Abstract
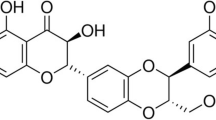
Post-traumatic stress disorder (PTSD) is the serious psychiatric disorder. Paeoniflorin (PF) produces the antidepressant-like properties. However, few studies are concerned about its anti-PTSD-like effects and mechanisms. To investigate these, the single prolonged stress (SPS) model was utilized. PTSD-like behavioral deficits in rats after exposure to SPS were improved by PF (10 and 20 mg/kg, i.p.), evidenced by blocking increased freezing time in contextual fear paradigm (CFP) and increased time and entries in open arms in elevated plus maze (EPM) test without affecting the locomotor activity in open field (OF) test. We also found that increased levels of corticosterone (Cort), corticotropin releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) after exposure to SPS were reversed by PF (10 and 20 mg/kg, i.p.) in serum, respectively. Moreover, the decreased levels of serotonin (5-HT) and 5-Hydroxyindoleacetic acid (5-HIAA) in prefrontal cortex and hippocampus were reversed by PF (10 and 20 mg/kg, i.p.), respectively. In summary, the anti-PTSD-like activities of PF were associated with the modulation of HPA axis and 5-HT system activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-traumatic Stress Disorder (PTSD) is a prevalent and chronic psychiatric disorder that develop from exposure to traumatic events (Muhtz et al. 2012). The patients with PTSD exhibit psychiatric comorbidities, such as major depression and generalized anxiety (Beristianos et al. 2016). So far, the pathology of PTSD is remain unclear. Researches into the underlying neurobiology of PTSD has implicated alterations of various neurotransmitters and neuroendocrine systems, including dysregulation of the monoaminergic neurotransmission and hypothalamic-pituitary-adrenal (HPA) axis (Fenchel et al. 2015; Kozaric-Kovacic 2008). The dysregulation of HPA axis may promote stress-related illnesses (e.g. depression, PTSD) (Raineki et al. 2016). Taking corticosterone (Cort) for example, administration of Cort in rodents could induce depressive-like behavior. Previous study showed that repeated Cort injection paradigm provided a useful and reliable mouse model within which to further study the role of stress and glucocorticoids in depressive illness (Zhao et al. 2008). Other studies also support that Cort induced anxiogenic- and depressive- like behavior as observed by increased immobility time in the tail suspension test and decreased sucrose consumption (Oliveira et al. 2017). Enhanced negative feedback inhibition of HPA axis may be a risk factor for PTSD. The levels of HPA stress hormones, such as Cort, corticotrophin releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), are closely associated with PTSD (de Kloet et al. 2012; Kao et al. 2015; Pervanidou and Chrousos 2012). These stress hormones result in the release and dysregulation of glucocorticoids and elevation in patients or animal models with PTSD (Yehuda et al. 2014).
Glucocorticoids exert the potential effects on prefrontal-mediated behaviors, including working memory, behavioral flexibility, executive function, et al. The prefrontal cortex is the executive control center of the brain, providing the top-down regulation of behavioral function. Thus, it is an important site for glucocorticoid actions and regulation of the HPA axis (McKlveen et al. 2013). Previous study determined that glucocorticoids act at the prefrontal cortex to inhibit HPA axis responses to psychogenic stress (Akana et al. 2001). In addition, The hippocampus is critical for processes involved memory, particularly contextual and spatial learning and memory retrieval. The effects of glucocorticoids in the hippocampus have long been recognized and studied in detail. Glucocorticoids are abundantly expressed in hippocampus, and memory processing is heavily influenced by circulating levels of glucocorticoids (Oitzl and de Kloet 1992; Roozendaal et al. 2001).
In addition, preclinical and clinical evidences also suggested that disturbed monoaminergic neurotransmission is one of important mechanisms underlying PTSD (Wilson et al. 2014). The hypothesis of monoamine indicated that monoamines, (e.g serotonin (5-HT), noradrenaline (NE), dopamine (DA), 5-Hydroxyindoleacetic acid (5-HIAA), Homovanillic acid (HVA), 3,4-Dihydroxyphenylacetic acid (DOPAC), adrenalin (AD), et al) are important neurotransmitters involved in the etiology of PTSD (Kozaric-Kovacic 2008). Actually, most of the anti-PTSD drugs act on more than one mechanism based on the monoamine hypothesis, such as inhibition of the reuptake of 5-HT and its metabolites. Evidences from various studies indicated that the levels of metabolic monoamine neurotransmitters (i.e 5-HT) in brain increased compared with that of controls after anti-PTSD treatments (Lin et al. 2016a, b; Zhang et al. 2012).
Selective serotonin reuptake inhibitors (SSRIs) (e.g sertraline (Ser) and paroxetine) are the first-line treatment options for PTSD (MacNamara et al. 2016). However, there are several drawbacks in SSRIs including a response / non-response with residual symptoms, a delayed onset of action, as well as severe side effects (Reid et al. 2015). Thus, searching the novel pharmacological therapy for anti-PTSD drugs is important.
Traditional Chinese medicine (TCM) draws more and more attentions and provides a prospective alternative to the treatment of PTSD based on its lower side effects and better compliance (Wang et al. 2009; Zhang 2014b). The root part of Paeonia lactiflora Pall (Ranunculaceae), called peony, is often used in Chinese herbal medicine for the treatment of depressive-like disorder (Mao et al. 2008). Among these components, paeoniflorin (PF) is usually referred as one of the most important active components of peony (Wang et al. 2014). PF has been widely studied as an anti-convulsant, anti-oxidant, anti-thrombotic agent, cognition enhancer or learning impairment-attenuating and neuroprotective agent (Li et al. 2014; Nam et al. 2013; Ye et al. 2001). Moreover, the pharmacological activity of PF is associated with alternation levels of HPA stress hormones and monoamines (Huang et al. 2015; Qiu et al. 2013). However, little information regards the anti-PTSD-like activities of PF. Consequently, it is reasonable to hypothesis that PF may also be effective in ameliorating stress-induced psychiatric conditions, i.e PTSD.
The present study is to evaluate the anti-PTSD-like effects of PF firstly. Following the preparation of SPS model (the classical PTSD model in rodent), the anti-PTSD-like properties of PF were assessed by behavioral tests. The role of HPA stress hormones and monoamines in anti-PTSD-like activities of PF was also investigated after the behavioral tests.
Materials and methods
Drugs
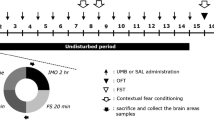
Sertraline (Ser) (St Louis, MO, U.S.A.) was prepared in 0.9% normal saline and injected intraperitoneally (i.p.) as a positive control (15 mg/kg) based on its anti-PTSD-like activities (Miao et al. 2014; Zhang et al. 2012, 2014a). PF (purity≥98%, St Louis, MO, U.S.A.) was also prepared in 0.9% normal saline and given once daily (5, 10 and 20 mg/kg, i.p.) from day 2 to 13 (Figs. 1 and 2). The selective doses of PF were based on its antidepressant-like effects (Qiu et al. 2013). The behavioral tests were performed 1 h after drugs treatment. The control group animals received 0.9% normal saline (i.p.) (Fig. 2).
Treatment and behavioral test schedules. Animals were subjected to SPS on day 1. From day 8 through 13, animals were performed testing sessions that were composed of various behavioral tests: contextual fear paradigm (CFP), elevated plus maze (EPM) test and open field (OF) test. PF (at doses of 5, 10 and 20 mg/kg, i.p.) and Ser (at a dose 15 mg/kg, i.p.) were administered daily from day 2 through 13. The drugs were administered 1 h before testing, respectively
Animals
The rats (Sprague-Dawley, male, 190 ± 10 g) were obtained from Vital River Laboratory Animal Technology Company (China) and maintained in a temperature (22–24 °C)- and humidity (50–60%)- controlled condition. The total number of the animals is sixty. All the animals were housed in a 12 h- light/dark cycle environment during the tests with water and food available freely. All the procedures were carried out based on the National Institute of Health Guide for the care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996) and approved by the institutional committee on animal care and use. All efforts were made to minimize the number of animals used and their suffering.
The preparation of SPS model
SPS is a classical PTSD animal model and widely used in PTSD studies (Jin et al. 2016; Miao et al. 2014; Zhang et al. 2012). Each rat was placed in a restrainer with tail-gate without impairing the circulation to limbs for 2 h. The bag size was adjusted based on the size of each one to achieve complete immobilization. Following the restraint, each rat was placed individually into an acrylic cylinder (height 45 cm, diameter 20 cm, that contained 23–25 cm of water and maintained at the temperature of 23–24 °C) to perform a 20-min forced swim. After a 15-min recuperation period, rats were exposed to the ether vapors until loss of consciousness and removed from the restrainer. The control group remained in a room adjacent to SPS rats for the duration of the SPS procedure.
Behavioral paradigms
Seven days after the exposure to SPS, behavioral tests were performed, including the contextual fear paradigm (CFP) (day 8 and 9), elevated plus-maze (EPM) test (day 11), and open-field (OF) test (day 13).
Contextual fear paradigm (CFP)
The CFP represents as the freezing response on re-experience to traumatic context, which was used as a measure of PTSD-associated fear memory (Eskandarian et al. 2013; Jin et al. 2016; Levkovitz et al. 2015; Zhang et al. 2012). Each rat was exposed to a 180-s conditioned context without stimulation. After that, a foot electric shock (0.8 mA, for 4 s) through the stainless steel grid floor was given. Each rat remained in the chamber for an additional 1 min before being returned to home cages. Twenty-four hours later, each one was placed in the conditioning chamber where it was exposed to the foot shock previously. The contextual fear response was determined as the time of freezing-like behavior by observers blinded to the treatment groups during a 5-min interval.
Elevated plus maze (EPM) test
The EPM test is a classical assessments to evaluate the PTSD-associated anxiogenic-like behavior in rodents (Santos et al. 2016; Wang et al. 2009; Zhang et al. 2014). The apparatus consisted of four arms (60 × 12 cm) with two open arms and two closed arms with dark walls (40 cm hight). The maze was 50 cm above the ground with the arms were connected by a central platform (12 × 12 cm). Each rat was placed in the platform facing the closed arms. The rats were scored as entering an open/closed arm when all four paws passed over the dividing line. The time and numbers of entries into the open arms were obtained as anxiety indices by observers who were blind to treatment conditions of the animals (Li et al. 2009; Wang et al. 2009).
Open field (OF) test
To evaluate the role of locomotor activity in the anti-PTSD-like activities of PF, the number of crossings, rears, and fecal pallets was determined as the previous literature (Xue et al. 2013). Each rat was placed in the corner of the plastic box (76 × 76 × 46 cm) that the base was divided into equal sectors for a 5-min acclimation duration. After that, the number of crossings (with four paws placed into a new square), rears (with both front paws raised from the floor), and fecal pallets was measured for 5 min.
Enzyme-linked immunosorbent assay (ELISA)
The rats were decapitated after the behavioral tests in 24 h and the blood was collected. The blood was sampled in the EDTA-free sterile tubes. Serum was then separated by centrifugation (1500 g, 30 min) at 4 °C. The supernatants were collected, clot and stored at −80 °C until the further analyses. The levels of Cort, CRH and ACTH in serum were determined according to the instructions of the ELISA kits (magnetic solid phase; TPI Inc., WA, USA). The standard/sample and conjugate were added to each well, and the plate was incubated for 1 h at 37 °C. After the washes and proper color development, the optical density was determined at 450 nm by an ELISA plate reader.
High-performance liquid chromatography with electrochemica detection (HPLC-ECD)
It is reported that the dysfunction of the prefrontal cortex and/or hippocampus is implicated in the pathogenesis of PTSD-associated behavioral deficits (van Rooij et al. 2017; Wen et al. 2017). Both brain regions play an important role in fear conditioning, emotional processing and explicit memory. Actually, the SPS procedure may enhance contextual fear and freezing behavior that may represent as the severity of anxiety due to the dysfunction of prefrontal cortex and hippocampus (Qiu et al. 2016; Zhang et al. 2012). More studies also supported that the disregulated levels of metabolic monoamine neurotransmitters (5-HT, NE, DOPAC, 5-HIAA, HVA, et al) in both brain regions may be one of the possible factors to the pathogenesis of PTSD (Wilson et al. 2014). Consequently, to further evaluate the neurochemical mechanisms involved in the anti-PTSD-like effects of PF, the levels of the metabolic monoamine neurotransmitters and metabolites in the prefrontal cortex and hippocampus were detected by HPLC-ECD based on the previous study (Wang et al. 2016). Also, the animals were decapitated after the behavioral tests in 24 h. The prefrontal cortex and hippocampus were dissected on ice by a binocular dissection microscope and homogenized in an ice-cold tissue lysis buffer. The samples were centrifuged (12,000×g, 30 min) at 4 °C and then filtered through a 0.45 μm pore membrane. The standard/sample solution was injected into the reversed-phase C18 column (250 mm × 4.6 mm, 5 μm). The separation was performed in an isocratic elution mode at a column temperature of 20 °C. The metabolic monoamine neurotransmitters and metabolites (5-HT, 5-HIAA, DOPAC, DA, AD, HVA and NE) in the both brain regions were caculated as ng/g wet weight of tissue.
Statistical analysis
The data were analyzed by GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA) and presented as the mean ± standard error of the mean (S.E.M). The statistical significance was analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests. Differences at an alpha value (p < 0.05) were considered statistically significant for tests.
Results
The anti-PTSD-like effects of PF in CFP
The effects of PF on PTSD-like associated contextual freezing behavior in rats were shown in Fig. 3. Following exposure to SPS, the freezing time was significantly elevated. In line with Ser (15 mg/kg, i.p.), the increased freezing time was markedly reversed by PF treatment (10 and 20 mg/kg, i.p.) (F (5,54) = 12.86, p < 0.05). These results indicated that PF treatment alleviated PTSD-like associated contextual freezing behavior in rats.
The anti-PTSD-like effects of PF in EPM test
As shown in Fig. 4, the percentage of time (F (5,54) = 7.114, p < 0.05; Fig. 4c) and entries (F (5,54) = 8.648, p < 0.05; Fig. 4d) into open arms was significantly reduced after SPS exposure in rats, while similar to Ser (15 mg/kg, i.p.), both parameters above were reversed by PF (10 and 20 mg/kg, i.p.). There was no significant difference in terms of total time (F (5,54) = 1.021, p > 0.05; Fig. 4a) and entries (F (5,54) = 0.7045, p > 0.05; Fig. 4b) in arms among groups. These results indicated that PTSD-associated anxiogenic behavior was ameliorated by PF in the EPM test.
Anti-PTSD-like effects of PF treatment in rats following exposure to SPS. The behavior was presented by percentages of time spent (c) in and entries (d) into open arms, as well as total time (a) and entries (b) in the arms. # p < 0.05, ## p < 0.01 vs. vehicle-treated SPS (−) group; * p < 0.05, ** p < 0.01 vs. vehicle treated SPS (+) group (n = 10)
Effects of PF on locomotor activity in rats
The effects of PF on locomotor activity were shown in Fig. 5. There was no significant effect on the number of line crossings (F (5,54) = 0.7815, p > 0.05, Fig. 5a), rears (F (5,54) = 0.1081, p > 0.05, Fig. 5b), or fecal pallets (F (5,54) = 0.2006, p > 0.05, Fig. 5c) among groups. These results indicated that neither PF treatment nor SPS modeling affected locomotor activity in rats.
Effects of PF on Cort, CRH and ACTH levels in rats
The effects of PF on Cort, CRH and ACTH levels in rats were shown in Fig. 6. Following exposure to SPS, levels of Cort (F (5,30) = 3.413, p < 0.05; Fig. 6a), CRH (F (5,30) = 4.420, p < 0.05; Fig. 6b) and ACTH (F (5,30) = 7.407, p < 0.05; Fig. 6c) in serum were significantly increased. In accordance with Ser (15 mg/kg, i.p), these effects were significantly reversed by treatment with PF (10 and 20 mg/kg, i.p), respectively. These results indicated that anti-PTSD-like effects of PF were associated with decreased levels of HPA stress hormone (Cort, CRH and ACTH).
Effects of PF on levels of metabolic monoamine neurotransmitters in the prefrontal cortex and hippocampus
As shown in Figs. 7 and 8, after exposure to SPS, the levels of 5-HT (F (5,30) = 2.952, p < 0.05; Fig. 7a) and 5-HIAA (F (5,30) = 3.240, p < 0.05; Fig. 7b) in prefrontal cortex were significantly decreased. Similar to Ser (15 mg/kg, i.g.), the decreased levels of 5-HT and 5-HIAA were significantly reversed by treatment with PF (10 and 20 mg/kg, i.p.), respectively. In line with the results of prefrontal cortex, the decreased levels of 5-HT (F (5,30) = 2.913, p < 0.05; Fig. 8a) and 5-HIAA (F (5,30) = 3.033, p < 0.05; Fig. 8b) in the hippocampus were also significantly reversed by treatment with PF (10 and 20 mg/kg, i.p.), respectively. However, NE (F (5,30) = 0.2920, p > 0.05, for prefrontal cortex, Fig. 7c; F (5,30) =0.4059, p > 0.05, for hippocampus, Fig. 8c), AD (F (5,30) = 0.2833, p > 0.05, for prefrontal cortex, Fig. 7d; F (5,30) = 0.03126, p > 0.05, for hippocampus, Fig. 8d), HVA (F (5,30) = 0.3000, p > 0.05, for prefrontal cortex, Fig. 7e; F (5,30) = 0.1837, p > 0.05, for hippocampus, Fig. 8e), DA (F (5,30) = 1.235, p > 0.05, for prefrontal cortex, Fig. 7f; F (5,30) = 0.1805, p > 0.05, for hippocampus, Fig. 8f), DOPAC (F (5,30) = 0.2823, p > 0.05, for prefrontal cortex, Fig. 7g; F (5,30) = 0.3818, p > 0.05, for hippocampus, Fig. 8g) in both brain regions were not significantly affected by SPS and PF treatments. These results indicated that anti-PTSD-like effects of PF were associated with the reversion of decreased levels of 5-HT and 5-HIAA.
Discussion
In the present study, we evaluated pharmacological profile and possible mechansims of PF in an animal PTSD model. The PTSD-like behavioral deficits were elicited in rats after exposure to SPS. However, similar to Ser, significant suppression of enhanced anxiety and contextual fear effects was induced by PF without affecting locomotor activity in rats. Moreover, the role of HPA stress hormones and monoamines in anti-PTSD-like effects of PF was also assessed. The findings indicated that anti-PTSD-like activities of PF were closely associated with decreased levels of Cort, CRH and ACTH in serum and increased levels of 5-HT/5-HIAA in the prefrontal cortex and hippocampus.
Accumulating evidences indicate that SPS has been defined as a valid PTSD animal model based on the fact that enhanced inhibition of the HPA axis in response to glucocorticoid administration after the exposure to SPS in rodents and exhibit a sustained exaggeration of the acoustic startle response, which has been reliably reproduced in patients with PTSD (Zhe et al. 2008). The presents study showed that a sustained PTSD-associated contextual fear behavior and anxiogenic-like activity was induced by SPS, which was evidenced by increased freezing time in the CPF and decreased exploration into open arms in the EPM test. One possible explanation for behavioral deficits was that SPS induced the acquisition of conditioned fear (Lin et al. 2016a). These responses were consistent with clinical symptoms observed in patients with PTSD who were subjected to re-experiencing aspects of a traumatic event or repeated traumatization may elicit stress-induced anxiogenic effects (Eagle et al. 2013). The SPS procedure has been shown to enhance contextual fear and freezing behavior that may represent as the assessment for the severity of anxiety due to dysfunction of prefrontal cortex and hippocampus (George et al. 2015; Han et al. 2013).
Although animals exposed to SPS exhibited freezing and anxiogenic-like behavior, SPS did not affect the locomotor activity in rats. This finding was consistent with previous studies that locomotor activity was not affected by SPS stress in rodents (Miao et al. 2014; Zhang et al. 2012), suggesting that freezing behavior to the context associated with aversive stress by SPS in rats was not generated by affecting locomotor activity.
Additionally, the aversive effects after SPS exposure were successfully blocked by PF (10 and 20 mg/kg i.p). The elevated freezing time in CFP test and the decreased exploration in open arm in EPM test were reversed by PF, indicating that PF ameliorated these behavioral changes produced after exposure to SPS. The dose ranges of PF were almost confirmed between CFP and EPM, and concordant with prior studies of PF treatment that showed the improvement on behavioral deficits of menopause depression in ovariectomized rats under chronic unpredictable mild stress (Huang et al. 2015). We also found that PF alleviated the fear and anxiogenic-like behavior in stressed animals without affecting locomotor activity, which was consistent with the antidepressant-like effects of PF that were not mediated by affecting locomotor activity (Qiu et al. 2013).
As demonstrated by studies, hyperactivity of the HPA axis that commonly seen in patients with PTSD, is reversed during clinically effective by anti-PTSD drugs (Jin et al. 2016). Among various molecular events, altering level of stress hormone is one of the significant mechanisms produced by anti-PTSD-like treatments (Jin et al. 2016; Krishnamurthy et al. 2013). The HPA axis includes a feedback loop that composed of the hypothalamus, pituitary as well as adrenal glands (Uschold-Schmidt et al. 2013). The HPA stress response is driven by neural mechanisms originally, invoking CRH release from hypothalamic paraventricular nucleus (PVN) neurons (Ondicova et al. 2014). Briefly, the hypothalamus releases arginine vasopressin and CRH in response to a stressor, and then activates the secretion of ACTH from the pituitary, which finally stimulates the secretion of Cort (in rodents) or cortisol (in humans) from the adrenal cortex (Hosseinichimeh et al. 2015). As observed in our present study, exposure to SPS significantly increased serum CRH, Cort and ACTH levels in rats, which was accompanied by conditioning fearful- and anxiogenic- like behavioral alterations. The finding were supported by that elevated levels of HPA stress hormones (e.g Cort and ACTH) in serum were significantly increased following subject to time-dependent sensitization (TDS) (Jin et al. 2016). Not only in PTSD, increased levels of CRH, Cort and ACTH in serum were also showed in menopause depression ovariectomized rats (Huang et al. 2015).
The present study showed that the elevated levels of CRH, Cort and ACTH in serum were blocked by SSRIs (Ser) that were consistent with other PTSD model (Jin et al. 2016). The similar activities had been found in PF (10 and 20 mg/kg) which may be a primary neuroendocrine mechanism underlying its behavioral effects. Other mental disorder study reported that the increased levels of stress hormones (CRH, CORT and ACTH) were blocked by PF at the similar dose (10 mg/kg) in depression (Huang et al. 2015). Collectly, it is indicated that anti-PTSD-like activities of PF were associated with the alternation levels of HPA stress hormones in serum.
In mammals, the HPA axis and the monoamines system are greatly involved in stress-related disorders, which closely interact in central nervous system (CNS) (particularly in the prefrontal cortex and hippocampus) (Fenchel et al. 2015; McKlveen et al. 2013). Consequently, the role of monoamines in the anti-PTSD-like effects of PF was evaluated. Following the exposure to SPS, the levels of 5-HT and 5-HIAA in both of the brain regions were significantly decreased. The results were supported by the other study showing that decreased serotonin levels (e.g 5-HT and 5-HIAA) were produced in a PTSD animal model (Zhang et al. 2012). The reduction of 5-HT is associated with the symptoms of fear, aggression, impulsivity, and sadness/depression (Fernandez and Gaspar 2012). Furthermore, the improvement of serotonergic antidepressants (e.g., SSRIs) on the symptoms above has been one explanation to support the role of serotonergic dysfunction in the pathology of PTSD (Bentefour et al. 2016). The efficacies of SSRIs in treating PTSD-associated symptoms are likely mediated by the improvement of serotonergic function and the subsequent amelioration in the modulation of impulsivity, anger, mood, and anxiety (Echiverri-Cohen et al. 2016). Our present study showed that similar to Ser (15 mg/kg), the decreased levels of 5-HT and 5-HIAA were blocked by PF (10 and 20 mg/kg), respectively. The present study also confirmed the possibility that the antidepressant-like effects of PF through the increased levels of 5-HT and 5-HIA in the hippocampus (Qiu et al. 2013). Thus, it is reasonable to speculate that the serotonergic activities of PF might be similar to that of SSRIs. However, further investigations on the presumptive adaptive changes in the receptors are still needed to elucidate.
Collectly, our findings indicate that a therapeutic effect on PTSD-like stress responding is produced by PF that is accompanied by the modulation of the serotonergic activation and the HPA axis. Thus, PF may play a significant role in the anti-PTSD-like effects. More experiments are needed to clarify the exact molecular mechanisms underlying its effects to better understand the neuropathological changes in PTSD. For instance, the further studies need to focus on the evaluation of metabolic monoamine neurotransmitters in more other brain regions, like amygdala which is also considered as one of regions that has been repeatedly implicated in the psychopathology of PTSD (Akiki et al. 2017). Previous study showed that the disregulated levels of metabolic monoamine neurotransmitters (i.e 5-HT, DA and NE) in the amygdala were associated with PTSD generation (Lin et al. 2016a, b). Moreover, a direct comparison of the activity of the plant extract (e.g peony) and the pure compound (PF) would be informative and interesting based on the fact that peony contains many of compounds (e.g PF, albiflorin, et al) that could be active in itself or in combination with others, although the anti-PTSD-like effects of PF have been preliminary evaluated in the present study. Thus, picking out more compounds instead of single could make more sense for coming to evidence-based used traditional medicine. In that case, the activities and concentrations among the components of the plant extractions can be compared, and a possible role of the pure compound for activities can also be assessed.
References
Akana SF, Chu A, Soriano L, Dallman MF (2001) Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol 13:625–637
Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B et al (2017) The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chron Stress (Thousand Oaks). https://doi.org/10.1177/2470547017724069
Bentefour Y, Rakibi Y, Bennis M, Ba-M'hamed S, Garcia R (2016) Paroxetine treatment, following behavioral suppression of PTSD-like symptoms in mice, prevents relapse by activating the infralimbic cortex. Eur Neuropsychopharmacol 26:195–207
Beristianos MH, Yaffe K, Cohen B, Byers AL (2016) PTSD and risk of incident cardiovascular disease in aging veterans. Am J Geriatr Psychiatry 24:192–200
de Kloet CS, Vermetten E, Rademaker AR, Geuze E, Westenberg HG (2012) Neuroendocrine and immune responses to a cognitive stress challenge in veterans with and without PTSD. Eur J Psychotraumatol 3
Eagle AL, Fitzpatrick CJ, Perrine SA (2013) Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res 256:591–597
Echiverri-Cohen A, Zoellner LA, Gallop R, Feeny N, Jaeger J, Bedard-Gilligan M (2016) Changes in temporal attention inhibition following prolonged exposure and sertraline in the treatment of PTSD. J Consult Clin Psychol 84:415–426
Eskandarian S, Vafaei AA, Vaezi GH, Taherian F, Kashefi A, Rashidy-Pour A (2013) Effects of systemic administration of oxytocin on contextual fear extinction in a rat model of post-traumatic stress disorder. Basic Clin Neurosci 4:315–322
Fenchel D, Levkovitz Y, Vainer E, Kaplan Z, Zohar J, Cohen H (2015) Beyond the HPA-axis: the role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. Eur Neuropsychopharmacol 25:944–957
Fernandez SP, Gaspar P (2012) Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology 62:144–154
George SA, Rodriguez-Santiago M, Riley J, Rodriguez E, Liberzon I (2015) The effect of chronic phenytoin administration on single prolonged stress induced extinction retention deficits and glucocorticoid upregulation in the rat medial prefrontal cortex. Psychopharmacology 232:47–56
Han F, Yan S, Shi Y (2013) Single-prolonged stress induces endoplasmic reticulum-dependent apoptosis in the hippocampus in a rat model of post-traumatic stress disorder. PLoS One 8:e69340
Hosseinichimeh N, Rahmandad H, Wittenborn AK (2015) Modeling the hypothalamus-pituitary-adrenal axis: a review and extension. Math Biosci 268:52–65
Huang H, Zhao J, Jiang L, Xie Y, Xia Y, Lv R (2015) Paeoniflorin improves menopause depression in ovariectomized rats under chronic unpredictable mild stress. Int J Clin Exp Med 8:5103–5011
Jin ZL, Liu JX, Liu X, Zhang LM, Ran YH, Zheng YY et al (2016) Anxiolytic effects of GLYX-13 in animal models of posttraumatic stress disorder-like behavior. Psychopharmacol 30:913–921
Kao CY, Stalla G, Stalla J, Wotjak CT, Anderzhanova E (2015) Norepinephrine and corticosterone in the medial prefrontal cortex and hippocampus predict PTSD-like symptoms in mice. Eur J Neurosci 41:1139–1148
Kozaric-Kovacic D (2008) Psychopharmacotherapy of posttraumatic stress disorder. Croat Med J 49:459–475
Krishnamurthy S, Garabadu D, Joy KP (2013) Risperidone ameliorates post-traumatic stress disorder-like symptoms in modified stress re-stress model. Neuropharmacology 75:62–77
Levkovitz Y, Fenchel D, Kaplan Z, Zohar J, Cohen H (2015) Early post-stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. Eur Neuropsychopharmacol 25:124–132
Li YF, Huang Y, Amsdell SL, Xiao L, O'Donnell JM, Zhang HT (2009) Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34:2404–2419
Li J, Ji X, Zhang J, Shi G, Zhu X, Wang K (2014) Paeoniflorin attenuates Aβ25-35-induced neurotoxicity in PC12 cells by preventing mitochondrial dysfunction. Folia Neuropathol 52:285–290
Lin CC, Tung CS, Lin PH, Huang CL, Liu YP (2016a) Traumatic stress causes distinctive effects on fear circuit catecholamines and the fear extinction profile in a rodent model of posttraumatic stress disorder. Eur Neuropsychopharmacol 26:1484–1495
Lin CC, Tung CS, Liu YP (2016b) Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology 233:1135–1146
MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB et al (2016) Emotion regulatory brain function and SSRI treatment in PTSD: neural correlates and predictors of change. Neuropsychopharmacology 41:611–618
Mao QQ, Ip SP, Tsai SH, Che CT (2008) Antidepressant-like effect of peony glycosides in mice. J Ethnopharmacol 119:272–275
McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB et al (2013) Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry 74:672–679
Miao YL, Guo WZ, Shi WZ, Fang WW, Liu Y, Liu J et al (2014) Midazolam ameliorates the behavior deficits of a rat posttraumatic stress disorder model through dual 18 kDa translocator protein and central benzodiazepine receptor and neurosteroidogenesis. PLoS One 9:e101450
Muhtz C, Wiedemann K, Kellner M (2012) Panicogens in patients with Post-Traumatic Stress Disorder (PTSD). Curr Pharm Des 18:5608–5618
Nam KN, Yae CG, Hong JW, Cho DH, Lee JH, Lee EH (2013) Paeoniflorin, a monoterpene glycoside, attenuates lipopolysaccharide-induced neuronal injury and brain microglial inflammatory response. Biotechnol Lett 35:1183–1189
Oitzl MS, de Kloet ER (1992) Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci 106:62–71
Oliveira TQ, Sousa CNS, Vasconcelos GS, de Sousa LC, de Oliveira AA, Patrocínio CFV et al (2017) Brain antioxidant effect of mirtazapine and reversal of sedation by its combination with alpha-lipoic acid in a model of depression induced by corticosterone. J Affect Disord 219:49–57
Ondicova K, Kvetnansky R, Mravec B (2014) Deafferentation of the hypothalamic paraventricular nucleus (PVN) exaggerates the sympathoadrenal system activity in stressed rats. Endocr Regul 48:135–143
Pervanidou P, Chrousos GP (2012) Posttraumatic stress disorder in children and adolescents: neuroendocrine perspectives. Sci Signal 5:pt6. https://doi.org/10.1126/scisignal.2003327
Qiu F, Zhong X, Mao Q, Huang Z (2013) The antidepressant-like effects of paeoniflorin in mouse models. Exp Ther Med 5:1113–1116
Qiu ZK, Liu CH, Gao ZW, He JL, Liu X, Wei QL et al (2016) The inulin-type oligosaccharides extract from morinda officinalis, a traditional Chinese herb, ameliorated behavioral deficits in an animal model of post-traumatic stress disorder. Metab Brain Dis 31:1143–1149
Raineki C, Chew L, Mok P, Ellis L, Weinberg J (2016) Short- and long-term effects of stress during adolescence on emotionality and HPA function of animals exposed to alcohol prenatally. Psychoneuroendocrinology 74:13–23
Reid AM, McNamara JP, Murphy TK, Guzick AG, Storch EA, Goodman WK et al (2015) Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial. J Psychiatr Res 71:140–147
Roozendaal B, Phillips RG, Power AE, Brooke SM, Sapolsky RM, McGaugh JL (2001) Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat Neurosci 4:1169–1171
Santos CJ, Ferreira AV, Oliveira AL, Oliveira MC, Gomes JS, Aguiar DC (2016) Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr Neurosci 29:1–7
Uschold-Schmidt N, Peterlik D, Füchsl AM, Reber SO (2013) HPA axis changes during the initial phase of psychosocial stressor exposure in male mice. J Endocrinol 218:193–203
van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, Ogbonmwan YE (2017) The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2017.09.005
Wang HN, Peng Y, Tan QR, Wang HH, Chen YC, Zhang RG et al (2009) Free and Easy Wanderer Plus (FEWP), a polyherbal preparation, ameliorates PTSD-like behavior and cognitive impairments in stressed rats. Prog Neuro-Psychopharmacol Biol Psychiatry 33:1458–1463
Wang QS, Gao T, Cui YL, Gao LN, Jiang HL (2014) Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol 52:1189–1195
Wang YL, Wang JX, Hu XX, Chen L, Qiu ZK, Zhao N et al (2016) Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol 179:9–15
Wen L, Xiao B, Shi Y, Han F (2017) PERK signalling pathway mediates single prolonged stress-induced dysfunction of medial prefrontal cortex neurons. Apoptosis 22:753–768
Wilson CB, Ebenezer PJ, McLaughlin LD, Francis J (2014) Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS One 9:e89104
Xue R, Jin ZL, Chen HX, Yuan L, He XH, Zhang YP et al (2013) Antidepressant-like effects of 071031B, a novel serotonin and norepinephrine reuptake inhibitor. Eur Neuropsychopharmacol 23:728–741
Ye J, Duan H, Yang X, Yan W, Zheng X (2001) Anti-thrombosis effect of paeoniflorin: evaluated in a photochemical reaction thrombosis model in vivo. Planta Med 67:766–767
Yehuda R, Pratchett LC, Elmes MW, Lehrner A, Daskalakis NP, Koch E et al (2014) Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus 4:20140048
Zhang YH (2014) The treatment and research for posttraumatic stress disorder with Chinese medicine. Eur J Psychotraumatol 5:26524
Zhang LM, Yao JZ, Li Y, Li K, Chen HX, Zhang YZ et al (2012) Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evid Based Complement Alternat Med 2012:623753
Zhang LM, Qiu ZK, Zhao N, Chen HX, Liu YQ, Xu JP et al (2014) Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol 17:1659–1669
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581:113–120
Zhe D, Fang H, Yuxiu S (2008) Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem Cytochem 41:89–95
Acknowledgements
This study was supported by a grant from Natural Science Foundation of Guangdong Province, China (No. 2017A030313448), National Natural Science Foundation of China (No. 81703731) and Project of Educational Commission of Guangdong Province, China (No. 2016KQNCX086).
Author Contribution
Wei Xiao, Qing-Hong Fan, Xiao-Meng Chai and Wei-hai Ye have participated in the revised manuscript and the proof. They had contributed to the revision. Thus, we invited them in the publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Rights and permissions
About this article
Cite this article
Qiu, ZK., He, JL., Liu, X. et al. Anxiolytic-like effects of paeoniflorin in an animal model of post traumatic stress disorder. Metab Brain Dis 33, 1175–1185 (2018). https://doi.org/10.1007/s11011-018-0216-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0216-4