Abstract
Reactive oxygen species have been implicated in seizure-induced neurodegeneration, and there is a correlation between free radical level and scavenger enzymatic activity in the epilepsy. It has been suggested that pilocarpine-induced seizures is mediated by an increase in oxidative stress. Current research has found that antioxidant may provide, in a certain degree, neuroprotection against the neurotoxicity of seizures at the cellular level. Alpha-tocopherol has numerous nonenzymatic actions and is a powerful liposoluble antioxidant. The objective of the present study was to evaluate the neuroprotective effects of alpha-tocopherol (TP) in rats, against oxidative stress caused by pilocarpine-induced seizures. 30 min prior to behavioral observation, Wistar rats were treated with, 0.9% saline (i.p., control group), TP (200 mg/kg, i.p., TP group), pilocarpine (400 mg/kg, i.p., P400 group), or the combination of TP (200 mg/kg, i.p.) and pilocarpine (400 mg/kg, i.p.). After the treatments all groups were observed for 6 h. The enzymatic activities, lipid peroxidation and nitrite concentrations were measured using speccitrophotometric methods and these data were assayed. In P400 group mice there was a significant increase in lipid peroxidation and nitrite levels. However, no alteration was observed in superoxide dismutase (SOD) and catalase activities. In the TP and pilocarpine co-administered mice, antioxidant treatment significantly reduced the lipid peroxidation level and nitrite content, as well as increased the SOD and catalase activities in rat hippocampus after seizures. Our findings strongly support the hypothesis that oxidative stress occurs in hippocampus during pilocarpine-induced seizures, indicate that brain damage induced by the oxidative process plays a crucial role in seizures pathogenic consequences, and imply that strong protective effect could be achieved using alpha-tocopherol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seizures and status epilepticus (SE) induced by pilocarpine in animal models are similar to human temporal lobe epilepsy in semiology and electrographic appearance. The epileptic model induced by pilocarpine is very useful for us to study the development and neuropathology of temporal lobe epilepsy [1, 2]. Neurochemical as well as enzymatic activities studies suggest that excitotoxic stimulation in SE induces excess production of reactive oxygen species, and finally leads to oxidative stress [3–5]. Through the regulation of reactive oxygen species production and maintenance of oxidative phosphorylation in mitochondria by enhanced restitution of high-energy phosphates, alpha-tocopherol (α-tocopherol) acts as chain-breaking antioxidant and protects cell membrane against oxidative damage [6]. In animals, alpha-tocopherol inhibits seizures induced by ferrous chloride, hyperbaric oxygen and penicillin. In these circumstances, the production of reactive oxygen species may have a very important role in the development of seizures itself [7, 8].
In recent years, a great deal of attention has been given to antioxidants consumption and their roles in reducing rates of chronic diseases such as epilepsy, cancer, coronary heart disease, stroke, diabetes, and arthritis [9–11]. It is suggested that protective effects of antioxidant compounds is partly due to antioxidant nutrients such as alpha-tocopherol and carotenoids which inhibit lipid per-oxidation and oxidative cell damage [12, 13]. Reactive oxygen species have been implicated in the development of seizures and SE induced by pilocarpine [14]. The mechanism underlying seizures-induced oxidative stress is not well understood yet, but several interpretations have been proposed, which include excitotoxicity associated with excessive neurotransmitter release, oxidative stress leading to free radical damage [14, 15]. Recently, several studies have examined the role of oxidative stress on pilocarpine-induced seizures which was thought possibly via the formation of free radicals [16].
Free radicals are generated during oxidative stress. It’s attractive as a possible mechanism to pilocarpine-induced seizures for many reasons. The brain processes large amounts of O2 in relatively small mass and has a high content of substrates available for oxidation in conjunction with low antioxidant activities, making it extremely susceptible to oxidative damage [17, 18]. In addition, certain regions of central nervous system, such as hippocampus, are particularly sensitive to oxidative stress because of their low endogenous levels of alpha-tocopherol, an important biochemical antioxidant, relatively to other brain regions [19, 20]. A depressed defense system may be adequate under normal circumstances. However, under pro-oxidative conditions such as those in seizures, the low antioxidant defenses capacity can predispose the brain to oxidative stress.
A variety of animal seizure models aid in documenting the effects of alpha-tocopherol and specifying its action [7, 21]. Ribeiro et al. [22] showed that alpha-tocopherol decreases pentylenetetrazol and methylmalonate-induced convulsions. Preliminary injection of the antioxidant alpha-tocopherol in rats abolished the effect of lipid peroxidation activation and decreased the number of seizures recorded on electrocorticogram in the course of the focus existence [21]. In hippocampal slice cultures, the amplitude of the evoked field potential responses was significantly decreased after experimental SE. Treatment with alpha-tocopherol prevented this dropping, which supports that it has neuroprotective effect [23]. The aim of present study was to examine the effects of alpha-tocopherol on oxidative stress in the hippocampus of adult rats prior to pilocarpine-induced seizures.
Materials and Methods
Animals and Experimental Procedures
Adult male Wistar rats (250–280 g) were maintained in a temperature controlled room (26 ± 1°C) with a 12 h light/dark cycle and food and water ad libitum (Nutrilabor, Campinas, Brazil). All experiments were performed according to the guide for the care and use of laboratory the US Department of Health and Human Services, Washington, DC (1985). The dosages of pilocarpine hydrochloride and alpha-tocopherol (Sigma, Chemical USA) are expressed at milligrams per kilogram of body weight, and were administered in a volume of 10 ml/kg injected intraperitoneally (i.p.). A total of 96 rats were treated with either 200 mg/kg alpha-tocopherol (i.p., TP) or 0.9% saline (i.p.). 30 min after the treatments 24 rats from each above group were randomized to pilocarpine hybrochloride administration (400 mg/kg, i.p., P400). Thus there are 4 groups of rats in this set of experiments: group 1, TP and P400 co-administration (n = 24); group 2, P400 plus saline treatment (n = 24); group 3, TP alone administration (n = 24); and group 4, saline treatment serves as control (n = 24). After the treatments, the animals were recorded in 30 × 30 cm chambers with: latency to first seizure (any one of the behavioral indices typically observed after pilocarpine administration: wild running, clonuses, tonus, clonic-tonic seizures), number of animals that died after pilocarpine administration. Previous work has shown that convulsions and deaths occurred within 1 and 24 h respectively post pilocarpine injection, so we decided to record the phenotypes of the animals for 6 h after pilocarpine administration. At the end of observations, the survivors were killed by decapitation and their brains were dissected on ice to remove hippocampus for histophatological analyses and determinations of lipid peroxidation level, nitrite content, superoxide dismutase and catalase activities. The pilocarpine administration rat group was constituted by those presented seizures, SE for over 30 min and non-phenotype survivors.
The drug dosages of pilocarpine (400 mg/kg) and alpha-tocopherol (200 mg/kg) were determined by previous study in our lab [5, 24] and the present study (data not shown). The drug doses used in this present study are not equivalent to those used by humans because rats have different metabolic rates.
Lipid Peroxidation Level Determinations in Hippocampus of Adult Rats Pretreated with Alpha-Tocopherol Prior to Pilocarpine-Induced Seizures
For all experimental procedures, 10% (w/v) homogenates of the area of the brain investigated were prepared for all groups. Lipid peroxidation levels in the TP plus P400 group (n = 6), P400 group (n = 6), TP group (n = 6) and control animal (n = 9) were analyzed by measuring the thiobarbituric-acid-reacting substances in homogenates, as previously described by Draper & Hadley [25]. Briefly, the homogenates were mixed with 1 ml 10% trichloroacetic acid and 1 ml 0.67% thiobarbituric acid, and were heated in a boiling water bath for 15 min, then butanol (2:1, v/v) was added to the solution. After centrifugation (800g, 5 min), TBARS determinations were performed spectrophotometrically at 535 nm and expressed as nmol of malondialdehyde (MDA)/g wet tissue.
Nitrite Content Determinations in Hippocampus of Adult Rats Pretreated with Alpha-Tocopherol Prior to Pilocarpine-Induced Seizures
To determine nitrite contents of control group (n = 9), TP plus P400 group (n = 6), P400 group (n = 6) and TP group (n = 6), the 10% (w/v) homogenates were centrifuged (800g, 10 min). The supernatants were collected, and nitric oxide production was determined based on the Griess reaction [26]. Briefly, 100 μl supernatant was incubated with 100 μl of the Griess reagent at room temperature for 10 min. A550 was measured using a microplate reader. Nitrite concentration was determined from a standard nitrite curve generated using NaNO2. The results above were expressed as nM.
Determinations of Superoxide Dismutase and Catalase Activities in Hippocampus of Adult Rats Pretreated with Alpha-Tocopherol Prior to Pilocarpine-Induced Seizures
The hippocampus was ultrasonically homogenized in 1 ml 0.05 M sodium phosphate buffer, pH 7.0. Protein concentration was measured by the method of Lowry et al. [27]. The 10% homogenates were centrifuged (800g, 20 min) and supernatants were used to assay superoxide dismutase (SOD) and catalase. SOD activity in the TP plus P400 group (n = 6), P400 group (n = 6) and TP group (n = 6) and control animals (n = 9) was assayed by using xanthine and xanthine oxidase to generate superoxide radicals [28]. They react with 2,4-iodophenyl-3,4-nitophenol-5-phenyltetrazolium chloride to form a red formazan dye. The degree of inhibition of this reaction was used to assess SOD activity. The standard assay substrate mixture contained 3.0 ml xanthine (500 μM), 7.44 mg cytochrome c, 3.0 ml KCN (200 μM), and 3.0 ml EDTA (1 mM) in 18.0 ml 0.05 m sodium phosphate buffer, pH 7.0. The sample aliquot (20 μl) was added to 975 μl of the substrate mixture plus 5 μl xanthine oxidase. After 1 min, the initial absorbance was recorded and the timer was started. The final absorbance after 6 min was recorded. The reaction was followed at 550 nm. Purified bovine erythrocyte SOD (Randox Laboratories, Belfast, Northern Ireland, UK) was used under identical conditions to obtain a calibration curve showing the percentage inhibition correlation of formazan dye formation and SOD activity. SOD activity was determined from this curve, and the results expressed as U/mg of protein.
Catalase activity was measured in the TP plus P400 group (n = 6), P400 group (n = 6) and TP group (n = 6) and control (n = 9) groups by the method that uses H2O2 to generate H2O and O2 [29]. Protein concentration was measured by the method of Lowry et al. [27]. The activity was measured by the degree of this reaction. The standard assay substrate mixture contained 0.30 ml H2O2 in 50 ml 0.05 M sodium phosphate buffer, pH 7.0. The sample aliquot (20 μl) was added to 980 μl of the substrate mixture. The initial and final absorbencies were recorded at 1 min and 6 min time-points respectively. The reaction was followed at 230 nm. A standard curve was established using purified catalase (Sigma, St Louis, MO, USA) under identical conditions. All samples were diluted with 0.1 mmol/L sodium phosphate buffer (pH 7.0), to provoke a 50% inhibition of the diluents rate (i.e. the uninhibited reaction). Results are expressed as mmol/min/mg of protein [29].
Histopathological Investigation in Hippocampus of Adult Rats Pretreated with Alpha-Tocopherol Prior to Pilocarpine-Induced Seizures
All groups were closely observed during 6 h for behavioral changes and convulsive state. After 6 h of observation, animals were sacrificed by decapitation 6 h after the treatment and brains were dissected out and fixed in formalin 10% [30, 31]. After an initial coronal section at the level of the optic nerve, 3–5 μm thick sections were prepared and stained with Hematoxylin & Eosin (HE) for light microscopy studies (100×). The degree of hippocampal damage severity was defined by a scale ranging from 0 (none) to 100 (total) by light microscopy and previously defined to be reliable for morphological analysis [32]. Brain damage presence was confirmed if hippocampus showed at least 50% involvement.
Statistical Analysis
Results of latency to first seizure, histopathological abnormalities and neurochemical alterations were compared by one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test (p < 0.05) (Graphpad program Intuitive, Software for Science, San Diego, CA). The number of animals that seized and the number that survived were calculated as percentages (seizures percentage and survival percentage, respectively), and compared with a nonparametric test (χ2).
Results
Pilocarpine induced the first seizure at 35.00 ± 0.70 min. All the animals studied showed generalized tonic-clonic convulsions with status epilepticus (SE), and 30% survived the seizures. All animals pretreated with alpha-tocopherol were observed for 6 h before pilocarpine injection and their manifested alterations in behavior, such as peripheral cholinergic signs (100%), tremors (50%), staring spells, facial automatisms, wet dog shakes, rearing and motor seizures (60%) developed progressively within 1–2 h into a long-lasting SE (60%). Table 1 shows that alpha-tocopherol (200 mg/kg) administration before pilocarpine treatment reduced by 25% the percentage of animals that seized (p < 0.0001), increased latency (212%) to the first seizure (109.09 ± 1.05 min) (p < 0.0001) and increased (40%) the survival (p < 0.0001) when compared to the pilocarpine only group. None of the control animals (saline or alpha-tocopherol) showed seizures (Table 1).
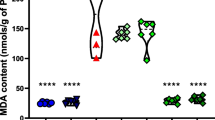
Effects of alpha-tocopherol in lipid peroxidation and nitrite concentrations during seizures induced by pilocarpine are presented in Figs. 1 and 2. Lipid peroxidation was markedly increased in pilocarpine group in comparison with the corresponding values of the saline group. During acute phase of seizures induced by pilocarpine a significant increase (90%) in thiobarbituric-acid-reacting substances (p < 0.0001) was observed. Seizures induced by pilocarpine produced a significant increase in hippocampal nitrite content (94%, p < 0.0001, Fig. 2). Rats pretreated with alpha-tocopherol showed decrease in lipid peroxidation level (79%, p < 0.0001) and nitrite content (56%, p < 0.0001) when to compared with the pilocarpine group (Fig. 1). In addition, the pretreatment with alpha-tocopherol, 30 min before administration of pilocarpine also reduced lipid peroxidation level (60%, p < 0.0001) and nitrite content (15%, p < 0.005) when compared to the control group (Figs. 1, 2). On the other hand, none of the control animals (saline or alpha-tocopherol) showed alterations in lipid peroxidation level and nitrite content (Figs. 1, 2).
Effects of alpha-tocopherol (TP) on status of lipid peroxidation level in hippocampus of adult rats prior to seizures induced by pilocarpine. Male rats (250–280 g, 2 months old) were treated with a single dose of pilocarpine (400 mg/kg, intraperitoneal, i.p., n = 6, P400), TP group with alpha-tocopherol (200 mg/kg, i.p., n = 6, TP group) and the control animals with 0.9% saline (i.p., n = 9, Control). The TP plus pilocarpine group was treated with alpha-tocopherol (200 mg/kg, i.p.) for 30 min prior to pilocarpine injection (400 mg/kg, i.p., n = 6, TP plus P400). Results are expressed as means ± S.E.M. for the number of animals shown inside in parenthesis. Differences in experimental groups were determined by two-tailed analysis of variance. a p < 0.05 as compared to control animals (t–Student–Neuman–Keuls test); b p < 0.05 as compared to P400 group (t–Student–Neuman–Keuls test)
Effects of alpha-tocopherol (TPA) on the nitrite content in hippocampus of adult rats prior to seizures induced by pilocarpine. Male rats (250–280 g, 2 months old) were treated with a single dose of pilocarpine (400 mg/kg, intraperitoneal, i.p., n = 6, P400), TP group with alpha-tocopherol (200 mg/kg, i.p., n = 6, TP group) and the control animals with 0.9% saline (i.p., n = 9, Control). The TP plus pilocarpine group was treated with alpha-tocopherol (200 mg/kg, i.p.) for 30 min prior to pilocarpine injection (400 mg/kg, i.p., n = 6, TP plus P400). Results are expressed as means ± S.E.M. for the number of animals shown inside in parenthesis. Differences in experimental groups were determined by two-tailed analysis of variance. a p < 0.05 as compared to control animals (t–Student–Neuman–Keuls test); b p < 0.05 as compared to P400 group (t–Student–Neuman–Keuls test)
Superoxide dismutase and catalase activities in the hippocampus during acute phase of seizures were not markedly altered in pilocarpine group, when compared to corresponding values to the control saline group. By the contrary, it was found a significant increase in hippocampal superoxide dismutase (40 and 43%) and catalase (51 and 53%) activities of rats pretreated with alpha-tocopherol in comparison to the pilocarpine and saline groups, respectively (p < 0.0001) (Figs. 3, 4). However, there were no enzyme alterations in TP group (Figs. 3, 4).
Effects alpha-tocopherol (TP) on the superoxide dismutase activities in hippocampus of adult rats prior to seizures induced by pilocarpine. Male rats (250–280 g, 2 months old) were treated with a single dose of pilocarpine (400 mg/kg, intraperitoneal, i.p., n = 6, P400), TP group with alpha-tocopherol (200 mg/kg, i.p., n = 6, TP group) and the control animals with 0.9% saline (i.p., n = 9, Control). The TP plus pilocarpine group was treated with alpha-tocopherol (200 mg/kg, i.p.) for 30 min prior to pilocarpine injection (400 mg/kg, i.p., n = 6, TP plus P400). Results are expressed as means ± S.E.M. for the number of animals shown inside in parenthesis. Differences in experimental groups were determined by two-tailed analysis of variance. a p < 0.05 as compared to control animals (t–Student–Neuman–Keuls test); b p < 0.05 as compared to P400 group (t–Student–Neuman–Keuls test)
Effects of alpha-tocopherol (TP) on catalase activities in hippocampus of adult rats prior to seizures induced by pilocarpine. Male rats (250–280 g, 2 months old) were treated with a single dose of pilocarpine (400 mg/kg, intraperitoneal, i.p., n = 6, P400), TP group with alpha-tocopherol (200 mg/kg, i.p., n = 6, TP group) and the control animals with 0.9% saline (i.p., n = 9, Control). The TP plus pilocarpine group was treated with alpha-tocopherol (200 mg/kg, i.p.) for 30 min prior to pilocarpine injection (400 mg/kg, i.p., n = 6, TP plus P400). Results are expressed as means ± S.E.M. for the number of animals shown inside in parenthesis. Differences in experimental groups were determined by two-tailed analysis of variance. a p < 0.05 as compared to control animals (t–Student–Neuman–Keuls test); b p < 0.05 as compared to P400 group (t–Student–Neuman–Keuls test)
Brain tissue examinations of the control (saline 0.9%), alpha-tocopherol groups (TP group) did not reveal hippocampal histopathological changes. On the other hand, P400 group presented neuronal loss, gliosis, and typical vacuolar degeneration in hippocampus region. Histopathological damages were observed in four (80%) rats of P400 group, and in one rat (20%) of the TP plus P400 group (Fig. 5).
Histopathological alterations in hippocampus of rats pretreated with alpha-tocopherol prior to pilocarpine-induced seizures. Severity of lesion was expressed as a mean ± S.E.M. of scores of damage based in a scale from zero (none) to 100 (total) percentage of hippocampus involvement. Brain damage was considered positive if there was at least 50% hippocampal involvement. Hematoxylin and eosin staining (HE). Magnification, 100×. Figures shown is from one representative experiment of n = 5
Discussion
Epilepsy is one of the most common neurologic problems all over the world, being associated with paroxysmal discharge of cerebral neurons and is characterized by several symptoms including alterations of behaviors and consciousness [33]. The molecular observations of epilepsy include the temporal correlation between free radical generation and the development of seizures in some pathological conditions, and the protective efficacy of antioxidative treatments against some types of seizures. Alpha-tocopherol, one of the effective antioxidant, not only has antioxidant functions, but also has functions in pro-oxidant, cell signaling and gene regulation [34, 35]. Previous studies indicated that alpha-tocopherol has anticonvulsant activity in several animal models, including in the ferrous chloride, hyperbaric oxygen model [35], kindling and PTZ models [36]. In this study, we demonstrated a role of alpha-tocopherol against oxidative stress generated in pilocarpine-induced seizures.
In the present study we investigated the influence of alpha-tocopherol on the level of lipid peroxidation, nitrite content and enzymatic activities of superoide dismutase and catalase in the rat hippocampus during pilocarpine-induced seizures. Generation of reactive oxygen species is currently viewed as one of the process through which epileptic activity exert their deleterious effects on brain [37]. These reactive oxygen species in the absence of an efficient defense mechanism cause peroxidation of membrane polyunsaturated fatty acids [38]. Brain is particularly susceptible to peroxidation due to simultaneous presence of high levels of polyunsaturated fatty acids and iron [39, 40] which are the targets of free radical damage. We showed the lipid peroxidation was rising in hippocampus homogenate of rats after 6 h of acute phase of seizures. The increase of lipid peroxidation was reflected by the rise of thiobarbituric-acid-reacting substances level which may be related to its intermediate free radicals formed during seizures induced by pilocarpine.
Literature has shown that pilocarpine-induced seizures led to changes in nitric oxide metabolism, and increased the production of its metabolites (nitrite and nitrate). The increased metabolites may interacts with glutamatergic receptors to produce part of its stimulatory action on the central nervous system [41, 42]. The reduction in nitrite content, after pretreatment with alpha-tocopherol, is most readily explained as a consequences of radical formation inhibiting, scavenges reactive oxygen species and lipid peroxidation products [43].
Histopathological studies of animals pretreated with alpha-tocopherol thirty min before pilocarpine injection showed a decrease of 60% in the number of animals that presented hippocampal damage after seizures (data not shown). We also observed that none of the animals which received alpha-tocopherol presented hippocampal damage. However, 80% of the animals which had seizures and that developed SE presented hippocampal damage. On the other hand, the hippocampus of rats pretreated with alpha-tocopherol presented a small damage extension (7%, data not shown). These findings support the theory of the oxidative stress involvement in the start of seizures by the increase of free radical production. Moreover, these results suggest a neuroprotective activity of alpha-tocopherol by the removal of free radicals produced during pilocarpine-induced seizures. Thus, the results suggest that oxidative stress mediated by pilocarpine exerts its pathologic effects during seizures and also that the neuroprotective and anticonvulsive role of alpha-tocopherol can be mediated by a reduction in lipid peroxidation levels and nitrite content. Possibly, this reduction is due to the modulatory activity of alpha-tocopherol in the antioxidant enzymes (superoxide dismutase and catalase) in the hippocampus of adult rats.
Superoxide dismutase and catalase activities do not protect against seizures induced by pilocarpine. However, there no changes in hippocampal superoxide dismutase activity during acute phase of seizures, suggesting that high amount of H2O2, released during the O2 − dismutation can inhibits the superoxide dismutase during this phase of seizures induced by pilocarpine. On the other hand, the catalase activity augmented in those animals presenting seizures, which suggests that H2O2 generated during superoxide dismutation would not be sufficiently removed from the hippocampus by catalase during acute phase of seizures. The increase in antioxidant enzymes activities induced by alpha-tocopherol might be explained as a necessary consequence of scavenging of O2 − produced by dismutation during acute period of seizures. Our data shows a possible neuroprotective effect of alpha-tocopherol through the scavenging of radical O2 −. This consequent scavenging of O2 − produces a decrease in the H2O2 levels generated by superoxide dismutation hippocampus, causing increase of the activities of the enzymes superoxide dismutase and catalase as neuroprotective action mechanism of this antioxidant.
Free radical formation elevations are frequently accompanied by an immediate compensatory increase in the activities of the free radical scavenging enzymes [44]. Previous studies have showed an increased in catalase activity in the hippocampus during a 24 h period of acute phase of seizures [4, 44]. Moreover, during the convulsive process, the neuronal changes are accompanied by alterations in the cerebral metabolic rate evidenced by modifications in the regional cerebral blood flow [45, 46].
In the present work, we did not observe any alteration in superoxide dismutase and catalase activities within 6 h of acute phases of seizures, which indicates the oxidative metabolism remains unaltered for at least 6 h of seizures induced by pilocarpine and the alpha-tocopherol pretreatment increases the antioxidant enzymes activity in rat hippocampus. The compensatory mechanisms of alpha-tocopherol against oxidative stress observed during seizures can be used for the explanation of its anticonvulsant action in behavioral studies. The seizures induced by pilocarpine are prevented by alpha-tocopherol, which implies free radical plays a role in controlling of seizures installation and propagation. Indeed, we found that alpha-tocopherol pretreatment is able to inhibit pilocarpine-induced seizures, SE and mortality of adult rats. In addition, these data also implies free radical formation has a relevant role in the propagation and/or maintenance of convulsive activity. The capability of alpha-tocopherol to increase antioxidant enzymes activities, and decrease free radical formation will finally lead to a significant decrease in the susceptibility to seizures induced by pilocarpine. These observations suggest alpha-tcopherol has promising anticonvulsant effect on pilocarpine induced seizures.
Herein, we clearly showed that alpha-tocopherol decreased the frequency of pilocarpine-induced seizures and increased the survival rate. In our knowledge, these effects of alpha-tocopherol on oxidative stress observed during acute phases of pilocarpine-induced seizures have not been reported before. Thus, these findings might have important implications for understanding the mechanism of epilepsy to promote new advances in the development of selective and targeted antiepileptic drugs. Alpha-tocopherol protected the hippocampus against neuronal damages regularly observed during seizures. Further investigations of the effects of alpha-tocopherol against necrosis, apoptosis and/or autophagy observed during the acute phase of this epilepsy model are in progress to confirm its neuroprotective effects.
References
Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L (1983) Limbic seizures produced by pilocarpine in rats: behavioural, eletroencephalographic and neuropathological study. Behav Brain Res 9:315–336
Treiman DM (1995) Electroclinical features of status epilepticus. J Clin Neurophysiol 12:343–362
Frantseva MV, Velazquez JL, Hwang PA, Carlen PL (2000) Free radical production correlates with cell death in an in vitro model of epilepsy. Eur J Neurosci 12:1431–1439
Freitas RM, Souza FCF, Vasconcelos SMM, Viana GSB, Fonteles MMF (2005) Oxidative stress in the hippocampus after status epilepticus in rats. FEBS J 272:1307–1312
Barros DO, Xavier SM, Barbosa CO, Silva RF, Maia FD, Oliveira AA, Freitas RM (2007) Effects of the vitamin E in catalase activities in hippocampus after status epilepticus induced by pilocarpine in Wistar rats. Neurosci Lett 416:227–230
Kotegawa M, Sugiyama M, Shoji T, Haramaki N, Ogura R (1993) Effect of α-tocopherol on high energy phosphate metabolite levels in rat heart by P-NMR using a Langendoff perfusion technique. J Mol Cell Cardiol 25:1067–1074
Levy SL, Burnham WM, Bishai A, Hwang PA (1992) The anticonvulsant effects of vitamin E: a further evaluation. Can J Neurol Sci 19:201–203
Ayyildiz M, Yildirim M, Agar E, Baltaci AK (2006) The effect of leptin on penicillin-induced epileptiform activity in rats. Brain Res Bull 68:374–378
Carr AC, Frei B (1999) Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69:1086–1107
Simon JA, Hudes ES, Tice JA (2001) Relation of serum α-tocopherol to mortality among US adults. J Am Coll Nutr 20:255–263
Ferreira PMP, Farias DF, Oliveira JTA (2008) Carvalho AFFU (2008) Moringa oleifera: Bioactive compounds and nutritional potential. Rev Nutr 21:431–437
Xavier SML, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM (2007) Vitamin C antioxidant in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci Lett 420:76–79
Steinmetz KA, Potter JD (1991) Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 2:427–442
Andreoli SP, Mallett CP (1997) Disassociation of oxidant-induced ATP depletion and DNA damage from early cytotoxicity in LLC-PK1 cells. Am J Physiol 272:F729–F735
Liang LP, Beaudoin ME, Fritz MJ, Fulton R, Patel M (2007) Kainate-induced seizures, oxidative stress and neuronal loss in aging rats. Neurosci 147:1114–1118
Heaton MB, Mitchell JJ, Paiva M (2000) Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res 24:512–518
McCord JM (1989) Superoxide radical: controversies, contradiction and paradoxes. Proc Soc Exp Biol Med 209:112–117
Walz R, Moreira JCF, Benfato MS, Quevedo J, Schorer N, Vianna MMR, Klamt F, Dal-Pizzol F (2000) Lipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine of kainic acid in Wistar rats. Neurosci Lett 291:179–182
Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10:1611–1626
Gottlieb M, Leal-Campanario R, Campos-Esparza MR, Sánchez-Gómez MV, Alberdi E, Arranz A, Delgado-García JM, Gruart A, Matute C (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis 23:374–386
Kryzhanovskii GN, Nikushkin EV, Braslavskii VE, Glebov RN (1980) Lipoperoxidation in the hyperactive focus of rat cerebral cortex. Biull Eksp Biol Med 89:14–16
Ribeiro MCP, de Avila DS, Schneider CYM, Hermesa FS, Furiana AF, Oliveira MS, Rubin MA, Lehmann MAM, Krieglstein J, Mello CF (2005) α-Tocopherol protects against pentylenetetrazol-and methylmalonate-induced convulsions. Epilepsy Res 66:185–190
Kovacs R, Kardos J, Heinemann U, Kann O (2005) Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures. J Neurosci 25:4260–4269
Freitas RM (2009) The evaluation of effects of lipoic acid on the lipid peroxidation, nitrite formation and antioxidant enzymes in the hippocampus of rats after pilocarpine-induced seizures. Neurosci Lett 455:140–144
Draper HH, Hadley M (1990) Malondialdehyde determination as an index of lipid peroxidation. Methods Enzymol 186:421–431
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germfree and conventional rat. Science 212:56–58
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Flohe L, Otting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93–104
Chance B, Maehly AC (1955) Assay catalases and peroxidases. Methods Enzymol 2:764–768
Marinho MMF, Sousa FCF, Bruin VMS, Vale MR, Viana GSB (1998) Effects of lithium, alone or associated with pilocarpine, on muscarinic and dopaminergic receptors and on phosphoinositide metabolism in rat hippocampus and striatum. Neurochem Int 33:299–306
Szyndler J, Bobtowicz TW, Skórzewska A, Maciejak P, Walkowiak J, Lechowicz W, Danuta T, Andrzej B, Adam P (2005) Behavioral, biochemical and histological studies in a model of pilocarpine-induced spontaneous recurrent seizures. Pharmacol Biochem Behav 1:15–23
Paxinos G, Watson C (1986) The rat brain in stereotaxie coordenates, 2nd edn. Academic Press, New York
Godlevskii LS, Stepanenko KI, Lobasyuk BA, Sarakhan EV, Bobkova LM (2004) The effects of electrical stimulation of the paleocerebellar cortex on penicillin-induced convulsive activity in rats. Neurosci Behav Physiol 34:797–802
Sudha K, Ashalatha VR, Rao A (2001) Oxidative stress and antioxidants in epilepsy. Clin Chimica Acta 303:19–24
Tucker JM, Townsend DM (2005) Alpha-tocopherol: roles in prevention and therapy of human disease. Biomed Pharmacother 59:380–387
Jerrett SA, JeVerson D, Mengel CE (1973) Seizure H2O2 and lipid peroxidase in brain during exposure to oxygen under high pressure. Aeroesp Med 44:40–44
Rauca C, Wiswedel I, Zerbe R, Gerburg K, Manfred K (2004) The role of superoxide dismutase and α-tocopherol in the development of seizures and kindling induced by pentylenetetrazol-infuence of the radical scavenger α-phenyl-N-tert-buthyl nitrone. Brain Res 109:203–212
Castagne V, Gastschi M, Lefevre K, Posada A, Clarke PGH (1999) Relationship between neuronal death and cellular redox status, focus on the developing nervous system. Prog Neurophysiol 59:397–423
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford Science Publications, London
Halliwell B, Gutteridge JMC (1989) Lipid peroxidation: a radical chain reaction. Free Rad Biol Med. Clarendon Press, Oxford pp 188–276
Maczurek A, Hager J, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Munch G (2008) Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliver Rev 60:1463–1470
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17:235–248
Tejada S, Roca C, Sureda A, Rial RV, Gamundí A, Esteban S (2006) Antioxidant response analysis in the brain after pilocarpine treatments. Brain Res Bull 69:587–592
Salo DC, Lin SW, Pacifici RE, Davies KJ (1988) Superoxide dismutase is preferentially degraded by proteolytic system from red blood cells following oxidative modification by hydrogen peroxide. Free Rad Biol Med 5:335–339
Tran TD, Jackson HD, Horn KH, Goodlett CR (2005) Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eye blink classical conditioning in rats. Alcohol Clin Exp Res 29:117–129
Dymond AM, Crandall PH (1976) Oxygen availability and blood flow in the temporal lobes during spontaneous epileptic seizures in men. Brain Res 102:191–196
Acknowledgments
This work was supported in part by grants from the Brazilian National Research Council (CNPq), Brazil. R. M. F is fellow from CNPq. We would like to thank Stenio Gardel Maia for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomé, A.R., Feng, D. & Freitas, R.M. The Effects of Alpha-Tocopherol on Hippocampal Oxidative Stress Prior to in Pilocarpine-Induced Seizures. Neurochem Res 35, 580–587 (2010). https://doi.org/10.1007/s11064-009-0102-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0102-x