Abstract
In the present study, we investigated the effects of a treadmill exercise on serum glucose levels and Ki67 and doublecortin (DCX) immunoreactivity, which is a marker of cell proliferation expressed during cell cycles except G0 and early G1 and a marker of progenitors differentiating into neurons, respectively, in the subgranular zone of the dentate gyrus (SZDG) using a type II diabetic model. At 6 weeks of age, Zucker lean control (ZLC) and Zucker diabetic fatty (ZDF) rats were put on a treadmill with or without running for 1 h/day/5 consecutive days at 22 m/min for 5 weeks. Body weight was significantly increased in the control (without running)-ZDF rats compared to that in the other groups. In the control groups blood glucose levels were increased by 392.7 mg/dl in the control-ZDF rats and by 143.3 mg/dl in the control-ZLC rats. However, in the exercise groups, blood glucose levels were similar between the exercise-ZLC and ZDF rats: The blood glucose levels were 110.0 and 118.2 mg/dl, respectively. Ki67 positive nuclei were detected in the SZDG in control and exercise groups. The number of Ki67 positive nuclei was significantly high in exercise groups compared to that in the control groups. In addition, Ki67 positive cells were abundant in ZLC groups compared to those in ZDF groups. DCX-immunoreactive structures in the control-ZDF rats were lower than that in the control-ZLC rats. In the exercise groups, DCX-immunoreactive structures (somata and processes with tertiary dendrites) and DCX protein levels were markedly increased in both the exercise-ZLC and ZDF rats compared to that in the control groups. These results suggest that a treadmill exercise reduces blood glucose levels in ZDF rats and increases cell proliferation and differentiation in the SZDG in ZLC and ZDF rats compared to those in control groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is one of the most common endocrine disorders, affecting almost 6% of the world’s population. DM is divided according to the action of beta cells: Type I DM is characterized into the degeneration of beta cells, while the main cause of type II DM is a progressive decline in insulin sensitivity with eventual insulin deficiency that results in sustained hyperglycemia [1–3]. The latter is most common in humans and has a proportion of more than 97% in diabetic patients [4]. The Zucker diabetic fatty (ZDF) rat is considered to be an excellent animal model of type II DM that presents a physiological and metabolic profile similar to those seen in humans, and male ZDF rats develop a phenotype of obesity, insulin resistance, and hyperglycemia [5].
Recently, it has been reported that hippocampal neurons are vulnerable to diabetes [6–9], and the diabetes occurs damages of dendrites, pre- and post-synaptic structures in CA3 neurons, and reduces the expression of insulin growth factors and their receptors in the hippocampus in type I DM model [8, 10–12]. In addition, DM reduces cell proliferation in the dentate gyrus in type I [10, 13, 14] and type II models [15, 16]. Exercise has been focused on metabolic diseases because the exercise reduces predisposing factors induced by diabetes, improving cardiovascular health, lipid-cholesterol balance, energy metabolism, glucose use, insulin sensitivity, inflammation and hippocampal plasticity [17–21].
Neural progenitors in the dentate gyrus proliferate, migrate, and differentiate into granule cells, which extend their axons and contact CA3 pyramidal neurons, becoming integrated into the hippocampal circuitry [22–24]. Although some researchers have reported that treadmill increased cell proliferation in the dentate gyrus of type I DM model [25, 26], there are no studies about correlations between diabetes and neurogenesis after exercise in type II models. Therefore, in the present study, we investigated cell proliferation and neuronal differentiation in the dentate gyrus of Zucker diabetic fatty (fa/fa, ZDF) rats and Zucker lean control (fa/+ or +/+, ZLC) rats using Ki67, a marker of cell proliferation expressed during the late G1, S, M, and G2 phases of the cell cycle [27] and doublecortin (DCX), a marker of progenitors differentiating into neurons.
Experimental Procedures
Experimental Animals
Male and female ZDF (fa/+) were purchased from Genetic Models (Indianapolis, ME) and mated each other. They were housed in a conventional state under adequate temperature (23°C) and humidity (60%) control with a 12-h light/12-h dark cycle, and free access to food and water. Purina 5008 rodent diets (7.5% fat) were provided as recommended by Genetic Models Co. (Purina, St. Louis, MO). The procedures for handling and caring for the animals adhered to the guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985, revised 1996). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
Genotyping of fa Gene and Experimental Design
Genotype of fa gene herein was determined with the strategy described in our previous study [15, 28]. Ten male ZLC and ZDF rats were randomly divided into 4 groups (n = 8 per group): control-ZLC, control-ZDF, exercise-ZLC and exercise-ZDF group. At 6 weeks of age, exercise-ZLC and exercise-ZDF rats were familiarized with the treadmill running on a motorized treadmill (Model 1050 LS Exer3/6; Columbus Instruments, Columbus, OH) for 15 min/day at 15 m/min for 5 consecutive days. After the familiarization, electrical stimulation to encourage the rats to run was disconnected to avoid pain stress. The rats were run for 1 h/day/5 consecutive days at 22 m/min for 5 weeks and the speeds accelerated 2 m/min per 2-weeks. The control ZLC and ZDF rats were put on the treadmill without running for 1 h/day/5 consecutive days at 22 m/min for 5 weeks. All animals were euthanized at 12 weeks of age.
Food Intake, Body Weight, and Blood Glucose Sampling
Total food intake and body weight over the course of the study were determined for each animal by summing the weekly averages. To measure blood glucose concentration, blood was sampled each morning (9:00 am) by “tail nick” using a 27 G needle and analyzed by using a blood glucose monitor (Ascensia Elite XL Blood Glucose Meter, Bayer, Toronto, ON, Canada).
Tissue Processing for Histology
For immunohistological analysis, five animals in each group were anesthetized with sodium pentobarbital and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative for 6 h. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter the frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30-μm coronal sections, and they were then collected into six-well plates containing PBS.
Immunohistochemistry for Ki67 and DCX
Immunohistochemical staining was conducted with our previous study [13]. The sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal goat or rabbit serum in 0.05 M PBS for 30 min. They were then incubated with diluted rabbit anti-Ki67 antibody (1:1,000, Abcam, Cambridge, UK) or diluted goat anti-DCX antibody (1:50, SantaCruz Biotechnology, Santa Cruz, CA) overnight at room temperature and subsequently exposed to biotinylated goat anti-rabbit IgG or rabbit anti-goat IgG and streptavidin peroxidase complex (1:200, Vector, Burlingame, CA). They were then visualized by staining with 3,3′-diaminobenzidine in 0.1 M Tris–HCl buffer (pH 7.2) and mounted on gelatin-coated slides. The sections were mounted in Canada Balsam (Kanto, Tokyo, Japan) following dehydration. A negative control test was carried out using pre-immune serum instead of primary antibody in order to establish the specificity of the immunostaining. The negative control resulted in the absence of immunoreactivity in any structures.
Western Blot Analysis
To confirm changes in DCX levels in the dentate gyrus of control and exercise groups, 3 animals in each group were sacrificed and used for Western blot analysis. After sacrificing them and removing the brain, dentate gyrus were then dissected with a surgical blade. The tissues were homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis (2-aminoethyl Ether)-N,N,N′,N′ tetraacetic acid (EGTA) (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (EDTA) (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM dithiothreitol (DTT). After centrifugation, the protein level was determined in the supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, USA). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. The aliquots were then loaded onto a 10% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min, followed by incubation with goat anti-DCX (1:200), peroxidase-conjugated rabbit anti-goat IgG (Sigma, St Louis, MO) and an ECL kit (Pierce Chemical, Rockford, IL).
Quantification of Data
All measurements were performed in order to ensure objectivity in blind conditions, by two observers for each experiment, carrying out the measures of experimental samples under the same conditions.
To elucidate the effects of exercise on cell proliferation in ZLC and ZDF rats, the corresponding areas of the dentate gyrus were measured from 10 sections per animal. Images of all Ki67 or DCX immunoreactive structures were taken from dentate gyrus through a BX51 light microscope (Olympus, Japan) equipped with a digital camera (DP71, Olympus, Japan) connected to a PC monitor. Dendritic complexity of DCX positive cells was analyzed using the accompanying software (NeuroExplore, MicroBrightField, Inc., VT,), calculating complexity including dendritic length and number of branches. The number of Ki67, DCX positive cells and total granule cells in all groups was counted in dentate gyrus using an image analyzing system equipped with a computer-based CCD camera (software: Optimas 6.5, CyberMetrics, Scottsdale, AZ). Cell counts were obtained by averaging the counts from the sections taken from each animal: A ratio of the count was calibrated as %.
The effects of exercise on cell differentiation in ZLC and ZDF rats were assessed by western blot analysis, and the quantification of the Western blotting was done using Scion Image software (Scion Corp., Frederick, MD), which was used to count the optical density.
Statistical Analysis
The data shown here represent the means of experiments performed for each experimental area. Differences among the means were statistically analyzed by one-way analysis of variance followed by Duncan’s new multiple range method in order to elucidate differences between ZLC and ZDF and/or control and exercise group.
Results
Genotyping of the fa gene and Changes in Food Intake, Body Weight and Blood Glucose Level
We confirmed the genotype of the fa gene in order to identify the homozygote and others such as previous studies [15, 28]. The food intake was not significantly different between ZLC and ZDF group. In addition, the exercise did not give any significant changes in the food intake (data not shown).
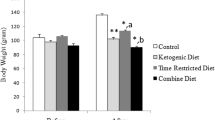
Unlike food intake, body weights showed significant difference between ZLC and ZDF group. In the ZDF rats, the body weight was not different at 6 weeks of age. The body weight in the control-ZDF group was much higher than that in the other groups at 10 weeks of age, and this pattern was maintained by 12 weeks of age. In the exercise groups, the body weight was similar between ZLC and ZDF rats (Fig. 1a).
Changes in body weight (a) and blood glucose levels (b) in control-ZLC (Co-ZLC), control-ZDF (Co-ZDF), exercise-ZLC (Ex-ZLC) and exercise-ZDF (Ex-ZDF) rats. Blood glucose concentration is significantly increased in the Co-ZLC and Co-ZDF groups with age. However, in the Ex-ZLC and Ex-ZDF group, the blood glucose levels are not significantly changed. The body weight is increased with age in all groups; however in the Co-ZLC, Ex-ZLC and Ex-ZDF groups, the slope of body weight is blunt compared to that in the ZDF group (n = 5 per group; *P < 0.05, significantly different from the ZLC group, # P < 0.05, significantly different from the control group). The bars indicate the means ± SE
In the control groups, blood glucose level was age-dependently increased and was 392.70 mg/dl and 143.30 mg/dl in the ZDF and ZLC rats, respectively, at 12 weeks of age. However, the exercise in ZLC and ZDF rats maintained the blood glucose levels with ages compared to those in the control groups: The blood glucose levels were 110.00 and 118.17 mg/dl in ZLC and ZDF groups, respectively, at 12 weeks of age (Fig. 1b).
Effects of Treadmill Exercise on Cell Proliferation in ZDF and ZLC Rats
In the control groups, Ki67 immunoreactive nuclei were detected in the subgranular zone of dentate gyrus and the number of Ki67 immunoreactive nuclei was abundant in ZLC group compared to that in the ZDF group (Fig. 2a, b and e). The exercise increased the number of Ki67 immunoreactive cells compared to that in the control groups. In addition, the number of Ki67 immunoreactive nuclei in the exercise-ZLC group was more abundant compared to that in the exercise-ZDF group (Fig. 2c, d and e).
Microphotographs of Ki67 positive cells in the dentate gyrus in control-ZLC (Co-ZLC), control-ZDF (Co-ZDF), exercise-ZLC (Ex-ZLC) and exercise-ZDF (Ex-ZDF) groups at 12 weeks of age. Ki67 positive cells are detected in the subgranular zone (arrows) of the polymorphic layer (PL). Note that Ki67 immunoreactive cells are abundant in the Ex-ZLC and Ex-ZDF groups compared to those in the Co-ZLC and Co-ZDF groups. GCL, granule cell layer; ML, molecular layer. Bar = 100 μm. (e) The relative number of Ki67 positive cells in the dentate gyrus of the Co-ZLC, Co-ZDF, Ex-ZLC and Ex-ZDF groups at 12 weeks of age (n = 5 per group; *P < 0.05, significantly different from the ZLC group, # P < 0.05, significantly different from the control group). The bars indicate the means ± SEM
Effects of Treadmill Exercise on Neuronal Differentiation in ZDF and ZLC Rats
In all groups, DCX immunoreactive cells were observed in the subgranular zone of the dentate gyrus; however, DCX protein levels were markedly different between groups (Figs. 3, 4, 5). In the control-ZDF group, DCX immunoreactive fibers were decreased compared to those in the control-ZLF group and DCX protein levels in the control-ZLC group were higher than in the control-ZDF group (Figs. 3a, b, 4a, b). In the control-ZDF group, DCX positive cells with tertiary dendrites were fewer than those in the control-ZLC group (Fig. 5). DCX immunoreactive processes in the exercise-ZLC group were well stained with DCX and extended into the outer two-thirds of the molecular layer of the dentate gyrus compared to those in the exercise-ZDF group (Figs. 3c, d, 4c, d). In these exercise groups, the number of DCX positive cells with tertiary dendrites and without tertiary dendrites was significantly increased compared to those in the control groups (Fig. 5). The number of DCX positive with tertiary dendrites was significantly decreased in the exercise-ZDF group compared to that in the exercise-ZLC group (Fig. 5).
Low magnification of DCX immunoreactive cells in the dentate gyrus in control-ZLC (Co-ZLC), control-ZDF (Co-ZDF), exercise-ZLC (Ex-ZLC) and exercise-ZDF (Ex-ZDF) groups at 12 weeks of age. DCX immunoreactive cells are detected in the subgranular zone (arrows) of the polymorphic layer (PL). DCX immunoreactive cells are abundant in the Ex-ZLC and Ex-ZDF groups compared to those in the Co-ZLC and Co-ZDF groups. GCL, granule cell layer; ML, molecular layer. Bar = 100 μm
High magnification of DCX immunoreactive cells in the upper blade of the dentate gyrus in control-ZLC (Co-ZLC), control-ZDF (Co-ZDF), exercise-ZLC (Ex-ZLC) and exercise-ZDF (Ex-ZDF) at 12 weeks of age. DCX immunoreactive cells have many well-stained tertiary dendrites (arrows) and a few poor dendrites (arrowheads) in the Ex-ZLC and Ex-ZDF groups. GCL, granule cell layer; ML, molecular layer. Bar = 25 μm
Analysis of granule cells and DCX positive cells with and without tertiary dendrites in the dentate gyrus of control-ZLC (Co-ZLC), control-ZDF (Co-ZDF), exercise-ZLC (Ex-ZLC) and exercise-ZDF (Ex-ZDF) rats. Note that the number of granule cells is similar in all groups, but DCX positive cells with and without tertiary dendrites are abundant in the Ex-ZLC and Ex-ZDF rats (n = 5 per group; *P < 0.05, significantly different from the ZLC group, # P < 0.05, significantly different from the control group). The bars indicate the means ± SEM
In addition, exercise increased the DCX protein levels compared to that in the control groups. The DCX protein levels in the exercise-ZLC group were higher than that in the exercise ZDF-group (Fig. 6).
Western blot analysis of DCX in the dentate gyrus of control-ZLC (Co-ZLC), control-ZDF (Co-ZDF), exercise-ZLC (Ex-ZLC) and exercise-ZDF (Ex-ZDF) rats. Relative optical density (ROD) as % of immunoblot band is also represented (n = 3 per group; *P < 0.05, significantly different from the ZLC group, # P < 0.05, significantly different from the control group). The bars indicate the means ± SEM
Discussion
ZDF rats show type II DM pathologies such as glucose tolerance at 12 weeks of age and are maintained for at least 6 months [5, 29, 30]. In a previous [15] and present study, we found that the blood glucose level was much higher in the 12-week-old ZDF rats than that in age-matched ZLC rats. However, the treadmill exercise reduced the blood glucose levels although the food intake was not significantly different between groups. This result was good agreement with the previous studies that exercise improved insulin sensitivity and attenuated the development of type II diabetes [31–36]. In addition, Király et al. [37] reported that improvement in glucose tolerance with exercise was not a result of reduced food intake, a reduction in body weight, or differences in plasma lipid profiles. The reduction of glucose levels may be associated with a compensatory function in pancreatic beta cells. In a swim exercise model of ZDF rats, regular exercise increased the rates of beta cell proliferation and beta cell mass compared with sedentary control animals [37].
Next, we observed Ki67 immunoreactive nuclei and DCX immunoreactive structures (somata and processes) in the subgranular zone of the dentate gyrus to investigate cell proliferation and differentiation in diabetes and influences after the exercise. For this study, we trained the ZLC and ZDF rats for 5 weeks because it has been reported that most benefits have been associated with longer-term exercise (3–12 weeks) [38–41]. Ki67 immunoreactive nuclei and DCX immunoreactive structures in the dentate gyrus in the control-ZDF rats were more than those in the control-ZLC rats. In addition, the exercise significantly increased the number of DCX positive cells with tertiary dendrites and without tertiary dendrites in the ZLC and ZDF rats. This result coincided with some studies on type I [10, 13, 14] or II [15] DM models. However, in this study, we found that the exercise significantly increased the density of cells in both the ZLC and ZDF rats. These results suggest that an exercise facilitate the neuronal differentiation in the subgranular zone of the dentate gyrus in both control and type II diabetic models. Unlike in the hippocampus, exercise decreased neuronal activity in the posterior hypothalamic area in the spontaneously hypertensive rats [42] and increased glutamic acid decarboxylase gene transcription in the posterior hypothalamic area after physical exercise [43]: This finally reduced sympathetic tone and resting blood pressure.
Exercise increases some neurotrophic factors such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) in the brain [44–46]. An intrahippocampal injection of BDNF receptor antagonist attenuates the beneficial effects of exercise on hippocampus-dependent learning, specifically blocking improvements in both the acquisition and the retention of a spatial learning task [47, 48]. In addition, exercise increases several afferent neurotransmitters systems to the hippocampus, including norepinephrine, serotonin, acetylcholine and GABA [49], which are important factors to modulate the memory function.
A recent report pointed out an association between memory alterations and a decrease in neuronal proliferation in the dentate gyrus in diabetic rodents [10], and some reports also demonstrated that the impairment of cognitive performance in diabetic animals was associated with the hippocampus in terms of both anatomy and phenotype [6, 50]. Therefore, the increase of DCX immunoreactive cells in the dentate gyrus may be related with compensation in the impairment of cognitive performance in diabetic animals because exercise in young and aged animals can facilitate both acquisition and retention in various hippocampus-dependent tasks including the Morris water maze [41, 47], radial arm maze [40], passive avoidance [39] and object recognition [38]. In human study, exercise was found to have a primary effect on cerebral blood volume (CBV) in the dentate gyrus, which is the subregion that supports adult neurogenesis. Moreover, exercise-induced increases in dentate gyrus CBV were found to correlate with postmortem measurements of neurogenesis [51].
In conclusion, neuronal differentiation is markedly decreased in the subgranular zone of the dentate gyrus in type II diabetic rats and treadmill exercise induces cell proliferation and differentiation in the dentate subgranular zone in both control and diabetic rats.
References
Lazar MA (2005) How obesity causes diabetes: not a tall tale. Science 207:373–375. doi:10.1126/science.1104342
Tahirovic I, Sofic E, Sapcanin A et al (2007) Reduced brain antioxidant capacity in rat models of betacytotoxic-induced experimental sporadic Alzheimer’s disease and diabetes mellitus. Neurochem Res 32:1709–1717. doi:10.1007/s11064-007-9410-1
Katyare SS, Patel SP, Modi HR (2008) Diabetic modulation of the temperature kinetics properties of cytochrome oxidase activity in rat brain mitochondria. Neurochem Res 33:422–429. doi:10.1007/s11064-007-9447-1
Adeghate E, Schattner P, Dunn E (2006) An update on the etiology and epidemiology of diabetes mellitus. Ann NY Acad Sci 1084:1–29. doi:10.1196/annals.1372.029
Peterson RG, Shaw WN, Neel MA, Little KA, Eichberg J (1990) Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR J 32:16–19
Gispen WH, Biessels GH (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23:542–549. doi:10.1016/S0166-2236(00)01656-8
Li ZG, Britton M, Sima AA, Dunbar JC (2004) Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci 76:249–262. doi:10.1016/j.lfs.2004.03.039
Magariños AM, McEwen BS (2000) Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci USA 97:11056–11061. doi:10.1073/pnas.97.20.11056
Piotrowski P, Wierzbicka K, Smiałek M (2001) Neuronal death in the rat hippocampus in experimental diabetes and cerebral ischaemia treated with antioxidants. Folia Neuropathol 39:147–154
Jackson-Guilford J, Leander JD, Nisenbaum LK (2000) The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett 293:91–94. doi:10.1016/S0304-3940(00)01502-0
Klein JP, Waxman SG (2003) The brain in diabetes: molecular changes in neurons and their implications for end-organ damage. Lancet Neurol 2:548–554. doi:10.1016/S1474-4422(03)00503-9
Li ZG, Zhang W, Grunberger G, Sima AAF (2002) Hippocampal neuronal apoptosis in type I diabetes. Brain Res 946:221–231. doi:10.1016/S0006-8993(02)02887-1
Beauquis J, Saravia F, Coulaud J et al (2008) Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol 210:359–367. doi:10.1016/j.expneurol.2007.11.009
Revsin Y, Saravia F, Roig P et al (2005) Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. Brain Res 1038:22–31. doi:10.1016/j.brainres.2004.12.032
Hwang IK, Yi SS, Kim YN et al (2008) Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res 33:394–400. doi:10.1007/s11064-007-9440-8
Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP (2008) Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11:309–317. doi:10.1038/nn2055
Carroll S, Dudfield M (2004) What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 34:371–418. doi:10.2165/00007256-200434060-00004
Kleim JA, Jones TA, Schallert T (2003) Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res 28:1757–1769. doi:10.1023/A:1026025408742
Muller AP, Cammarota M, de Oliveira Dietrich M et al (2008) Different effect of high fat diet and physical exercise in the hippocampal signaling. Neurochem Res 33:880–885. doi:10.1007/s11064-007-9530-7
Pedersen BK (2006) The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 42:105–117. doi:10.1042/bse0420105
Schrauwen P, Westerterp KR (2000) The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr 84:417–427
Cameron HA, Woolley CS, McEwen BS, Gould E (1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56:337–344. doi:10.1016/0306-4522(93)90335-D
Hastings NB, Gould E (1999) Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol 413:146–154. doi:10.1002/(SICI)1096-9861(19991011)413:1<146::AID-CNE10>3.0.CO;2-B
Stanfield BB, Trice JE (1988) Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res 72:399–406
Kim HB, Jang MH, Shin MC et al (2003) Treadmill exercise increases cell proliferation in dentate gyrus of rats with streptozotocin-induced diabetes. J Diabetes Complicat 17:29–33. doi:10.1016/S1056-8727(02)00186-1
Lee HH, Shin MS, Kim YS et al (2005) Early treadmill exercise decreases intrastriatal hemorrhage-induced neuronal cell death and increases cell proliferation in the dentate gyrus of streptozotocin-induced hyperglycemic rats. J Diabetes Complicat 19:339–346. doi:10.1016/j.jdiacomp.2005.03.006
Cooper-Kuhn CM, Kuhn HG (2002) Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res 134:13–21. doi:10.1016/S0165-3806(01)00243-7
Yi SS, Hwang IK, Kim YN et al (2008) Enhanced expressions of arginine vasopressin (avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem Res 33:833–841. doi:10.1007/s11064-007-9519-2
Etgen GJ Jr, Jensen J, Wilson CM, Hunt DG, Cushman SW, Ivy JL (1997) Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Am J Physiol 272:E864–E869
Tokuyama Y, Sturis J, DePaoli AM et al (1995) Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 44:1447–1457. doi:10.2337/diabetes.44.12.1447
Christ CY, Hunt D, Hancock J, Garcia-Macedo R, Mandarino LJ, Ivy JL (2002) Exercise training improves muscle insulin resistance but not insulin receptor signaling in obese Zucker rats. J Appl Physiol 92:736–744. doi:10.1063/1.1487455
Etgen GJ, Oldham BA (2000) Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism 49:684–688. doi:10.1016/S0026-0495(00)80049-9
Hevener AL, Reichart D, Olefsky J (2000) Exercise and thiazolidinedione therapy normalize insulin action in the obese Zucker fatty rat. Diabetes 49:2154–2159. doi:10.2337/diabetes.49.12.2154
Kibenge MT, Chan CB (2002) The effects of high-fat diet on exercise-induced changes in metabolic parameters in Zucker fa/fa rats. Metabolism 51:708–715. doi:10.1053/meta.2002.32727
Pold R, Jensen LS, Jessen N et al (2005) Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54:928–934. doi:10.2337/diabetes.54.4.928
Shima K, Zhu M, Noma Y et al (1997) Exercise training in Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus: effects on the B-cell mass, insulin content and fibrosis in the pancreas. Diabetes Res Clin Pract 35:11–19. doi:10.1016/S0168-8227(96)01357-5
Király MA, Bates HE, Yue JT et al (2007) Attenuation of type 2 diabetes mellitus in the male Zucker diabetic fatty rat: the effects of stress and non-volitional exercise. Metabolism 56:732–744. doi:10.1016/j.metabol.2006.12.022
O’Callaghan RM, Ohle R, Kelly AM (2007) The effects of forced exercise on hippocampal plasticity in the rat: a comparison of LTP, spatial- and non-spatial learning. Behav Brain Res 176:362–366. doi:10.1016/j.bbr.2006.10.018
Radak Z, Toldy A, Szabo Z et al (2006) The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int 49:387–392. doi:10.1016/j.neuint.2006.02.004
Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE (2006) Exercise-induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochem Int 48:9–16. doi:10.1016/j.neuint.2005.08.006
Van Praag H, Shubert T, Zhao C, Gage FH (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25:8680–8685. doi:10.1523/JNEUROSCI.1731-05.2005
Beatty JA, Kramer JM, Plowey ED, Waldrop TG (2005) Physical exercise decreases neuronal activity in the posterior hypothalamic area of spontaneously hypertensive rats. J Appl Physiol 98:572–578. doi:10.1152/japplphysiol.00184.2004
Little HR, Kramer JM, Beatty JA, Waldrop TG (2001) Chronic exercise increases GAD gene expression in the caudal hypothalamus of spontaneously hypertensive rats. Brain Res Mol Brain Res 95:48–54. doi:10.1016/S0169-328X(01)00239-X
Fabel K, Fabel K, Tam B et al (2003) VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J NeuroSci 18:2803–2812. doi:10.1111/j.1460-9568.2003.03041.x
Lopez-Lopez C, LeRoith D, Torres-Aleman I (2004) Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA 101:9833–9838. doi:10.1073/pnas.0400337101
Trejo JL, Carro E, Torres-Aleman I (2001) Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21:1628–1634
Vaynman S, Ying Z, Gomez-Pinilla F (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J NeuroSci 20:2580–2590. doi:10.1111/j.1460-9568.2004.03720.x
Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F (2006) Exercise differentially regulates synaptic proteins associated to the function of BDN. Brain Res 1070:124–130. doi:10.1016/j.brainres.2005.11.062
Ma Q (2008) Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull 24:265–270. doi:10.1007/s12264-008-0402-1
Flood JF, Mooradian AD, Morley JE (1990) Characteristics of learning and memory in streptozocin-induced diabetic mice. Diabetes 39:1391–1398. doi:10.2337/diabetes.39.11.1391
Pereira AC, Huddleston DE, Brickman AM et al (2007) An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 104:5638–5643. doi:10.1073/pnas.0611721104
Acknowledgements
The authors would like to thank Mr. Seok Han, Mr. Seung Uk Lee and Ms. Hyun Sook Kim for their technical help in this study. This work was supported by the stem cell research program of Ministry of Science & Technology, grants (M10641450002-07N4145-00210).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yi, S.S., Hwang, I.K., Yoo, KY. et al. Effects of Treadmill Exercise on Cell Proliferation and Differentiation in the Subgranular Zone of the Dentate Gyrus in a Rat Model of Type II Diabetes. Neurochem Res 34, 1039–1046 (2009). https://doi.org/10.1007/s11064-008-9870-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9870-y