-dependent manner with the most potential neuroprotection from 7-days pretreatment, whereas metformin treatment at the onset of ischemia showed no effects. These observations were further confirmed by assessing cell apoptosis. These data confirm the requirement of prestroke administration, and further suggested that a pretreatment time window no less than 7 days was required for the neuroprotection of metformin against acute brain injury. We found 3 or 30 mg/kg/day for 7-days pretreatment were not effective. Similarly, recent study suggested that the biological effects of metformin may be attributable to its cumulative dosage based on the dosage administrated daily [15, 16]. Therefore, it seems the cumulative metformin dosage may closely relate with its neuroprotection against ischemic brain injury. Moreover, our results found 30 and 100 mg/kg/day metformin pretreatment for shorter time did not show neuroprotection, indicating the time window cannot be reduced by increasing metformin dosage. These results extend the conception that the cumulative dynamics of metformin dosage may be a key of the therapy for stroke by metformin pretreatment.
Our study showed that a relatively higher dosage of 30 mg/kg pretreatment did not offer neuroprotection. Similar results were reproduced by other studies [6, 9, 17], in which low-dose of metformin pre-conditioning may constitute a safer and efficient way to protect brain injury. Higher dosage of metformin lead to lactic acid accumulation [9], which results in acidosis and cell apoptosis [18]. Besides, higher dosage of metformin reinforced ischemia-induced AMPK activation, which was demonstrated detrimental for neuronal survival [12]. The dosage of 10 mg/kg metformin in mice is approximately 50 mg daily (60 kg of body weight) in human, according to the body surface area normalization [19]. This dosage is approximately 20 % of the clinical prescribed dosage of metformin by considering its relative bioavailability, which is normally range from 500 to 2000 mg daily. Although metformin has being considered as a safe drug [20], adverse effects are documented due to long-term use or overdose [21, 22]. Our observation thus suggested that a low dosage of metformin pretreatment for a relative long duration could be competent for cerebral ischemia therapy.
Interestingly, we found metformin pretreatment failed to rescue ischemia-reperfusion-induced brain injury. Treatment of metformin after reperfusion even made severer brain infarct. These results thus implied that the neuroprotection from metformin pretreatment may depend on the pathological conditions of cerebral ischemia. In consist, we found a sustained AMPK activation with permanent ischemia, whereas AMPK activation decreased with reperfusion [23]. Despite the fact that blood supply restore remains the principle therapy for cerebral ischemia, only a few patients are able to receive the thrombolysis therapy [24]. Therefore, the present study pointed to a potential application of metformin for treatment of cerebral ischemia as a preventive neuroprotectant without thrombolysis and the administration might be withdrawn once reperfusion was established.
We interestingly found that 7-days pretreatment of 10 mg/kg metformin significantly decreased the total and phosphorylated AMPK levels in ischemic brains. This reduction was not that remarkable for other dosages with 7-days pretreatment or with 10 mg/kg with shorter duration. These data suggested an association between AMPK reduction and metformin’s neuroprotection. This assumption was further supported by using Compound C, an AMPK inhibitor, which reinforced the neuroprotection of metformin. Nevertheless, 3-days metformin pretreatment reduced the ischemic brain injury without significantly reduced the pAMPK level. Indeed, acute metformin treatment reduced ischemic brain injury by pre-activating AMPK [5], however, we found that the AMPK activation cannot extend to 7 days after metformin pretreatment in intact brains. Therefore, the present study suggested that metformin may have bi-directional impacts on AMPK activation. Given that in most cases metformin is chronically administrated, AMPK may be inactivated in the ischemic brains with long-term metformin treatment. The AMPK subunits are widely expressed throughout the brain. We determined the AMPKα subunits in the present study. AMPK α subunits are mainly neuronal localized in mouse brain, and AMPK α2 is highly expressed in activated astrocytes, which are found in ischemic brains [25]. Metformin was showed to suppress the C6 glioma cells activation [26]. Thus it is plausible that both neurons and astrocytes may contribute to the AMPKα subunits regulation in brains. Further studies are needed to address these issues. The present data implied that AMPK inactivation can be a potential marker indicating metformin’s neuroprotection.
The AMPK signaling is typically activated by energy deprivation like ischemia, which was confirmed by our data. In ischemic brains, transient AMPK activation was clearly proved deleterious for neuronal survival [11, 12]. In contrast, there were evidences indicating chronic metformin pretreatment lead to reduced pAMPK levels and offer potent neuroprotection [12, 27]. How metformin pretreatment reduced AMPK phosphorylation was not determined. We surprisingly found that 7-days pretreatment of 10 mg/kg metformin significantly decreased rather than reinforced the total AMPK level in ischemic brains. Recent investigations indicated that AMPK subunits were ubiquitylated for degradation [28–30]. In addition, metformin was reported to modify post-translation proteins by ubiquitination [31]. We found both the AMPK α1 and α2 subunits were not transcriptionally downregulated. Although directed evidences are still lacking, we assume that the post-translational mechanisms response to AMPK reduction with metformin treatment. Immediate degradation of AMPK α was found when overexpressed in COS-7 cells, and the turnover of α subunit auto-inhibited its catalytic activity [32]. Hence it is plausible that total AMPK decrease will reduce its kinase activity. Metformin is well-accepted as an AMPK activator, whereas our results clearly indicated that long-term metformin administration may reduce AMPK activity, at least in ischemic brains. A long-term and low dosage metformin could be an unexpected approach to shut down the deleterious AMPK signaling during ischemia. Therefore the effects of metformin in AMPK regulations must be confirmed in further studies. In addition, it cannot be exclude that AMPK-independent mechanisms may also contribute to the neuroprotection of metformin.
Taken together, the present study identified a pretreatment time window effective for stroke intervention with metformin. The AMPK inactivation is involved in the availability of the time window. Both the metformin dosage and blood reperfusion after ischemia attenuated the neuroprotection possibly by altering the AMPK inactivation. These data suggested the potential values of long-term metformin treatment in reducing ischemic brain injury and brought forth the notion that the potential neuroprotection of metformin was limited by the administration manner.
References
Cheng YY, Leu HB, Chen TJ, Chen CL, Kuo CH, Lee SD, Kao CL (2014) Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: a 4-year follow-up study. J Stroke Cerebrovasc Dis 23:e99–e105
Liu Y, Tang G, Li Y, Wang Y, Chen X, Gu X, Zhang Z, Wang Y, Yang GY (2014) Metformin attenuates blood–brain barrier disruption in mice following middle cerebral artery occlusion. J Neuroinflammation 11:177
Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A (2015) Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis 30:747–754
Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, Zhou X, Qin Z, Jia J, Zhen X (2014) Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun 40:131–142
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L (2014) Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171:3146–3157
Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M (2014) Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metab Brain Dis 29:47–58
Stroke Therapy Academic Industry Roundtable (1999) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30:2752–2758
Venna VR, Li J, Hammond MD, Mancini NS, McCullough LD (2014) Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci 39:2129–2138
Li J, Benashski SE, Venna VR, McCullough LD (2010) Effects of metformin in experimental stroke. Stroke 41:2645–2652
Li W, Qu Z, Prakash R, Chung C, Ma H, Hoda MN, Fagan SC, Ergul A (2013) Comparative analysis of the neurovascular injury and functional outcomes in experimental stroke models in diabetic Goto-Kakizaki rats. Brain Res 1541:106–114
McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV (2005) Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280:20493–20502
Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD (2007) Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38:2992–2999
Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, Wang G, Chen Z (2013) Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9:1321–1333
Zhang X, Yuan Y, Jiang L, Zhang J, Gao J, Shen Z, Zheng Y, Deng T, Yan H, Li W, Hou WW, Lu J, Shen Y, Dai H, Hu WW, Zhang Z, Chen Z (2014) Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 10:1801–1813
Kuzik N, Myette-Cote E, Carson V, Slater L, Boule NG (2015) Evaluating the effects of metformin use on height in children and adolescents: a meta-analysis of randomized clinical trials. J Am Med Assoc Pediatr 169:1032–1039
Tseng CH (2015) Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol Oncol 138:147–153
Vazquez-Manrique RP, Farina F, Cambon K, Sequedo MD, Parker AJ, Millan JM, Weiss A, Deglon N, Neri C (2016) AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Hum Mol Genet 25(6):1043–1058
Shen Z, Jiang L, Yuan Y, Deng T, Zheng YR, Zhao YY, Li WL, Wu JY, Gao JQ, Hu WW, Zhang XN, Chen Z (2015) Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci Ther 21:271–279
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Diabetes Prevention Program Research Group (2012) Long-term safety, tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study. Diabetes Care 35:731–737
Spiller HA, Quadrani DA (2004) Toxic effects from metformin exposure. Ann Pharmacother 38:776–780
Forrester MB (2008) Adult metformin ingestions reported to Texas poison control centers, 2000–2006. Hum Exp Toxicol 27:575–583
Li M, Zhao J, Hu Y, Lu H, Guo J (2010) Oxygen free radicals regulate energy metabolism via AMPK pathway following cerebral ischemia. Neurol Res 32:779–784
Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, Karp H, LaBresh KA, Malarcher A, Mensah G, Moomaw CJ, Schwamm L, Weiss P, Paul Coverdell Prototype Registries Writing G (2005) Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke 36:1232–1240
Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72:1707–1716
Isakovic A, Harhaji L, Stevanovic D, Markovic Z, Sumarac-Dumanovic M, Starcevic V, Micic D, Trajkovic V (2007) Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci 64:1290–1302
Venna VR, Li J, Benashski SE, Tarabishy S, McCullough LD (2012) Preconditioning induces sustained neuroprotection by downregulation of adenosine 5′-monophosphate-activated protein kinase. Neuroscience 201:280–287
Pineda CT, Ramanathan S, Fon Tacer K, Weon JL, Potts MB, Ou YH, White MA, Potts PR (2015) Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160:715–728
Lee JO, Lee SK, Kim N, Kim JH, You GY, Moon JW, Jie S, Kim SJ, Lee YW, Kang HJ, Lim Y, Park SH, Kim HS (2013) E3 ubiquitin ligase, WWP1, interacts with AMPKalpha2 and down-regulates its expression in skeletal muscle C2C12 cells. J Biol Chem 288:4673–4680
Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J, Li JZ, Wu J, Zhou HM, Li P (2008) Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. EMBO J 27:1537–1548
Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, Wong OG, Cheung AN, Ngan HY (2013) AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/beta-catenin signaling activity. PLoS One 8:e53597
Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA (1998) Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem 273:35347–35354
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81373393, 81273506, 81273490 and 81402907), Zhejiang Provincial Natural Science Foundation (LR15H310001, LY15H090004), the Research Project of Department of Education of Zhejiang Province (Y201329908) and the Program for Zhejiang Leading Team of S&T Innovation Team (2011R50014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All experiments were approved by and conducted in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Tian Deng and Yan-Rong Zheng have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, T., Zheng, YR., Hou, WW. et al. Pre-stroke Metformin Treatment is Neuroprotective Involving AMPK Reduction. Neurochem Res 41, 2719–2727 (2016). https://doi.org/10.1007/s11064-016-1988-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1988-8