Abstract
We investigated interaction of GM3 with N-acetylglucosamine (GlcNAc) termini of N-linked glycans of epidermal growth factor receptor (EGFR), as the underlying mechanism for inhibitory effect of GM3 on EGFR activation, using ldlD cells transfected with EGFR gene. These cells, defective in UDP-Gal/UDP-GalNAc 4-epimerase, are incapable of synthesizing galactose (Gal)-containing glycans, unless Gal is provided in culture (+Gal). Key observations: (1) Expression of GlcNAc termini was high in −Gal cells, and strongly reduced in +Gal cells. (2) Comparative study of inhibitory effect of exogenously-added GM3 on EGFR activation in +Gal versus −Gal cells indicated that higher level of GlcNAc termini on EGFR is correlated with greater inhibitory effect of GM3. (3) GM3-, but not GM1-, coated beads bound to EGFR in lysate of −Gal cells, which have highly exposed GlcNAc termini. Such binding was inhibited in the presence of EDTA, similarly to other carbohydrate-carbohydrate interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept that cell growth is regulated by growth factors and their receptors, through activation of protein kinase associated with the receptor, was established initially by studies of epidermal growth factor receptor (EGFR) [1, 2]. Following early findings of an association between phenotypic changes and changes of ganglioside expression [3–6], subsequent studies revealed functional effects of gangliosides on receptor-associated tyrosine kinases for epidermal growth factor (EGFR) [7], platelet-derived growth factor [8], fibroblast growth factor [9], neuronal growth factor [10], insulin [11], etc.
Ganglioside effect in these studies was based mainly on exogenous addition of ganglioside. Endogenous GM3 effect on EGFR was studied in ldlD cells transfected with EGFR gene (“ldlD/EGFR”) [12]. ldlD cells, a mutant cell line derived from Chinese hamster ovary (CHO) cells, are deficient in the enzyme UDP-Gal/UDP-GalNAc 4-epimerase. When these cells are grown in glucose-based media, they cannot synthesize enough UDP-Gal and UDP-GalNAc to allow normal synthesis of glycolipids and glycoproteins. All glycosylation defects observed in ldlD cells can be fully corrected by exogenous addition of Gal and GalNAc [13]. ldlD/EGFR cells displayed clear inhibition of cell growth and EGFR tyrosine kinase under +Gal condition compared to −Gal condition [12], demonstrating that endogenously- expressed GM3 has an inhibitory effect, and supporting our previous finding that GM3 inhibits EGFR tyrosine kinase activity [7].
Recent studies on the molecular mechanism underlying the inhibitory effect of GM3 on EGFR tyrosine kinase suggest that carbohydrate-to-carbohydrate interaction between GM3 and N-acetylglucosamine (GlcNAc) termini of N-linked glycans of EGFR plays a key role in this process, in A431 cells [14, 15].
The purpose of the present study is to clarify the mechanism of such interaction, using EGFR-transfected ldlD cells.
Experimental Procedure
Cells, Antibodies and Reagents
ldlD14, a UDP-Gal/UDP-GalNAc 4-epimerase deficient variant of Chinese Hamster Ovary cell line, was established [13] and kindly donated by M. Krieger (Massachusetts Institute of Technology, Cambridge, MA). ldlD14 cells and their transfectants were cultured in Ham’s F12 culture medium supplemented with 5% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. Glycosylation status of these cells was manipulated by culturing in serum-free Ham’s F12 containing ITS (insulin/transferrin/selenium) (BD Biosciences, Bedford, MA) alone, or in the presence of Gal (10, 20, 50, 100, or 200 μM).
Mouse IgG1 anti-EGFR mAb Ab-5 (Clone H11) was from Thermo Fisher Scientific (Waltham, MA). Mouse IgG2b anti-phospho-Tyr (PY-20) mAb was from Biosciences (San Jose, CA). Mouse IgG anti-γ-tubulin mAb was from Sigma–Aldrich (St. Louis, MO). Mouse IgM mAb J1, which selectively recognizes GlcNAc termini of glycoproteins and glycosphingolipids, was established in our lab [16]. Goat anti-mouse IgG, conjugated with horseradish peroxidase (HRP), was from Southern Biotech (Birmingham, AL). FITC-labeled goat anti-mouse IgM + IgG was from Biosource/Life Technologies (Carlsbad, CA). HRP- or FITC-conjugated lectin GS-II were from EY Labs (San Mateo, CA). Gangliosides GM3 and GM1 were from Matreya (Pleasant Gap, PA). Human recombinant EGF was from Earth Chemical (Tokyo, Japan). Methotrexate hydrate (MTX), and other reagents unless described otherwise, were from Sigma–Aldrich.
Transfection of ldlD Cells
Plasmid pXER, an expression vector containing human EGFR gene, was kindly donated by R. Davis (Univ. of Massachusetts Medical School, Worcester, MA). ldlD cells were transfected with the plasmid using Fugene 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN), according to the manufacturer’s protocol. After drug selection by culturing in 5% FBS/Eagle’s minimum essential medium (EMEM, Sigma–Aldrich) containing 0.5 μM MTX, and screening for EGFR expression by Western blot or cell surface staining, the transfectants were cloned by colony selection.
SDS–PAGE and Western Blot
Western blot analysis was performed as described previously [17]. In brief, cells were lysed in RIPA buffer (1% Triton X-100, 150 mM NaCl, 25 mM Tris pH 7.4, 5 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM tetrasodium pyrophosphate, 50 mM sodium fluoride, 1 mM Na3VO4, 2 mM phenylmethanesulfonyl fluoride, 0.076 U/ml aprotinin), and protein concentration was determined using Micro-BCA kit (Thermo Fisher/Pierce; Rockford, IL). Proteins separated by SDS–PAGE were transferred onto PVDF membranes (Millipore; Billerica, MA). Membranes were blocked with 1% BSA in 0.1% Tween 20 TBS (10 mM Tris–HCl, 150 mM NaCl, pH 8.0), blotted with primary mAb, incubated with appropriate HRP-conjugated secondary antibody, and visualized using Supersignal Chemiluminescence substrate kit (Thermo Fisher/Pierce).
Analysis of GlcNAc Termini on the Cell Surface by Flow Cytometry and Fluorescence Microscopy
ldlD/EGFR cells were cultured in Ham’s F12 medium containing 5% FBS for 24 h. Medium was replaced by serum-free F12 medium with ITS, in the presence or absence of 200 μM Gal. After further 48 h incubation, cells were detached and aliquots (1 × 105 cells) were incubated with mAb J1, followed by incubation with secondary FITC-conjugated antibody, or incubated with GS-II-FITC, and analyzed with flow cytometer (Epics XL, Beckman Coulter, Brea, CA). Alternatively, cells were seeded onto 12-mm diameter glass coverslips in 24-well tissue culture plates, and cultured under the condition described above. After washing, the cells were fixed with 2% paraformaldehyde/PBS, washed with PBS, blocked with 1% BSA/0.1% NaN3/PBS, and stained with J1 or GS-II as described above. Cell nuclei were stained with Hoechst 44442 (Invitrogen, Carlsbad, CA). Stained cells were washed, mounted with Glycergel (Dakocytomation, Carpinteria, CA), and photographed with fluorescent microscope (Leica DM6000B, Leica Microsystems Ltd., Heerbrugg, Switzerland).
EGFR Activation Induced by EGF
EGF-dependent EGFR activation was assessed by tyrosine phosphorylation of the receptor using anti-phosphotyrosine mAb PY20. Cells were incubated under various conditions as described above for 3 days, then stimulated with 100 ng/ml EGF for 30 min at 37°C in 5% CO2. Cells were washed 3× with cold PBS containing 1 mM sodium vanadate, lysed, and subjected to SDS–PAGE and Western blot.
Exogenous Addition of GM3 to ldlD/EGFR Cells
GM3 in methanol/chloroform (2:1) was dried under nitrogen stream, and re-suspended in Ham’s F12 medium by sonication at room temp for 3 h. ldlD/EGFR cell monolayers were grown in Ham’s F12 medium with ITS in the presence or absence of 200 μM Gal for 3 days, then incubated with 50 μM GM3 for 2 h. EGF-dependent EGFR activation was analyzed as described above.
Phagokinetic Gold Sol Assay for Motility
Cell motility was measured by gold-sol assay as described previously [17, 18]. In brief, cells were detached with trypsin/EDTA, and resuspended in serum-free medium containing soybean trypsin inhibitor (Sigma–Aldrich), washed with F12 medium, and seeded onto gold sol-coated wells in F12 medium alone, or in the presence of 20 μM Gal and/or 10 ng/ml EGF. After 18 h incubation, the track area of 30 cells was photographed, and cleared areas on gold sol were measured using Scion Image program.
Interaction of GSL-Coated Polystyrene Beads with EGFR
The interaction was assessed as described previously [14]. Briefly, polystyrene beads (sulfate latex, 1 μm diameter, Invitrogen) were coated with ganglioside as follows. Beads were washed with PBS, and suspended in ethanol. Gangliosides were dried and dissolved in ethanol/distilled water (9:1). Beads and gangliosides were mixed and rotated overnight at 4°C. Coated beads were blocked with 0.1% gelatin, mixed with cell lysate, and bound EGFR was detected by Western blot.
Results
Endogenous GM3 Inhibits Phosphorylation of EGFR, and EGF Induced-Cell Motility
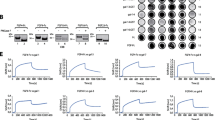
ldlD14 cells, a mutant from CHO cells, lack UDP-Gal/UDP-GalNAc 4-epimerase and are unable to synthesize UDP-Gal or UDP-GalNAc from glucose [13, 19]. This deficiency can be fully reversed by addition of Gal and GalNAc in culture medium. Using the mutant cell lines, we can modify cell surface glycan expression. For example, the cells do not express endogenous ganglioside GM3 on the cell surface, unless Gal is added to culture medium (+Gal condition) [13, 20–22]. To extend our previous finding that N-glycan structure of EGFR plays an essential role in GM3-dependent inhibition of EGFR activation, we transfected human EGFR gene to ldlD14 cells, and established ldlD/EGFR cells. Flow cytometry analysis indicated that ldlD/EGFR cells express high surface level of EGFR (Fig. 1a). Incubation of ldlD/EGFR cells with EGF enhanced EGFR phosphorylation on tyrosine residue, and such enhancement was reduced under +Gal condition, i.e., when they express GM3 (Fig. 1b), as reported previously by Weis & Davis [12].
Phosphorylation of EGFR in transfected cells. a ldlD14 and ldlD/EGFR cells (1 × 105 per well) were cultured in 6-well plates, in Ham’s F12 medium with 5% FBS for 2 days. Cells were detached with trypsin/EDTA. 1 × 105 cells were incubated with anti-EGFR mAb for 2 h, washed 3× with 1% BSA/0.1% NaN3/PBS, then incubated with FITC-labeled secondary antibody, fixed with 2% paraformaldehyde/PBS, and subjected to flow cytometry. Gray histogram: ldlD14 cells. Empty histogram: ldlD/EGFR cells. LFI log fluorescence intensity. b Phosphorylation of EGFR induced by EGF in ldlD/EGFR cells under different glycosylation status. ldlD/EGFR cells (1 × 105 per well) were seeded into 12-well plates in Ham’s F12 medium with 5% FBS, and cultured overnight. Medium was changed to serum-free medium supplemented with ITS alone, or ITS + Gal (20 μM). After growth for 48 h, medium was replaced by Ham’s F12 only, or by medium containing 20 μM Gal, and the cells were further cultured overnight. The cells were then added with 100 ng/ml EGF, incubated at 37°C in 5% CO2 for 30 min, washed with cold PBS containing 1 mM sodium vanadate, and lysed with RIPA buffer. Proteins (8 μg/well) were subjected to SDS–PAGE, and blotted with anti-phosphotyrosine, PY20, as described under Experimental Procedures. After stripping with Re-blot Plus Mild Solution (Millipore), membrane was re-blotted with anti-EGFR. Experiments were performed in triplicate, and representative Western blot results are shown (left panel). Upper band phosphorylated EGFR expression. Lower band EGFR expression. EGF-induced phosphorylation of EGFR was quantified as density of phospho-EGFR divided by that of EGFR, by densitometry using Scion Image. Data shown are mean ± SD from triplicate experiments (right panel). Lane 1 ITS alone. Lane 2 ITS + Gal. **P = 0.001–0.005
Next, we investigated the effect of GM3 expression on haptotactic cell motility in response to EGF stimulation. Without addition of EGF, ldlD/EGFR cells showed low motility, and +Gal condition had no significant effect (Fig. 2, columns 1, 2). In the presence of EGF, the cells showed increased motility (column 3). Such enhancement was significantly reduced under +Gal condition, which induces GM3 synthesis (column 4).
EGF-dependent cell motility. ldlD/EGFR cells (5 × 104 per well) were seeded into 12-well plates in Ham’s F12 medium with 5% FBS, and monolayer cells were cultured in serum-free medium as described above. EGF-dependent cell motility was measured by gold-sol assay as described in Experimental Procedures. (1) ITS alone, −EGF. (2) ITS + Gal, −EGF. (3) ITS, +EGF. (4) ITS + Gal, +EGF. Mean ± SD of cleared area (squared pixels) is shown. ***P < 0.001
Expression of GlcNAc Termini of N-Linked Glycans in ldlD/EGFR Cells
During the maturation process of N-linked glycans, the majority of complex-type and hybrid-type N-linked glycans undergo addition of β-linked Gal residues to GlcNAc termini, to form the ubiquitous building block Galβ1-4GlcNAc, i.e., bi- to penta- antennary structures [23, 24]. Without addition of Gal, ldlD/EGFR cells do not complete this process, and express high level of GlcNAc termini. Lectin GS-II [25] or mAb J1 [16], directed to non-substituted β-GlcNAc, was used to determine GlcNAc termini on cell surface by flow cytometry or fluorescence microscopy.
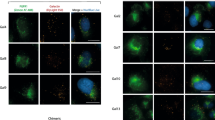
LdlD/EGFR cells cultured under −Gal condition showed high levels of GS-II sites, as expected (Fig. 3a). Addition of 20 μM Gal, which is used to induce GM3 expression, caused no detectable change in binding of GS-II. Addition of 50 or 100 μM Gal caused some reduction of binding, but much less than the blocking effect of 200 μM Gal. Addition of 200 μM Gal to culture medium significantly reduced binding of GS-II, as detected by flow cytometry (Fig. 3a). ldlD/EGFR cells showed similarly high levels of J1 binding sites under −Gal condition, and addition of 200 μM Gal completely blocked the J1 binding (Fig. 3a). Cell staining analysis with monolayer cells gave similar results (Fig. 3b). These findings are consistent with those from GM3 binding experiments as described below (Fig. 5).
Analysis of GlcNAc termini on surface in ldlD/EGFR cells. a ldlD/EGFR cells (1 × 105) were cultured in 6-well plates. Glycosylation status of the cells was altered by addition of ITS, with various concentrations of Gal (0, 20, 50, 100, 200 μM), as described in Experimental Procedures. After 3 days incubation, cells were detached with trypsin/EDTA as described in the Fig. 2 legend. For GS-II-FITC staining, 1 × 105 cells were incubated with GS-II-FITC for 1 h at room temp, washed with 1% BSA/0.1% NaN3/PBS, and fixed with 2% paraformaldehyde/PBS. For mAb J1 staining, cells were incubated with J1 for 2 h at 4°C, washed with 1% BSA/0.1% NaN3/PBS, incubated with FITC-labeled secondary antibody for 1 h at 4°C, washed, and fixed as above. Stained cells were subjected to flow cytometry. Empty histograms: GS-II-FITC (left) or mAb J1 (right) staining. Gray histograms: normal mouse IgM + FITC-labeled goat anti-mouse IgM + IgG (same in each panel). LFI log fluorescence intensity. b ldlD/EGFR cells (1 × 104 per well) were seeded onto 12-mm diameter glass coverslips in 24-well tissue culture plates. Glycosylation status of the cells was altered by addition of ITS with or without 200 μM Gal, as described in the Fig. 3 legend and Experimental Procedures. Cells were fixed with 2% paraformaldehyde in PBS for 15 min at room temp, washed with PBS, blocked with 1% BSA/0.1% NaN3/PBS for 30 min, stained with mAb J1 for 2 h at room temp, followed by incubation with FITC-labeled secondary antibody for 1 h at room temp, or staining with GS-II-FITC for 2 h. Cells were then washed, incubated with Hoechst-44442 (Invitrogen) for 15 min, and mounted with Glycergel (Dakocytomation). Stained cells were photographed with Leica DFC camera. Left Cell staining with GS-II-FITC and Hoechst-44442. Right Cell staining with J1 and Hoechst-44442. Lane 1 ITS alone. Lane 2 ITS + Gal
Effect of Highly Exposed GlcNAc Termini on Inhibitory Effect of GM3 on EGFR Activation
Since we are able to modify GlcNAc termini on N-glycans by culturing cells with 200 μM Gal, we examined the effect of such modification on the inhibitory effect of exogenous addition of GM3 on EGFR activation. For this purpose, we analyzed the inhibitory effect of exogenously-added GM3 (50 μM) on EGF-induced EGFR phosphorylation. Under −Gal condition, ldlD/EGFR cells showed highly exposed GlcNAc termini detected by GS-II binding, and clearly reduced EGF-dependent EGFR activation when exogenous GM3 was added (Fig. 4a). When cells were cultured with 200 μM Gal, expression of GlcNAc termini on EGFR, assessed by GS-II blotting, was strongly reduced (Fig. 4a, b), indicating that some GlcNAc termini of N-glycans of EGFR are indeed blocked by Gal elongation. Under +Gal condition, EGF-dependent EGFR activation, detected with PY20, was greatly enhanced (Fig. 4a, c), consistent with our previous results with A431 cells using inhibitors of N-glycan processing [14]. Under both conditions, EGFR expression was not significantly altered.
Effect of reduced GlcNAc termini of EGFR on GM3-dependent inhibition of EGFR activation. GM3 in chloroform/methanol 2:1 was dried under nitrogen stream, dissolved in Ham’s F12 serum-free medium, and sonicated for 3 h. ldlD/EGFR cells (1 × 105/well in 6-well plates) were incubated for 72 h in serum-free medium supplemented with ITS alone (lane 1), or ITS + Gal (200 μM) (lane 2). Cells were treated with 50 μM GM3 for 2 h, added with 100 ng/ml EGF, and incubated at 37°C in 5% CO2 for 30 min. After washing, cell lysate was prepared. GlcNAc termini on EGFR were determined using GS-II-HRP. EGFR and phosphorylated EGFR were determined as described in the Fig. 1b legend, and Experimental Procedures. γ-tubulin staining was used as loading control. a Representative results of Western blot for GlcNAc termini (GS-II), PY20, EGFR, and γ-tubulin. b Relative expression of GlcNAc termini, quantified by densitometry using Scion Image, and normalized with γ-tubulin expression. c EGF-induced phosphorylation of EGFR was quantified as density of phospho-EGFR divided by that of EGFR, by densitometry using Scion Image. Data shown are mean ± SD from triplicate experiments. **P = 0.001–0.005. Lane 1 ITS alone. Lane 2 ITS + Gal
Binding of GM3 to EGFR Through Highly Exposed GlcNAc Termini
Next, we examined whether GM3 binds selectively to exposed N-linked GlcNAc termini of EGFR, using the procedure described previously [14]. Polystyrene beads coated with GM3, or GM1 as control, were mixed with lysate from cells grown in the presence or absence of 200 μM Gal, and bound EGFR was detected by Western blot analysis using anti-EGFR mAb. Non-coated control beads, and GM1-coated beads, showed no binding to EGFR (Fig. 5a, lanes 1, 6, 7). GM3-coated beads showed clear binding to EGFR, which has highly exposed N-linked GlcNAc termini, from −Gal cells (lane 4), but not +Gal cells (lane 5). Binding of GM3 to N-linked GlcNAc termini was strongly inhibited by co-incubation with EDTA (lane 2), consistent with our previous finding with A431 cells that interaction of GM3 with N-linked GlcNAc termini requires divalent cation [14], similarly to other carbohydrate- carbohydrate interactions [26–28]. Binding of GM3 to EGFR in lysate prepared from +Gal cells showed a concentration-dependent response (Fig. 5b); i.e., binding was somewhat reduced at 50 μM Gal, and disappeared completely at 200 μM Gal. The amount of EGFR in each cell lysate used for the binding assays was consistent; i.e., it was similar among the blots shown in Fig. 5a, and similar among the blots shown in Fig. 5b. This finding indicates that addition of Gal does not significantly alter expression of EGFR.
Effect of modification of N-glycan on EGFR on its interaction with GM3. a Polystyrene beads (1.37 × 108) and ganglioside GM1 or GM3 (16 nmol each) were mixed and rotated overnight at 4°C. After washing 3×, coated beads were blocked with 0.1% gelatin for 1 h at room temp, and then collected by centrifugation. The beads were resuspended in TBS (+) buffer (150 mM NaCl, 10 mM Tris–HCl, 0.9 mM CaCl2, 0.5 mM MgSO4, 0.1 mM MnCl2, pH 8.0), and added with 30 μg protein of cell lysate prepared from ldlD/EGFR cells cultured in F12 medium containing ITS alone or ITS + Gal (200 μM). EDTA was added to assess divalent-cation dependent interaction. The mixture was vortexed and tumbled overnight at 4°C. Beads were collected by centrifugation, and washed with TBS (+). Bound proteins were extracted by boiling in SDS–PAGE sample buffer, and subjected to SDS–PAGE and Western blot for EGFR, as described in Experimental Procedures. Each cell lysate (containing 5 μg protein) used for binding assay was analyzed for EGFR, and shown as “input”. Lane 1 ITS alone, non-coated beads. Lane 2 ITS alone, beads coated with GM3, in the presence of EDTA. Lane 3 ITS + Gal, beads coated with GM3, in the presence of EDTA. Lane 4 ITS alone, beads coated with GM3. Lane 5 ITS + Gal, beads coated with GM3. Lane 6 ITS alone, beads coated with GM1. Lane 7 ITS + Gal, beads coated with GM1. Cont.: control. b ldlD/EGFR cells were cultured with various concentrations of Gal (0–200 μM). Cell lysate was prepared and mixed with GM3-coated beads, and the bound EGFR was revealed by SDS–PAGE and Western blot as described above. Each cell lysate (containing 5 μg protein) used for binding assay was analyzed for EGFR, and shown as “input”. EGFR in lane 2, 3, 4, 5 shifted to higher molecular weight. Lane 1 ITS alone. Lane 2 ITS + 10 μM Gal. Lane 3 ITS + 50 μM Gal. Lane 4 ITS + 100 μM Gal. Lane 5 ITS + 200 μM Gal. cont.: control
Discussion
Following our initial finding that GM3 inhibits EGF-dependent tyrosine kinase in human epidermoid carcinoma A431 cells [7], a series of studies were performed on regulatory effects of gangliosides on tyrosine kinases associated with various growth factor receptors [8–11]. These previous studies were based on the effect of exogenous addition of gangliosides on tyrosine kinases associated with various growth factor receptors. Effect of endogenous GM3 on tyrosine kinase activity was studied by M. Davis, using ldlD cells transfected with EGFR gene [12]. However, the mechanism of a possible interaction of GM3 with EGFR has been unclear in these cells, including ldlD cells.
The major focus of the present study is a possible interaction of GM3 with GlcNAc termini of N-linked glycans of EGFR, using ldlD cells transfected with EGFR (ldlD/EGFR). These cells do not express GM3 or other glycans containing Gal or GalNAc in Ham’s F12 media containing ITS, but express GM3 and various other N-linked glycans when Gal is added to medium (+Gal condition). In these cells, GM3 is the major GSL and other higher gangliosides or other GSLs are very minor components [20], assumed to be similar to original CHO cells from which ldlD cells were derived [19]. Furthermore, these cells are capable of synthesizing the core structure of N-linked glycan, but incapable of chain elongation of N-linked glycan, i.e., the cells express N-linked glycans with β-GlcNAc termini under −Gal condition, whereas GlcNAc termini are masked by β-Gal under +Gal condition. Thus, the studies with these cells grown in under +Gal versus −Gal condition are useful for evaluation of possible interaction of GM3 with GlcNAc termini of N-linked glycans of EGFR, and effect on EGF-induced tyrosine kinase.
In our model experiment using glycans isolated from ovalbumin, GM3 was found to bind to “Os Fr.B” having 5–6 GlcNAc termini, showed less binding to glycan having 3 GlcNAc termini, and no binding to glycan having 2 GlcNAc termini [29]. Further studies with A431 cells indicated that the mechanism for the inhibitory effect of GM3 on EGFR tyrosine kinase was based on carbohydrate-to-carbohydrate interaction between GM3 and GlcNAc termini of N-linked glycan of EGFR [14]. The present study, using a different approach with ldlD/EGFR cells, led to the same conclusion.
The generally accepted mechanism for growth factor-induced activation of tyrosine kinase is that originally proposed by Schlessinger, based on receptor-to-receptor interaction to form dimer [30, 31], in which involvement of GSLs or glycans was not considered. It is possible that such dimeric interaction may be inhibited by GM3 or other gangliosides. Along this line of study, GM3 was shown to interact equally well with monomeric versus dimeric EGFR, and GM3 equally inhibited tyrosine kinase of these two types of EGFR [32]. This suggests that GM3 may interact directly, and inhibit EGF tyrosine kinase, regardless of its association with monomeric versus dimeric form of EGFR. Further extensive studies are necessary to examine this possibility.
Our previous study showed that high motility of A431 cells was reduced by treatment with EtDO-P4 [33], an inhibitor of GlcCer synthesis, which blocks ganglioside expression. The findings suggested that EGFR is complexed with gangliosides and tetraspanins, to maintain high cell motility, and that such complex is dissociated by elimination of gangliosides by EtDO-P4 [34]. These results are different from the enhanced motility of EGF-stimulated ldlD/EGFR cells, and its inhibition by GM3 expression, as observed in the present study, but both sets of results illustrate cell motility control through EGFR function.
A few studies have suggested that changes in N-linked glycans of EGFR may play an essential role in regulation of EGFR kinase. Binding of Phaseolus vulgaris-E lectin, which recognizes bisecting β1-4GlcNAc structure, to EGFR of human glioblastoma-astrocytoma U373MG cells, blocked EGF binding and EGFR autophosphorylation [35]. These results were confirmed by transfection of β1-4GlcNAc transferase (GlcNAcT-III) gene in the cells, which caused decrease of EGF binding and inhibition of EGFR autophosphorylation [36]. This findings may be limited to U373MG cells, and may not be applicable to GM3 effect on EGFR in A431 cells or ldlD/EGFR cells. However, it will be interesting to study the status of GlcNAcT-III versus β1-6GlcNAcT-V, and their correlation with EGFR-associated tyrosine kinase activity, in A431, ldlD/EGFR, and other cell lines, since GlcNAcT-III and -V are reported to be competitive [37–41]. GlcNAcT-V produces side chain branch, GlcNAcβ1-6Manα1-6Manα, leading to tetra- to penta-antennary structure of N-linked glycans, which can be detected by binding to Phaseolus vulgaris-L lectin [42].
Further detailed studies on the structural basis for interaction of GM3 with specific N-linked glycans carrying multivalent GlcNAc termini of EGFR will clarify the mechanism by which GM3 localized in membrane microdomains inhibits EGFR tyrosine kinase activity.
Abbreviations
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- FBS:
-
Fetal bovine serum
- Gal:
-
Galactose
- GlcNAc:
-
N-acetylglucosamine
- ITS:
-
Insulin/transferrin/selenium
- ldlD/EGFR:
-
ldlD cells transfected with EGFR gene
- SDS–PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Cohen S, Carpenter G, King L (1980) Epidermal growth factor receptor-protein kinase interactions: co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem 255:4834–4842
Ushiro H, Cohen S (1980) Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A431 cell membranes. J Biol Chem 255:8363–8365
Hakomori S, Murakami WT (1968) Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci USA 59:254–261
Sakiyama H, Gross SK, Robbins PW (1972) Glycolipid synthesis in normal and virus-transformed hamster cell lines. Proc Natl Acad Sci USA 69:872–876
Chandrabose KA, Graham JM, Macpherson IA (1976) Glycolipid glycosyl transferases of a hamster cell line in culture. II. Subcellular distribution and the effect of culture age and density. Biochim Biophys Acta 429:112–122
Langenbach R, Kennedy S (1978) Gangliosides and their cell density-dependent changes in control and chemically transformed C3H/10T1/2 cells. Exp Cell Res 112:361–372
Bremer EG, Schlessinger J, Hakomori S (1986) Ganglioside-mediated modulation of cell growth: specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem 261:2434–2440
Bremer EG, Hakomori S, Bowen-Pope DF et al (1984) Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem 259:6818–6825
Toledo MS, Suzuki E, Handa K et al (2004) Cell growth regulation through GM3-enriched microdomain (glycosynapse) in human lung embryonal fibroblast WI38 and its oncogenic transformant VA13. J Biol Chem 279:34655–34664
Mutoh T, Tokuda A, Miyada T et al (1995) Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci USA 92:5087–5091
Nojiri H, Stroud MR, Hakomori S (1991) A specific type of ganglioside as a modulator of insulin-dependent cell growth and insulin receptor tyrosine kinase activity: possible association of ganglioside-induced inhibition of insulin receptor function and monocytic differentiation induction in HL60 cells. J Biol Chem 266:4531–4537
Weis FMB, Davis RJ (1990) Regulation of epidermal growth factor receptor signal transduction: role of gangliosides. J Biol Chem 265:12059–12066
Kingsley DM, Kozarsky KF, Hobbie L et al (1986) Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell 44:749–759
Yoon S, Nakayama K, Hikita T et al (2006) Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc Natl Acad Sci USA 103:18987–18991
Kawashima N, Yoon SJ, Itoh K et al (2009) Tyrosine kinase activity of epidermal growth factor receptor is regulated by GM3 binding through carbohydrate to carbohydrate interactions. J Biol Chem 284:6147–6155
Symington FW, Fenderson BA, Hakomori S (1984) Fine specificity of a monoclonal anti-testicular cell antibody for glycolipids with terminal N-acetyl-D-glucosamine structure. Mol Immunol 21:877–882
Guan F, Handa K, Hakomori S (2009) Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc Natl Acad Sci USA 106:7461–7466
Mitsuzuka K, Handa K, Satoh M et al (2005) A specific microdomain (“glycosynapse 3”) controls phenotypic conversion and reversion of bladder cancer cells through GM3-mediated interaction of alpha3beta1 integrin with CD9. J Biol Chem 280:35545–35553
Krieger M (1983) Complementation of mutations in the LDL pathway of receptor-mediated endocytosis by cocultivation of LDL receptor-defective hamster cell mutants. Cell 33:413–422
Ono M, Handa K, Withers DA et al (1999) Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9, with concurrent glycosylation. Cancer Res 59:2335–2339
Ono M, Handa K, Sonnino S et al (2001) GM3 ganglioside inhibits CD9-facilitated haptotactic cell motility: co-expression of GM3 and CD9 is essential in down-regulation of tumor cell motility and malignancy. Biochemistry 40:6414–6421
Kawakami Y, Kawakami K, Steelant WFA et al (2002) Tetraspanin CD9 is a “proteolipid”, and its interaction with a3 integrin in microdomain is promoted by GM3 ganglioside, leading to inhibition of laminin-5-dependent cell motility. J Biol Chem 277:34349–34358
Kobata A (1996) Cancer cells and metastasis: the Warren-Glick phenomenon: basis of tumorigenesis and metastasis. In: Montreuil J, Vliegenthart JFG, Schachter H (eds) Glycoproteins and disease. Elsevier Science, Amsterdam, pp 211–227
Yamashita K, Kamerling JP, Kobata A (1982) Structural study of the carbohydrate moiety of hen ovomucoid: occurrence of a series of pentaantennary complex-type asparagine-linked sugar chains. J Biol Chem 257:12809–12814
Shankar Iyer PN, Wilkinson KD, Goldstein IJ (1976) An N-acetyl-D-glycosamine binding lectin from Bandeiraea simplicifolia seeds. Arch Biochem Biophys 177:330–333
Eggens I, Fenderson BA, Toyokuni T et al (1989) Specific interaction between Lex and Lex determinants: a possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem 264:9476–9484
Kojima N, Fenderson BA, Stroud MR et al (1994) Further studies on cell adhesion based on Lex-Lex interaction, with new approaches: embryoglycan aggregation of F9 teratocarcinoma cells, and adhesion of various tumour cells based on Lex expression. Glycoconj J 11:238–248
Handa K, Kojima N, Hakomori S (2000) Analysis of glycolipid-dependent cell adhesion based on carbohydrate-carbohydrate interaction. Meth Enzymol 312:447–458
Yoon S, Nakayama K, Takahashi N et al (2006) Interaction of N-linked glycans, having multivalent GlcNAc termini, with GM3 ganglioside. Glycoconj J 23:639–649
Schlessinger J (1988) Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci (TIBS) 13:443–447
Ullrich A, Schlessinger J (1990) Signal transduction by receptors with tyrosine kinase activity. Cell 61:203–212
Zhou Q, Hakomori S, Kitamura K et al (1994) GM3 directly inhibits tyrosine phosphorylation and de-N-acetyl-GM3 directly enhances serine phosphorylation of epidermal growth factor receptor, independently of receptor-receptor interaction. J Biol Chem 269:1959–1965
Lee L, Abe A, Shayman JA (1999) Improved inhibitors of glucosylceramide synthase. J Biol Chem 274:14662–14669
Park S-Y, Yoon S-J, Freire-de-Lima L et al (2009) Control of cell motility by interaction of gangliosides, tetraspanins, and epidermal growth factor receptor in A431 vs. Kb epidermoid tumor cells. Carbohydr Res 344:1479–1486
Rebbaa A, Yamamoto H, Moskal JR et al (1996) Binding of erythroagglutinating phytohemagglutinin lectin from Phaseolus vulgaris to the epidermal growth factor receptor inhibits receptor function in the human glioma cell line, U373 MG. J Neurochem 67:2265–2272
Rebbaa A, Yamamoto H, Saito T et al (1997) Gene transfection-mediated overexpression of b1,4 GlcNAc bisecting oligosaccharide structure in a glioma cell line, U373MG, inhibits EGF receptor function. J Biol Chem 272:9275–9280
Schachter H (1986) Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol 64:163–181
Kang R, Saito H, Ihara Y et al (1996) Transcriptional regulation of the N-acetylglucosaminyltransferase V gene in human bile duct carcinoma cells (HuCC-T1) is mediated by Ets-1. J Biol Chem 271:26706–26712
Buckhaults P, Chen L, Freigen N et al (1997) Transcriptional regulation of N-acetylglucosaminyltransferase V by the src oncogene. J Biol Chem 272:19575–19581
Yoshimura M, Nishikawa A, Ihara Y et al (1995) Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci USA 92:8754–8758
Yoshimura M, Ihara Y, Matsuzawa Y et al (1996) Aberrant glycosylation of E-cadherin enhances cell-cell binding to suppress metastasis. J Biol Chem 271:13811–13815
Cummings RD, Kornfeld S (1982) Characterization of structural determinants required for the high-affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem 257:11230–11234
Acknowledgments
This work was supported in part by NIH National Cancer Institute grant 2 R01 CA080054. The authors thank Monty Krieger for donation of ldlD14 cells, Roger Davis for donation of plasmid pXER, and Steve Anderson for help in preparing the manuscript and figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Dr. Robert K. Yu.
Rights and permissions
About this article
Cite this article
Guan, F., Handa, K. & Hakomori, Si. Regulation of Epidermal Growth Factor Receptor Through Interaction of Ganglioside GM3 with GlcNAc of N-Linked Glycan of the Receptor: Demonstration in ldlD Cells. Neurochem Res 36, 1645–1653 (2011). https://doi.org/10.1007/s11064-010-0379-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0379-9