Abstract
The dysregulation of hypothalamic–pituitary–adrenal axis and noradrenergic, serotonergic and glutamatergic systems are thought to be involved in the pathophysiology of post-traumatic stress disorder. The effect of selective M1 muscarinic receptor antagonist, pirenzepine on anxiety indices was investigated by using elevated plus maze, following exposure to trauma reminder. Upon receiving the approval of ethics committee, Sprague–Dawley rats were exposed to dirty cat litter (trauma) for 10 min and 1 week later, the rats confronted to a trauma reminder (clean litter). The rats also received intraperitoneal pirenzepine (1 or 2 mg/kg/day) or saline for 8 days. Noradrenaline (NA) concentration in the rostral pons was analyzed by HPLC with electrochemical detection. The anxiety indices of the rats subjected to the trauma reminder were increased when compared to control rats (p < 0.05). Pirenzepine treatment in traumatized rats displayed similar anxiety indices of non-traumatized rats treated with physiological saline. Although freezing time was prolonged with pirenzepine in traumatized groups the change was not found statistically significant. The NA level was 1.5 ± 0.1 pg/mg in non-traumatized rats and increased to 2.4 ± 0.2 pg/mg in traumatized rats. Bonferroni post hoc test revealed that the NA content of the rostral pons of the traumatized rats treated with physiological saline was significantly higher than the content of other groups (p < 0.01). We conclude that NA content in the rostral pons increases in respect to confrontation to a trauma reminder which can be reversed by M1 antagonist pirenzepine indicating the roles of M1 receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-traumatic stress disorder (PTSD) is characterized by direct or indirect exposure to a traumatic event eliciting an extremely fearful reaction and subsequently accompanied by more than 1 month of dysfunction and the presence of symptoms characterized by: increased re-experiencing of past stress, numbing/avoidance behavior and persistent hyperarousal and hypervigilance with increased autonomic response [1]. The pathophysiology underlying these pathological responses of PTSD have been suggested to occur due to dysregulation of several biological systems that may include corticotropin releasing hormone (CRH) and hypothalamic–pituitary–adrenal (HPA) axis abnormalities, as well as dysfunction in noradrenergic, serotonergic and glutamatergic systems [2].
Noradrenaline (NA) is a centrally acting catecholamine, acting mostly on the sympathetic nervous system. The majority of noradrenergic neurons in the central nervous system are found in the locus ceruleus (LC) of the brainstem. LC has projections into several brain areas that have roles in emotion, memory and stress–response, such as the amygdala, hippocampus, thalamus and prefrontal cortex [3]. It was found that NA has a role in attention, learning and memory that are cognitive functions involved in a number of psychiatric disorders [4]. It was suggested that there may be hyperactive noradrenergic function in PTSD.
Although the role of monoamines in post-traumatic stress disorder is now well recognized [5, 6], increasing evidence suggests an important role for cholinergic neurotransmission in the anxiety, the cognitive function and in the stress-related psychiatric disorders [7, 8]. The cholinergic basal forebrain complex innervates the cortex and the hippocampus [9, 10] to influence cortical arousal, consciousness, memory and learning [11, 12]. The cholinergic manipulations can regulate memory [13] and behavioral arousal [12], while muscarinic antagonists increase anxiety/fear responding in rats [14, 15] and enhance HPA-axis stress responsiveness [16, 17].
Predator scent test, a useful method serving to study the pathology of the disease [18] and various aspects of the model was validated previously [19]. Our previous data indicated that the cat litter test is a reproducible experiment where the rats subjected to dirty cat litter (the trauma) had longer freezing times and higher anxiety indices when exposed to the situational reminder, the clean cat litter [20]. Our previous manuscript showed the contribution of muscarinic receptors in the pathophysiology of post-traumatic stress disorder. Western blot data showed increases in M2 and M5 expression in the frontal cortex and M1 receptors expression increased and M4 subtype decreased in the hippocampus. In the amygdaloid complex of rats, we also detected a down-regulation of M4 receptors. Fluoxetine and propranolol only corrected the changes occurred in the frontal cortex [20].
In the literature, enhanced activity or elevated levels of NA in cerebrospinal fluid was demonstrated in the PTSD [21] but not in the LC, another critical region in the development of PTSD. In the present study we hypothesized that the NA content of the LC increases during the development of PTSD model and pirenzepine, a selective M1 muscarinic receptor antagonist reverses both this neurochemical finding and other behavioral parameters of the model.
Materials and Methods
Animals and Experimental Conditions
The institutional ethical committee approval was obtained before the experiments were initiated (MÜHDEK approval no: 98.2012.mar). Female Sprague–Dawley rats weighing 200–250 g supplied from Marmara University Animal Center (DEHAMER) were used in the study. The rats were habituated to the housing conditions for 10 days with a reversed 12 h light/dark cycle at 21 ± 3 °C and 50 ± 5 % humidity. There was unlimited access to standard rat chow and water. All experiments were performed in the dark phase at 10:00 a.m. using a dim light source.
Predator Scent Test
The rats were placed on 125 ml of dirty cat litter for 10 min in a plexiglass cage (30 cm × 30 cm × 40 cm) to produce stress paradigm. The cat litter had been used for 2 days by the same cat and had been sifted for stools as previously described [20, 22–24]. The control animals were exposed to fresh, unused litter for the same time duration. Clean cat litter was used as situational reminder and the rats were subjected to the reminder 1 week following the onset of the stress. The behavioral experiments were recorded using an overhead video camera and behavioral parameters were later scored from the recordings. Intraperitoneal treatments (the 8th dose) were given 10 min before the predator test.
Drugs and Solutions
The rats received intraperitoneal (i.p.) injections of physiological saline or pirenzepine (1 and 2 mg/kg; Sigma, USA) for 1 week. The drugs were dissolved in physiological saline. The treatments were given once daily, at the same time of the day.
Elevated Plus Maze Experiments
The rats were placed on an elevated plus maze for 5 min immediately after they had been subjected to the situational reminder. The elevated plus maze had two open (50 cm × 10 cm) and two closed (50 cm × 10 cm) arms. The closed arms were surrounded by 40 cm long walls. The height of the maze was 50 cm from the ground. The labyrinth was cleaned with 5 % alcohol solution before the rats were placed on it. Each rat was placed in the central square of the plus maze facing the open arms. An arm entry was defined as an animal entering the arm with all four feet and the number of entries into open and enclosed arms was scored as described previously [25]. The anxiety index (Nanxiety) was calculated by using the following parameters and the formula:
a = cumulative time spent in open arms (s), b = open arm entries, c = total arm entries.
The cumulative freezing time was also recorded and evaluated. Upon completion of the experiments all the rats were sacrificed with a high dose of pentobarbital and the selected brain regions were dissected and kept at −80 °C for HPLC analysis.
Preparation of the Tissue Samples
After decapitation, the rat brain was collected to separate the rostral pons, which was weighed and ground into homogenate in the presence of 0.1 mol/l perchloric acid (20 μl per 1 mg tissue sample). After centrifugation at 18,000 r/min (4 °C) for 20 min, the supernatant was collected and stored at −80 °C until assay. The NA content in the homogenates was expressed as pg/mg tissue.
Chromatographic System and High Performance Liquid Chromatography Analysis of NA in Tissue Homogenates
The NA concentrations were quantified in brain tissue extracts by HPLC with electrochemical detection. The chromatographic system consists of a pump (Jasco PU 980, Tokyo, Japan) with 100 μl sample loop and Rheodyne valve, C18 reverse-phase colon (15 cm length, 4.6 mm diameter and 5 μm pore size), electrochemical detector (GBC LC 1260, Australia) with a glassy carbon working and Ag/AgCl2 reference electrodes where the working potential set to 700 mV and a computer. The chromatographic analysis was carried out with a software (Borwin Chromatograph, version 1.2, France).
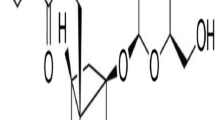
The mobile phase is a mixture of 6 % acetonitrile (Labscan, Ireland) and 75 nmol/l NaH2PO4, 50 mmol/l EDTA, 1 mmol/l octyl sodium sulfate. The pH of the mobile phase was adjusted to 4. The flow rate of the pump was set to 0.9 ml/min. Manual injections of external standards (noradrenaline bitartrate; Steradine) and samples were given within a volume of 20 μl at room temperature. The retention time of NA was 4.38 ± 0.04 min. Total duration of the chromatogram was 15 min. NA measurements were expressed as pg/ml in the samples. The minimum detectable amount on column was 60 pg (Fig. 1).
Statistical Analysis
All data are expressed as mean ± SEM. Two-way analysis of variance and the Bonferroni post hoc test were used for the analysis of anxiety indices, HPLC analysis of NA and freezing times in all groups. For all statistical calculations, significance was considered to be a value of p < 0.05.
Results
The Effects of Pirenzepine on Behavioral Parameters
The anxiety indices of the rats subjected to the trauma reminder 1 week after the predator scent test were found to be markedly higher than those of the control rats (subjected to only clean cat litter during trauma and trauma reminding sessions; Fig. 1; p < 0.05). Pirenzepine treatment at 2 different doses did not affect the anxiety indices in non-traumatized rats as shown in Fig. 2. Two-way analysis of variance detected an interaction for trauma (df = 1, F = 8.029; p = 0.0082) and Bonferroni post hoc test revealed that traumatized rats treated with physiological saline were different from all groups (p < 0.05). Pirenzepine treatments were not found to be different from non-traumatized rats treated with physiological saline, indicating that pirenzepine is effective in suppressing the anxiety.
The effect of physiological saline or pirenzepine (1 and 2 mg/kg) on anxiety indices (Nanxiety) calculated from behavioral data collected from elevated plus maze experiments (n = 8 per group). The filled bars represent the rats subjected to the predator scent (dirty cat litter) and the trauma reminder (clean litter) 1 week later. The control rats indicated with empty bars were only exposed to clean litter twice. The rats received injections daily for 1 week and the last injection (the 8th dose) was administered 10 min before the experiments. *Different from all groups, p < 0.05; †source of variation
When the freezing times were analyzed by using two-way analysis of variance trauma was found to the source of variation (df = 1, F = 10.27; p = 0.0029). Bonferroni post hoc test detected a no significant difference in the mean cumulative freezing time in all groups and pirenzepine did not affect the freezing time in traumatized groups although it was observed as it prolonged the freezing time but the statistical comparison denoted no significant change (Fig. 3).
The effect of physiological saline or pirenzepine (1 and 2 mg/kg) on cumulative freezing times recorded during elevated plus maze experiments (n = 8 per group). The filled bars represent the rats subjected to the predator scent (dirty cat litter) and the trauma reminder (clean litter) 1 week later. The control rats indicated with empty bars were only exposed to clean litter twice. The rats received injections daily for 1 week and the last injection (the 8th dose) was administered 10 min before the experiments. †Source of variation
The NA level was 1.5 ± 0.1 pg/mg in non-traumatized rats and increased to 2.4 ± 0.2 pg/mg in traumatized rats. The analysis of the NA levels in the homogenates of the rostral pons revealed that both trauma (df = 1, F = 8.037; p = 0.0078) and treatments (df = 2, F = 6.268; p = 0.0049) were found to be the source of variation. No interaction was detected between trauma and the treatments. Bonferroni post hoc test revealed that the NA content of the rostral pons of the traumatized rats treated with physiological saline was significantly higher than the content of other groups (p < 0.01). The NA levels in traumatized rats treated with pirenzepine at both doses were similar to non-traumatized control groups (Fig. 4).
The effect of physiological saline or pirenzepine (1 and 2 mg/kg) on noradrenaline levels collected from elevated plus maze experiments (n = 8 per group). The filled bars represent the rats subjected to the predator scent (dirty cat litter) and the trauma reminder (clean litter) 1 week later. The control rats indicated with empty bars were only exposed to clean litter twice. The rats received injections daily for 1 week and the last injection (the 8th dose) was administered 10 min before the experiments. The data are expressed as pg/mg tissue. *Different from all groups, p < 0.05; †source of variation
Discussion
Noradrenaline activity in the pathophysiology of post-traumatic stress disorder was implicated in preclinical and clinical studies [2–5]. We investigated the role of noradrenergic system within the LC on post-traumatic stress disorder by exposing rats to predator stress test and analyzing the concentration of NA in LC of traumatized and non-traumatized control rats. Muscarinic receptor antagonist pirenzepine was tested whether it interferes with the noradrenergic activity of LC in post-traumatic stress disorder. We concluded that the anxiety induced by the stress reminder parallels with NA increases in the rostral pons that is reversed by pirenzepine.
In our experiments, we not only showed that the cat litter test is a reproducible experiment, but also demonstrated that noradrenergic input into the rostral pons was increased. The latter finding may be an important neurochemical evidence for the method. NA is produced primarily by neurons in the LC, which is located at the pontomesencephalic junction at the floor of the fourth ventricle in the pontine region of the brain [26]. NA plays many roles in several behavioral events, including the stress response, attention, memory, the sleep–wake cycle, decision making and regulation of sympathetic system. Neurochemical interactions occur within the LC through numerous neurotransmitters including glutamate, gamma-aminobutyric acid (GABA), glycine, serotonin, dopamine, corticotropin-releasing factor (CRF), enkephalin, acetylcholine and substance P [27–33].
The role of NA has been identified in stress and stress-related psychiatric disorders [34]. In rodents, it was shown that uncontrollable stress increases NA turnover, resulting in transient reductions in tissue content of NA [35, 36]. The homogenates of whole-brain tissue obtained from rats exposed to escapable shock have shown to contain higher concentrations of NA than the homogenates collected from their yoked, inescapably shocked controls [37].
Muscarinic receptors have been studied in several neurodegenerative disorders but their contribution in PTSD has not been fully understood. The involvement of muscarinic receptors in PTSD rat model was demonstrated previously [20]. In this previous study, it was noted that M2 and M5 expression increased in the frontal cortex where M1 receptors increased and M4 subtype decreased in the hippocampus. A down-regulation of M4 receptors in the amygdaloid complex was also detected. It was also demonstrated that fluoxetine and propranolol only corrected the changes that occurred in the frontal cortex. In another study, the role of cholinergic pathways in mediating associative memory in post-traumatic stress disorder was investigated [38]. It was demonstrated that systemic or intracranial injections of the muscarinic M1 receptor antagonist pirenzepine could reduce anxiety in different experimental models [39, 40]. A study performed by using M4 knockout mice showed the presence of increased anxiolysis with a normal long-term memory compared to that of the wild type but on the contrary, M2 knock-out mice were not behaviorally different [41]. In the hippocampus of M4 knock-out mice, the acetylcholine release was also reported to be increased [42]. The autonomic responses to stress was studied and it was reported that electrical stimulation of the amygdaloid complex resulted in cardiovascular responses through M1 receptors [43]. Thus, we examined whether M1 receptor antagonist pirenzepine treatment could change the concentration of NA in the LC in rats with post-traumatic stress disorder. Previously, pirenzepine was shown to act as an antidepressant in an animal model of depression by blocking the endogenous acetylcholine at the M1 receptor [44]. Pirenzepine exhibited tonic cholinergic anxiolytic action mediated through by postsynaptic M1 receptors in the social interaction test through dorsal hippocampal cholinergic modulation of behaviour [45]. In a placebo controlled, cross-over trial performed with major depressive or bipolar disorder patients, it was implicated that scopolamine produced rapid antidepressant effects by blockade of cholinergic muscarinic receptors [46]. Our unpublished observations with atropine showed that atropine was ineffective in correcting the increases in the anxiety index produced with cat litter [20], but in this present study pirenzepine, a selective M1 inhibitor was shown to suppress the anxiety indices of traumatized rats when exposed to the reminder. This finding may be an evidence that the M1 receptors were more commonly involved in the model. Our previous study also demonstrated that expression of M4 receptors decreased after trauma. As M4 muscarinic receptors function as inhibitory autoreceptors for acetylcholine [47–51], the decrease in M4 expression may be assumed to occur as a result of increased cholinergic activity in the hippocampus and the amygdaloid complex. Pirenzepine dose might have affected the freezing time inversely due to this autoreceptor activity through its M4 receptor affinity [52]. The antagonist affinity constant (pKB value) of pirenzepine to M1 and M4 receptors are 7.8–8.5 and 7.1–8.1, respectively [52]. This may suggest that the dose of pirenzepine required to affect M4 receptors is slightly smaller than the dose required to stimulate M1 receptors. Although we did not find a statistical significance but we can see in the freezing time graph that the freezing time for 2 mg/kg is shorter (Fig. 3). There is no doubt that pirenzepine acted as an M1/M4 antagonist but our results may imply that anxiety relieving effects were mediated through M1 receptors. These findings imply that mixed M1 and M4 partial agonists may be more useful therapeutic alternatives.
Cholinergic system was also shown to play roles in the modulation of aversively motivated tasks, such as contextual fear conditioning. In contextual fear conditioning (CFC), an experimental animal exposed to an aversive stimulus that was applied before being determined by the environmental context, the animal shows a conditioned fear response, that is characterized by somatomotor immobility, called as freezing. The involvement of muscarinic receptors in the acquisition and consolidation of tasks in CFC was also studied. Administration of scopolamine was shown to interfere with the acquisition CFC [53]. The wide distribution of muscarinic M1 receptors in the brain regions suggests that cholinergic transmission has role in learning and memory processes [54–56].
When threatened, primates commonly demonstrate behavioral inhibition or freezing behavior. Freezing is an automatic response characterized by complete absence of motor and vocal activity except those necessary for respiration [57]. Many reports show that freezing is the most common defense response in rats exposed to unavoidable fear stimuli [58]. Thus the increased freezing time in traumatized rats was observed without treatment effect of pirenzepine. We also observed that pirenzepine did not produce any significant change in locomotor activity experiments with 1 or 2 mg/kg doses (data not shown).
The limitation of this study is NA content of other brain regions especially in the hippocampus, the amygdala and the frontal cortex were not studied. Further studies performed in other brain regions and neurotransmitter systems are required to delineate the pathophysiology of the disease.
In conclusion, our data showed that NA content in the rostral pons increased in traumatized rats with cat litter and this increase may serve as an additional neurochemical evidence for the model. Pirenzepine suppressed the anxiety index but it was not that effective in decreasing the prolonged freezing times.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, pp 463–468
Ravindran LN, Stein MB (2009) Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res 1293:24–39
Vermetten E, Bremner JD (2002) Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety 16:14–38
Wolf OT (2008) The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst) 127:513–531
Elzinga BM, Bremner JD (2002) Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord 70:1–17
Harvey BH, Brand L, Jeeva Z, Stein D (2006) Cortical/hippocampal monoamines, HPA-axis changes and aversive behavior following stress and restress in an animal model of post-traumatic stress disorder. Physiol Behav 87:881–890
Lopez JF, Akil H, Watson SJ (1999) Neural circuits mediating stress. Biol Psychiatry 46:1461–1471
Kaufer D, Friedman A, Seidman S, Soreq H (1998) Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393:373–377
Lamprea MR, Cardenas FP, Silveira R, Morato S, Walsh TJ (2000) Dissociation of memory and anxiety on a repeated elevated plus maze paradigm: forebrain cholinergic mechanisms. Behav Brain Res 117:97–105
Laborszky L, Duque A (2000) Local synaptic connections of basal forebrain neurons. Behav Brain Res 115:143–158
Sarter M, Bruno JP (2000) Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencaphalic and brainstem afferents. Neuroscience 95:933–952
Picciotto MR, Alreja M, Jentsch JT (2002) Acetylcholine. In: Charney D, Coyle JT, Nemeroff C, Davis KL (eds) Neuropsychopharmacology: the fifth generation of progress. American College of Neuropsychopharmacology, USA, pp 3–14
Pepeu G, Giovannini GM (2004) Changes in acetylcholine extracellular levels during cognitive processes. Learn Mem 11:21–27
Smythe JW, Murphy D, Bhatnagar S, Timothy C, Costall B (1998) The effects of intrahippocampal scopolamine infusions on anxiety in rats as measured by the black–white box test. Brain Res Bull 45:89–93
Hess C, Blozovski D (1987) Hippocampal muscarinic cholinergic mediation of spontaneous alternation and fear in the developing rat. Behav Brain Res 24:203–214
Jacobson L, Sapolsky R (1991) The role of the hippocampus in feedback regulation of the hypothalamic–pituitary–adrenocortical axis. Endocr Rev 12:118–134
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84
Armario A, Escorihuela RM, Nadal R (2008) Long-term neuroendocrine and behavioral effects of a single exposure to stress in adult animals. Neurosci Behav Rev 32:1121–1135
Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z (2012) Animal model for PTSD: from clinical concept to translational research. Neuropharmacology 62(2):715–724
Aykaç A, Aydın B, Cabadak H, Gören MZ (2012) The change in muscarinic receptor subtypes in different brain regions of rats treated with fluoxetine or propranolol in a model of post-traumatic stress disorder. Behav Brain Res 232:124–129
Geracioti TD Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr, Kasckow JW (2001) CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 158(8):1227–1230
Cohen H, Matar MA, Richter-Levin G, Zohar J (2006) The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann NY Acad Sci 1071:335–350
Matar MA, Cohen H, Kaplan Z, Zohar J (2006) The effect of early poststressor intervention with sertraline on behavioral responses in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 31:2610–2618
Mazor A, Matar MA, Kaplan Z, Kozlovsky N, Zohar J, Cohen H (2009) Gender-related qualitative differences in baseline and post-stress anxiety Responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World J Biol Psychiatry 10:856–869
Pelow S, Chopin P, File SE, Briley M (1985) Validation of open-closed arm entries in an elevated plus maze as measure of anxiety in the rat. J Neurosci Methods 14:149–167
Dahlström A, Fuxe K (1964) Localization of monoamines in the lower brain stem. Experientia 20(7):398–399
Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ (1997) Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther 281(1):163–172
Ennis M, Aston-Jones G (1986) A potent excitatory input to the nucleus locus coeruleus from the ventrolateral medulla. Neurosci Lett 71(3):299–305
Fodor M, Görcs TJ, Palkovits M (1992) Immunohistochemical study on the distribution of neuropeptides within the pontine tegmentum—Particularly the parabrachial nuclei and the locus coeruleus of the human brain. Neuroscience 46(4):891–908
Jodo E, Aston-Jones G (1997) Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res 768(1–2):327–332
Luppi PH, Charlety PJ, Fort P, Akaoka H, Chouvet G, Jouvet M (1991) Anatomical and electrophysiological evidence for a glycinergic inhibitory innervation of the rat locus coeruleus. Neurosci Lett 128(1):33–36
Sakai K (1991) Physiological properties and afferent connections of the locus coeruleus and adjacent tegmental neurons involved in the generation of paradoxical sleep in the cat. Prog Brain Res 88:31–45
Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ (2001) Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience 106(2):375–384
Anand A, Charney DS (2000) Norepinephrine dysfunction in depression. J Clin Psychiatry 61(Suppl 10):16–24
Anisman H, Sklar LS (1979) Catecholamine depletion in mice upon reexposure to stress: mediation of the escape deficits produced by inescapable shock. J Comp Physiol Psychol 93:610–625
Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH (1981) Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res Rev 3:167–205
Weiss JM, Stone EA, Harrell N (1970) Coping behavior and brain norepinephrine level in rats. J Comp Physiol Psychol 72:153–160
Anisman H, Pizzino A, Sklar LS (1980) Coping with stress, norepinephrine depletion and escape performance. Brain Res 191:583–588
Swenson RM, Vogel WH (1983) Plasma Catecholamine and corticosterone as well as brain catecholamine changes during coping in rats exposed to stressful footshock. Pharmacol Biochem Behav 18:689–693
Degroot A, Nomikos GG (2005) Fluoxetine disrupts the integration between anxiety and aversive memories. Neuropsychopharmacology 30:391–400
Degroot A, Nomikos GG (2006) Genetic deletion of muscarinic M4 receptors is anxiolytic in the shock-probe burying model. Eur J Pharmacol 531:183–186
Tzavara ET, Bymaster FP, Felder CC, Wade M, Gomeza J, Wess J, McKinzie DL, Nomikos GG (2003) Disregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry 8:673–679
Aslan N, Goren Z, Onat F, Oktay S (1997) Carbachol-induced pressor responses and muscarinic M1 receptors in the central nucleus of amygdala in conscious rats. Eur J Pharmacol 333:63–67
Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG (2001) Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience 104:791–798
File SE, Gonzales LE, Andrews N (1998) Endogenous acetylcholine in the dorsal hippocampus reduces anxiety through actions on nicotinic and muscarinic1 receptors. Behav Neurosci 112:352–359
Furey ML, Khanna A, Hoffman EM, Drevets WC (2010) Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology 35(12):2479–2488
Levey AI, Kitt C, Simonds W, Price D, Brann MR (1991) Identification and localization of muscarinic receptor subtype proteins in rat brain. J Neurosci 11(10):3218–3226
Moreira KM, Hipolide DC, Nóbrega JN, Bueno OF, TuWk S, Oliveira MG (2003) DeWcits in avoidance responding after paradoxical sleep deprivation are not associated with altered [3H] pirenzepine binding to M1 muscarinic receptors in rat brain. Brain Res 977(1):31–37
Wei J, Walton EA, Milici A, Buccafusco JJ (1994) m1–m5 muscarinic receptor distribuition in rat CNS by RT-PCR and HPLC. J Neurochem 63:815–821
Macedo CE, Martinez RC, Brandăo ML (2006) Conditioned and unconditioned fear organized in the inferior colliculus are differentially sensitive to injections of muscimol into the basolateral nucleus of the amygdala. Behav Neurosci 120:625–631
Bolles RC, Collier AC (1976) The effect of predictive cues of freezing in rats. Anim Learn Behav 4:6–8
Caulfield MP, Birdsall NJM (1998) International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. ASPET 50:279–290
Anagnostaras SG, MarenS Fanselow MS (1995) Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem 64:191–194
Misslin R (2003) The defense system of fear: behavior and neurocircuitry. Clin Neurophysiol 33:55–66
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11(10):3218–3226
Levey AI (1993) Immunological localization of m1–m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci 52:441–448
Sánchez G, Alvares Lde O, Oberholzer MV, Genro B, Quillfeldt J, da Costa JC, Cerveñansky C, Jerusalinsky D, Kornisiuk E (2009) M4 muscarinic receptors are involved in modulation of neurotransmission at synapses of Schaffer collaterals on CA1 hippocampal neurons in rats. J Neurosci Res 87(3):691–700
Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB (1993) Development of antisera selective for M4 and M5 muscarinic cholinergic receptors: distribution of M4 and M5 receptors in rat brain. Mol Pharmacol 43(2):149–157
Acknowledgments
This research was supported by Marmara University Research Fund (SAG-C-YLP-110412-0065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terzioğlu, B., Kaleli, M., Aydın, B. et al. Increased Noradrenaline Levels in the Rostral Pons can be Reversed by M1 Antagonist in a Rat Model of Post-traumatic Stress Disorder. Neurochem Res 38, 1726–1733 (2013). https://doi.org/10.1007/s11064-013-1076-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1076-2