Abstract
Temporal lobe epilepsy (TLE), the most common form of epilepsy is often resistant to pharmacological treatment. Neuronal loss observed in epileptic brain may be result of an overproduction of free radicals (oxidative stress). Oxidative stress is characterized by an imbalance between antioxidant defenses and oxidizing agents (free radicals), which can lead to tissue injury. The n-3 PUFAs are important for the development and maintenance of central nervous system functions. Research by our group has shown that chronic treatment with fish oil, immediately after status epilepticus (SE), exhibits both neuroprotective effects and effects on neuroplasticity. The main purpose of this research was to evaluate if fish oil exhibits a protective effect against oxidative stress. Animals were subjected to TLE model by pilocarpine administration. After 3 h of SE they were randomly divided into the following groups: control animals treated daily with vehicle or with 85 mg/kg of fish oil and animals with epilepsy treated daily with vehicle or with 85 mg/kg of fish oil. After 90 days, superoxide anion production, enzymatic activity of superoxide dismutase (SOD) and catalase (CAT) and protein expression of NAD(P)H oxidase subunits (p47PHOX and gp91PHOX) were analyzed. Our results showed evidences that reactive oxygen species are increased in animals with epilepsy and that fish oil supplementation could counteract it. Fish oil supplementation promoted protection against oxidative stress by multiple ways, which involved the reduction of activity and expression of NAD(P)H oxidase subunits and increased the activity and expression of antioxidants enzymes, contributing to well-known neuroprotective effect in epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporal lobe epilepsy (TLE), the most common form of epilepsy in humans, is often resistant to pharmacological treatment. The pilocarpine model of epilepsy, firstly described by Turski et al. (1984) has been widely used due to its similarity with human TLE and its multifaceted technical approaches (Curia et al. 2008).
Excitotoxicity and selective neuronal loss are a common finding in pilocarpine model of TLE and is related with excessive or prolonged activation of excitatory amino acid receptors, in particular glutamate receptors. It points reactive oxygen species (ROS) production as an important consequence involved with the glutamatergic excitotoxicity, usually referred as oxidative stress (Bondy and Lee 1993; Bonfoco et al. 1995; Shulz et al. 1995; Vincent and Mulle 2009). Ischemia and hypoxia can exacerbate the brain damage. However, experimental evidence shows that prolonged SE cause neuronal death even when animals are kept well oxygenated (Nevander et al. 1985). The biological effects of free radicals can be controlled in vivo by antioxidant enzymes that include glutathione reductase (GR), glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) (Valko et al. 2006). Imbalance between free radical formation and antioxidant defensive system can lead to neuronal damage and cell death through cell membrane lipid destruction and cleavage of DNA (Fujikawa 2005; Vincent and Maiese 1999).
Free radicals such as ROS can also be generated during normal cellular respiration and/or metabolic process by specific enzymes such as NAD(P)H oxidase. The NAD(P)H oxidase is a multi-subunit enzyme that catalyzes the reduction of molecular oxygen to generate superoxide radicals. It was discovered in neutrophils but the classical paradigm of NADPH oxidase function only in immunological system has become outdated (Babior 2004). Recently, Pestana et al. (2010) demonstrated the participation of ROS generated by NADPH oxidase in neuronal death in the pilocarpine model of epilepsy.
The n-3 polyunsaturated fatty acids (n-3 PUFA) are important for the development and maintenance of central nervous system functions (McCann and Ames 2005; Inni 2007). Clinical and experimental investigations have demonstrated that the duration and frequency of epileptic seizures can be reduced as a consequence of long-term n-3 PUFA supplementation (Schlanger et al. 2002). Previous studies in our laboratory demonstrated that chronic treatment with fish oil started immediately after the onset of status epilepticus (SE) produced neuroprotective and neuroplastic effects by reduction of cell death e enhanced the GABAergic transmission in hippocampus (Ferrari et al. 2008), but the mechanisms underlying those effects are still unknown. It has been described that n-3 PUFA can suppress production of ROS (superoxide and hydrogen peroxide) in stimulated leukocytes (Fisher et al. 1990) and up-regulates gene expression of antioxidant enzymes and down-regulates genes associated with production of ROS (Takahashi et al. 2002). Based on these facts, we tested the hypothesis that fish oil could act against oxidative stress in the pilocarpine model of TLE.
Material and methods
Animals
All animals were treated according to protocols for animal care established by the Federal University of São Paulo, and all efforts were made to minimize animal suffering (CEUA, 188439). Adult male Wistar rats with 220–280 g were housed under standard controlled conditions (7:00 am/7:00 pm light/dark cycle; 20–22 °C; 45–55 % humidity) with food and water ad libitum.
Induction of epilepsy
Epilepsy was induced according to the procedure described previously. In brief, 30 min after methylscopolamine injection (1 mg/kg, sc - Sigma, MO, USA), experimental animals received pilocarpine (350 mg/kg, ip - Sigma, MO, USA) e control received salina 0.9 %. To terminate or limit behavioral seizures induced by pilocarpine, diazepam (10 mg/kg – Cristalia, Compaz) was administered subcutaneously 3 h after the onset of status epilepticus, simultaneously with fish oil or vehicle administration. After that, the animals were then allowed to evolve through the silent period to the chronic phase (Cavalheiro 1995).
Fish oil treatment
Animals were randomly divided into the following groups:
-
1)
control vehicle (CV, n = 9): animals treated daily with vehicle (cremophor 0.009 %);
-
2)
control fish oil (CFO, n = 9): animals treated daily with 85 mg/kg fish oil;
-
3)
epilepsy vehicle (EV, n = 9): animals with epilepsy treated daily with vehicle;
-
4)
epilepsy fish oil (EFO, n = 9): animals with epilepsy treated with 85 mg/kg fish oil.
For 90 days animals received vehicle (cremophor 0.009 %) or fish oil (PROEPA®, 85 mg/kg). The fish oil capsule (1 g), containing the polyunsaturated fatty acids DHA (120 mg/1 g) and EPA (180 mg/1 g), was dissolved in Cremophor (Sigma ®) 0.009 % in distilled water yielding a final concentration of 21.25 mg/mL of fish oil, which corresponded to 3.82 mg/mL EPA and 2.55 mg/mL DHA. Vehicle solution (V) consisted of the same amount of Cremophor and water. The animals received 1 mL of the fish oil solution or vehicle solution for 250 g of body weight. The solutions were administrated between 11:00 and 12:00 AM by oral gavage daily. The volume administered was adjusted according to animal weight, which was verified three times a week during the treatment period. For all the procedures, the animals were killed by decapitation. The hippocampus of all animals was processed for enzymatic activity assays and western blot analysis. The trunk blood was collected in tubes and centrifuged at 2500 rpm for 15 min. The serum was collected and stored in freezer for enzymatic activity assays.
For the anion superoxide production, five animals per group were used and for antioxidants enzymatic activities and protein expression analysis, four animals per group were used.
Superoxide production
The superoxide production was measured using dihydroethidium (DHE) dye fluorescence method. Coronal brain slices with 200 μm measured from bregma (AP: -3.30 mm) (Paxinos and Watson 1998) were incubated for 6 min with artificial cerebrospinal fluid (ACSF) solution (10 mM glucose, 1.25 mM MgSO4.7H2O, 1.25 mM KH2PO4, 5 mM KCl, 125 mM NaCl, 24 mM NaHCO3, 2.5 mM CaCl2.2H2O) contained 100 μM/L of DHE fluorescence dye (Invitrogen Corporation, CA, USA). The fluorescence intensity of hippocampal regions (CA1 and CA3) was visualized on Zeiss Axiovert 780 confocal microscope (excitation: 480 nm and emission: 570 nm) and analyzed using “ZEN imaging software”. For fluorescence quantification analysis, specific program “Image J” (Wayne Rasband National Institutes of Health, USA) was used. Data were expressed considering the relationship between fluorescence intensity / area.
Enzymatic activity assays
Hippocampus samples were homogenized in 50 mM phosphate buffer (50 mM KH2PO4 and 50 mM K2HPO4, 1:1.5 (v/v) pH 7.4 and the samples are centrifuged at 2500 rpm for 10 min at 4 °C to remove insoluble cell debris. As hippocampus, serum samples protein amount was determined by the method of Bradford (1976) using bovine albumin as standard.
SOD activity
The commercial test Kit RANSOD (RANDOX, Randox Laboratories Ltd, United Kingdon) was used to determine the activity of superoxide dismutase. The superoxide dismutase activity was measured by the degree of inhibition of this reaction. Firstly, it was determinate the absorbance of blank, standard and samples. Subsequently, xanthine oxidase was added and absorbance monitored for 3 min at 505 nm in a spectrophotometer at 37 °C. One unit of SOD activity was defined as the amount of enzyme that inhibited the reaction rate by 50 %.
CAT activity
Catalase activity was assayed according to the method of Aebi (1984). This method is based on monitoring the decomposition of H2O2 determined by spectrophotometer at 420 nm for 5 min and calculated by change of absorbance per unit time.
Western blot analysis
Hippocampus samples were homogenized in lysis buffer with protease inhibitor cocktail (100 mM Tris-Base, pH 7.5, 10 mM EDTA, 10 % SDS, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM sodium orthovanadate). After that, Laemmli buffer (bromophenol blue 0.1 %, 1 M sodium phosphate pH 7.0, 50 % glycerol, 10 % SDS) containing dithiothreitol (DTT) was added to each sample and the mixture was boiled for 5 min. After boiling, the mixture was subjected to polyacrylamide gel 8 % electrophoresis (SDS-PAGE) system MINI-BIO-RAD PROTEIN®. The separated proteins were transferred electrophoretically (BIO-RAD ® cube) using a transfer buffer (25 mM Tris-Base, 192 mM glycine, 20 % methanol, 0.02 % SDS). The transfer efficiency was evaluated using Ponceau dye. The polyvinylidene difluoride membranes (PVDF, Millipore, Bedford, MA, USA) were incubated for 2 h at room temperature in blocking buffer (5 % nonfat dry milk, 1 M Tris base, 5 M NaCl, 0.05 % Tween 20) to reduce non-specific binding. The primary antibodies used were anti-p47PHOX, anti-gp91PHOX (Upstate-Millipore, Bedford, MA, USA), anti-SOD, anti-catalase e anti-tubulin (Santa Cruz Biotecnology Inc., Califórnia, USA). The chemiluminescence kit ECL (GE Healthcare Life Sciences, Buckingham Shire, UK) was used for autoradiograph detection with X-ray film (IBF Indústria Brasileira de Filmes, São Paulo, Brasil).
Statistical analysis
Data were expressed as mean ± standard error. Statistical analysis was performed using two-way ANOVA (groups: control versus experimental and treatment: vehicle versus fish oil) followed by Bonferroni post-hoc test. p values of 0.05 or less were considered significant. The analyses were effectuated using commercial program (Prism 5.03 for windows).
Results
Pilocarpine treatment sequentially induced the following behavioral changes: akinesia, facial automatisms, and limbic seizures consisting of forelimb clonus with rearing, salivation, masticatory jaw movements and falling. This type of behavior built-up progressively into motor limbic seizures that recurred repeatedly and rapidly developed into SE. After SE, animals were comatose or unresponsive to their environment and akinetic. Behavior returned to normal over a 3- to 5-day period (Cavalheiro 1995). Spontaneous and recurrent seizures in rats with epilepsy during the chronic period were characterized by: facial automatisms, forelimb clonus, rearing, loss of postural control and generalized clonic seizures lasting 40–60 s.
Superoxide production
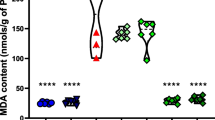
The superoxide anion production was significantly higher in CA1 and CA3 hippocampal regions in experimental group as compared to control (F(1;16) = 12.8; p = 0.0025 and F(1;16) = 5.2, p = 0.039, respectively) Fig. 1a and b. The superoxide anion production significantly decreased in CA1 and CA3 hippocampal regions in animals with epilepsy supplemented with fish oil as compared with animals with epilepsy that received vehicle (t = 3.029, p < 0.05 and t = 2.95, p < 0.05, respectively).
Immunofluorescence of ethidium in Ca1 (a) and Ca3 (b) of hippocampal formation of groups CV, CFO, EV and EFO. The reactive oxygen species production was significantly higher in CA1 and CA3 hippocampal regions in animals with epilepsy as compared to control. The reactive oxygen species production significantly decreased in CA1 and CA3 hippocampal regions in animals with epilepsy supplemented with fish oil (EFO) as compared with animals with epilepsy that received vehicle (EV). **p = 0.0025, *p = 0.039, #p < 0.05
Enzymatic activity assays
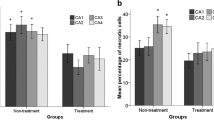
In hippocampus, a significant difference between groups was noted for SOD activity (F(1;12) = 18.2; p = 0.0013, Fig. 2a) and catalase activity (F(1;12) = 14.8; p = 0.0027, Fig. 2b). The enzymatic activities for both enzymes were higher in experimental group as compared to control. Effect of interaction between groups versus treatment was no observed neither to SOD activity (F(1,12) = 1.3; NS, Fig. 2a) nor to catalase activity (F(1,12) = 0.1; NS, Fig. 2b). The treatment with fish oil did not affect the SOD activity (F(1;12) = 0.23; NS). However, for catalase activity, a significant effect of treatment was noted (F(1;12) = 11.1; p = 0.0008, Fig. 2b). Fish oil supplementation significantly increased the catalase activity in both groups.
Superoxide dismutase (SOD) and catalase (CAT) activities in hippocampus and serum of control and experimental groups. In hippocampus, SOD and CAT activities were significantly higher in experimental group as compared to control (**p = 0.0013, **p = 0.0027, respectively). Fish oil supplementation did not change the SOD activity but significantly increased the CAT activity in both groups (*p < 0.05). In serum, SOD activity was significantly higher in experimental group as compared to control (***p = 0.001) with no difference for CAT activities. Effect of interaction between groups versus treatment was noted for both enzymes. Fish oil treatment decreased enzymatic activity in control group and increased it in animals with epilepsy
In hippocampus, the data indicates that the activity of antioxidants enzymes are increased in animals with epilepsy and that the fish oil supplementation enhanced that protection to counteract the over production of free radicals.
In serum, effect of interaction between groups versus treatment was noted for SOD (F(1,12) = 16.9; p = 0.0017, Fig. 2c) and catalase activities (F(1,12) = 5.2; p = 0.04; Fig. 2d). Fish oil treatment decreased enzymatic activities in control group and increased it in animals with epilepsy. In addition, SOD activity was significantly higher in experimental group as compared to control (F(1,12) = 5.2; p = 0.001) with no difference for catalase activities (F(1,12) = 0.048; NS). The results obtained in serum suggest that fish oil supplementation increased the activity of antioxidant enzymes to improve the protection against the free radical production.
Western blot analysis
The protein expression of NAD(P)H oxidase subunit p47PHOX was significantly different between groups (F(1,12) = 8.3; p = 0.013, Fig. 3a). The p47PHOX expression was higher in experimental group as compared to control group but it was unaffected by the treatment (F(1,12) = 1.5; NS, Fig. 3a). No effect of interaction between groups versus treatment was noted (F(1,12) = 0.1; NS).
Western blott in hippocampus of control and experimental groups. Each bar represents mean ± SEM from the ratio a p47PHOX/Tubulin, b gp91PHOX/Tubulin, c SOD/Tubulin and d catalase/Tubulin. The expression of NAD(P)H oxidase subunits p47PHOX (a) and gp91PHOX (b) were significantly higher in experimental group as compared to control (*p = 0.013, ***P = 0.003). In experimental group, the fish oil supplementation did not change the expression of p47PHOX but significantly decreased of the gp91PHOX. For SOD expression (c) a significant effect of interaction between groups versus treatment was observed. Fish oil supplementation decreased SOD expression in control and increased it in experimental group. For CAT expression (d) no difference was noted between groups, neither to the treatment nor to the effect of interaction between groups versus treatment
For the gp91PHOX expression a significant effect of interaction between groups versus treatment was observed (F(1,12) = 6.6; p = 0.024; Fig. 3b). The gp91PHOX expression increased in the experimental group but decreased with the fish oil treatment. In addition, the gp91PHOX expression was higher in experimental group as compared to control (F(1,12) = 15.7; p < 0.0003).
The results suggest that the expression of NAD(P)H oxidase subunits are increased in the animals with epilepsy which could be underlying the increased superoxide anion production. Fish oil supplementation decreased gp91PHOX expression most probably to counteract the free radical production. For SOD protein expression in hippocampus a significant effect of interaction between groups versus treatment was observed (F(1,12) = 5.2; p = 0.04, Fig. 3c). Fish oil decreased SOD protein expression in control and increased it in experimental group.
For catalase protein expression no difference was noted for groups, neither to the treatment nor to the effect of interaction between factors (F(1,12) = 1.6; NS, F(1,12) = 2.8; NS, F(1,12) = 0.002; NS, respectively).
Discussion
In the pilocarpine model of temporal lobe epilepsy, it was investigated the oxidative stress and the impact of fish oil supplementation. The main results showed that reactive oxygen species are increased in animals with epilepsy and that fish oil supplementation could counteract it. In order to investigate the probable mechanisms underlying this effect, it was investigate the enzymatic activities for antioxidants enzymes in hippocampus and serum, and protein expression for NAD(P)H oxidase subunits and antioxidants enzymes in hippocampus.
Corroborating with the current literature, it was noted an increase in superoxide production in the hippocampus of animals with epilepsy, which could be partially explained by increased expression of NAD(P)H oxidase subunit p47PHOX and gp91PHOX. In this sense, Patel et al. (2005) showed in kainic-induced SE a time-dependent translocation of NAD(P)H oxidase subunits from hippocampal cytosol to membrane that coincided with microglial activation, suggesting that seizure activity activates the membrane NAD(P)H oxidase complex resulting in an increase of superoxide formation. In addition, Pestana et al. (2010) showed that the NADPH oxidase inhibitor (apocynin) effectively decreased both ROS production and neurodegeneration in the pilocarpine model of epilepsy.
Interestingly, our results showed an increased CAT activity, most probably to counteract the increased free radicals production, with no changes in their expression. Corroborating with our results, Rumià et al. (2013) showed that the neocortex of drug-resistant epilepsy patients displayed increased level of superoxide anion and as well as increased enzymatic activity for catalase. It was suggested catalase as the main antioxidant enzyme in human epileptic neocortex. Freitas et al. (2005) using the pilocarpine model of TLE observed an increased lipid peroxidation and catalase activity in the hippocampus during the phase of spontaneous recurrent seizures.
In our study, SOD activity increased in the hippocampus and serum of animals with epilepsy. However, controversial results have been reported in the literature. Rumià et al. (2013) reported that SOD activity remained unchanged in human epileptic neocortex and Bellissimo et al. (2001) using pilocarpine model of temporal lobe epilepsy observed decreased SOD activity in hippocampus during the chronic phase.
The effect of fish oil supplementation on oxidative stress remains to be elucidated. The results showed that fish oil supplementation decreased reactive oxygen species production in CA1 and CA3 of hippocampal formation of animals with epilepsy, which could be explained by reduction of the gp91PHOX expression, increased SOD expression, increased SOD and catalase activities in hippocampus and SOD activity in serum. Corroborating with our results, Liu et al. (2012) showed in pentylenetetrazole-induced seizures that DHA reduced the levels of NO in the brain and liver, as well as increased SOD and CAT activities in both tissues. Other studies reported that fish oil supplementation provides protection against lipid peroxidation. Hypercholesterolemic rats supplemented with DHA exhibited an increase in CAT and GPx activities in the brain, which favored a protection against lipid peroxidation (Hossain et al. 1999). Also, animals subjected to traumatic brain injury and supplemented with fish oil diet exhibited decrease on oxidative damage in hippocampus (Wu et al. 2004). The molecular mechanism underlying those effects remains to be elucidated and further investigation are needed.
Taken as a whole, the results showed evidences that reactive oxygen species are increased in animals with epilepsy and that fish oil supplementation could counteract the oxidative stress. Fish oil supplementation promoted protection against oxidative stress by multiple ways, which involved the reduction of activity and expression of NAD(P)H oxidase subunits and the increased the activity and expression of antioxidants enzymes, contributing to well-known neuroprotective effect in epilepsy.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Babior BM (2004) NADPH oxidase. Curr Opin Immunol 16:42–47. doi:10.1016/j.coi.2003.12.001
Bellissimo MI, Amado D, Abdalla DS, Ferreira EC, Cavalheiro EA, Naffah-Mazzacoratti MG (2001) Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats. Epilepsy Res 46:121–128. doi:10.1016/S0920-1211(01)00269-8
Bondy SC, Lee DK (1993) Oxidative stress induced by glutamate receptors agonists. Brain Res 610:229–233. doi:10.1016/0006-8993(93)91405-H
Bonfoco E, Krainic D, Ankarcrona M, Nocotera P, Lipton AS (1995) Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N- methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci 92:7162–7166
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cavalheiro EA (1995) The pilocarpine model of epilepsy. Ital J Neurol Sci 16:33–37
Curia G, Longo D, Biagini G, Jones RS, Avoli M (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172(2):143–157. doi:10.1016/j.jneumeth.2008.04.019
Ferrari D, Cysneiros RM, Scorza CA, Arida RM, Cavalheiro EA, de Almeida ACG, Scorza FA (2008) Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: quantification with immunohistochoemical for calcium-binding proteins. Epilepsy Behav 13:36–42. doi:10.1016/j.yebeh.2008.01.001
Fisher M, Levine PH, Weiner BH (1990) Dietary n-3 fatty acid supplementation reduces superoxide production and chemiluminescence in a monocyte-enriched preparation of leukocytes. Am J Clin Nutr 51:804–808
Freitas RM, Vasconcelos SM, Souza FC, Viana GS, Fonteles MM (2005) Oxidative stress in the hippocampus after pilocarpine-induced status epilepticus in Wistar rats. FEBS J 272:1307–1312. doi:10.1111/j.1742-4658.2004.04537.x
Fujikawa DG (2005) Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 7(3):S3–S11. doi:10.1016/j.yebeh.2005.08.003
Hossain MS, Hashimoto M, Gamoh S, Masumura S (1999) Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem 72(3):1133–1138. doi:10.1046/j.1471-4159.1999.0721133.x
Inni SM (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137(4):855–859. doi:10.1016/j.brainres.2008.08.078
Liu SH, Chang CD, Chen PH, Su JR, Chen CC, Chaung HC (2012) Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res 1451:19–26. doi:10.1016/j.brainres.2012.02.060
McCann JC, Ames BN (2005) Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 82(2):281–295
Nevander G, Ingvar M, Auer R, Siesjö BK (1985) Status epilepticus in well-oxygenated rats causes neuronal necrosis. Ann Neurol 18(3):281–290. doi:10.1002/ana.410180303
Patel M, Li QY, Chang LY, Crapo J, Liang LP (2005) Activation of NADPH oxidase and extracellular superoxide production in seizure-induced hippocampal damage. J Neurochem 92:123–131. doi:10.1111/j.1471-4159.2004.02838.x
Paxinos JP, Watson C (1998) The rat brain in stereotaxic coordinates. Academic, San Diego
Pestana RF, Kinjo ER, Hernandes MS, Britto LRG (2010) Reactive oxygen species generated by NAD(P)H oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci Lett 484:187–191. doi:10.1016/j.neulet.2010.08.049
Rumià J, Marmol F, Sanchez J, Giménez-Crouseilles J, Carreño M, Bargalló N, Boget T, Pintor L, Setoain X, Donaire A, Saez GT, Ribalta T, Ferrer E, Puig-Parellada P (2013) Oxidative stress markers submitted to epilepsy surgery. Epilepsy Res 107(1–2):75–81. doi:10.1016/j.eplepsyres.2013.08.020
Schlanger S, Shinitzky M, Yam D (2002) Diet enriched with omega–3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia 43:103–104. doi:10.1046/j.1528-1157.2002.13601.x
Shulz JN, Henshaw DR, Siwek D, Jenkins BG, Ferrante RJ, Cipolloni PB, Kowall NW, Rosen BR, Beal MF (1995) Involvement of free radicals in excitotoxicity in vivo. J Neurochem 64:2239–2247. doi:10.1046/j.1471-4159.1995.64052239.x
Takahashi M, Tsuboyama-Kasaoka N, Nakatani T (2002) Fish oil feeding alters liver gene expressions to defend against PPAR alpha activation and ROS production. Am J Physiol Gastrointest Liver Physiol 282:G338–G348. doi:10.1152/ajpgi.00376.2001
Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L (1984) Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res 321(2):237–253
Valko M, Rhodes CJ, Izakovic MM (2006) Free radicals metals and antioxidants on oxidative stress-induced cancer. Chem Biol Interact 160:1–40. doi:10.1016/j.cbi.2005.12.009
Vincent AM, Maiese K (1999) Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res 246:290–300. doi:10.1006/excr.1998.4282
Vincent P, Mulle C (2009) Kainate receptors in epilepsy and excitotoxicity. Neuroscience 158:309–323. doi:10.1016/j.neuroscience.2008.02.066
Wu A, Ying Z, Gomez-Pinilla F (2004) Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 21(10):1457–1467. doi:10.1089/neu.2004.21.1457
Acknowledgments
This work was supported financially by FAPESP, CInAPCe-FAPESP, FAPESP/CNPq/MCT-Instituto Nacional de Neurociência Translacional and CNPq.
Conflict of interest
The authors declare that there is no conflict of interest with any financial organization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nejm, M.B., Haidar, A.A., Marques, M.J.G. et al. Fish oil provides protection against the oxidative stress in pilocarpine model of epilepsy. Metab Brain Dis 30, 903–909 (2015). https://doi.org/10.1007/s11011-015-9666-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9666-0