Abstract
Arginine vasopressin (AVP) is known to a neuropeptide that plays important roles in water conservation, sodium homeostasis, and in the regulation of serum osmolality. Several studies have reported that the elevated AVP level is related with diabetes mellitus as an acute or chronic stressor using type 1 diabetes mellitus animal models. However, it is unclear as to how the immunoreactivity and protein level of AVP in the brain is regulated in animal models of type 2 diabetes mellitus. In the present study, Zucker diabetic fatty (ZDF) rats were employed as a type 2 diabetes mellitus model and were compared with Zucker lean control (ZLC) rats with respect to AVP protein expression. Furthermore, in order to verify the regulation of AVP expression before and after the onset of diabetes mellitus, pre-diabetic rats (4 week-old) and obese-diabetic rats (12 week-old) were used. Blood glucose levels and water consumption were also measured and the results showed significantly high in 12 week-old ZDF than any other groups. AVP expression levels in the paraventricular nucleus and supraoptic nucleus were found to be significantly higher in 12 week-old ZDF rats than in 12 week-old ZLC rats and than in 4 week-old rats by immunostaining and western blotting. Enhanced expression of AVP in these animals may be associated with type 2 diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a common endocrine disorder that may induce dysfunction of the hypothalamus-pituitary-adrenal (HPA) axis due to its role as a stressor [1]. During acute and chronic stressful stimuli induced by diabetes mellitus, several neuropeptides levels are altered in the brain, e.g., arginine vasopressin (AVP), Neuropeptide Y, pro-opiomelanocortin, cocaine-amphetamine-regulated transport, corticotropin-releasing hormone, and adrenocorticotropin (ACTH) [1–6]. AVP is known to play an important role in water conservation, sodium homeostasis, cardiovascular homeostasis, vasoconstriction, antidiuresis, and in the regulation of serum osmolality [1, 7]. Moreover, AVP is synthesized in magnocellular neurons of the paraventricular nucleus (PVN) and in the supraoptic nucleus (SON) of the hypothalamus, and to be subsequently transported axonally and released into the systemic circulation in response to elevated osmotic pressure in the local microenvironment and in the plasma from the nerve terminals in the posterior pituitary [8, 9]. AVP is also released locally from cell bodies and dendrites in the SON with a longer delay and duration in response to systemic osmotic challenge [10, 11]. Several studies have reported that an inappropriately elevated AVP level in hypothalamus could be associated with diabetes mellitus, and elevated AVP is enhanced in patients with type 1 and 2 diabetes mellitus and in animal models of experimental or genetic diabetes mellitus [7, 8, 12–15].
The majority of animal models previously used have involved the peripheral administration of streptozotocin (STZ) to induce type 1 diabetes. Nevertheless, obese (fa/fa) Zucker diabetic fatty (ZDF) rats are also considered to be a good model for type 2 diabetes mellitus. ZDF rats have a missense mutation in the extracellular domain of all leptin receptor isoforms, which reduces signal transduction and may affect leptin transport into the brain [16, 17]. Moreover, obese ZDF rats between 6 and 10 weeks old spontaneously and rapidly develop diabetes. ZDF rats have been used extensively since the late 1980s to investigate the mechanisms of diabetes [18].
In order to determine the existence of a relation between AVP and type 2 diabetes mellitus, the brains of pre-diabetic 4-week-old male ZDF and Zucker lean controls (ZLC), and of obese diabetic 12-week-old male ZDF and ZLC rats were compared. Under diabetic stress, an apparent increase in the synthesis of AVP within PVN and SON was demonstrated in STZ-induced diabetes. However, there has not been introduced with ZDF rats according to age matched (4- and 12-week) under the condition of type 2 diabetes. Therefore, in this study, the AVP expression levels of PVN and SON were examined in ZLC and ZDF by immunostaining and western blotting. It was hoped that the results obtained would enable us to better understand how AVP might affect regulation of diabetes mellitus.
Experimental procedures
Experimental animals, check of blood glucose level and water consumption
Male and female ZDF (fa/+) were purchased from Genetic Models (Indianapolis, USA) and mated each other. They were housed in a conventional state under adequate temperature (23°C) and humidity (60%) control with a 12-h light/12-h dark cycle, and free access to food and water. Purina 5008 rodent diets (7.5% fat) were provided as recommended by Genetic Models Co. (USA) [19, 20]. The procedures for handling and caring for the animals adhered to the guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study. Every animal of each group was fasted for 3 h before sacrifice. The body weight and blood glucose levels of animals were checked before sacrifice with a validated one touch basic glucose measurement system. Daily water consumptions were recorded for 72 h in 4- and 12-week-old ZLC and ZDF (n = 5, each group).
Genotyping of fa gene
Genotype of fa gene herein was determined with the strategy described previous our study [20]. DNA was isolated from 3 mm tail snips. The tissue was placed in 0.5 ml of extraction buffer (100 mM/l Tris-HCl, pH 8.5, 5 mM/l EDTA, 0.2% SDS, 200 mM/l NaCl, and 200 μg/ml proteinase K) and incubated overnight at 55°C in a shaking water bath. After 15 min centrifuged at 15,500g, the supernatant was added to 0.5 ml of ice-cold isopropanol, and the tubes were shaken to form a precipitant. The tubes were centrifuged at 15,500g for 3 min, and the supernatant was discarded. The pellets were washed two times with cold 70% ethanol and air-dried. The DNA was dissolved in 500 μl of H2O at 55°C for 1 h, and the concentration was determined spectrophotometrically. Polymerase chain reactions (PCRs) were performed using the Promega PCR Core system I, 100 ng of DNA, 14 pM of each primer, and 30 units of Taq enzyme with the following cycling conditions: 92°C for 2 min, 49 cycles at 92°C for 30 s, 65°C for 30 s, 68°C for 3 min, then 5 min at 68°C. The amplified product of 1.8-kb was digested with Msp I (New England Biolabs, Beverly, USA).
Cresyl violet staining
To observe the overall morphology of PVN and SON, ZLC and ZDF rats in 4-week-old and 12-week-old rats (n = 5 at each time point) were used for cresyl violet staining. In brief, the sections were mounted on gelatin-coated microscopy slides. Cresyl violet acetate (Sigma, MO) was dissolved at 1.0% (w/v) in distilled water, and glacial acetic acid was added to this solution. Before and after staining for 2 min at room temperature, the sections were washed twice in distilled water. The fixed brain tissues were dehydrated by immersing for 2 h in 50%, 70%, 80%, 90%, 95% and 100% ethanol baths in succession at room temperature. After dehydration, the sections were mounted with Canada balsam (Kato, Japan).
Immunohistochemistry for AVP
Immunohistochemistry was performed under the same conditions in each group. Sections were treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and then 10% normal horse serum in 0.05 M PBS for 30 min. They were then incubated with diluted rabbit anti-AVP antibody (diluted 1:2,000, Phoenix, CA, USA) overnight at room temperature and then exposed to biotinylated goat anti-rabbit IgG and streptavidin peroxidase complex (diluted 1:200, Vector, USA). Sections were then visualized by staining with 3,3′-diaminobenzidine in 0.1 M Tris-HCl buffer (pH 7.2), and mounted on gelatin-coated slides and dehydrated. A negative control test was carried out using pre-immune serum instead of primary antibody in order to establish the specificity of immunostaining. The tissue in negative control contained no AVP immunoreactivity in any structure.
Western blot analysis
To confirm changes in AVP level in the PVN and SON of ZDF and ZLC rats, five animals in ZLC and ZDF groups in 4- and 12-week-old rats were sacrificed and used for western blot analysis. After sacrifice and brain removal, brains were serially and transversely sectioned at 400 μm on a vibratome (Leica, Germany); Hypothalamic regions were then dissected using a surgical blade. Tissues were homogenized in 50 mM PBS (pH 7.4) containing 1 mM EGTA (pH 8.0), 0.2% NP-40, 10 mM EDTA (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM dithiothreitol (DTT). After centrifugation, protein levels were determined in supernatants using Micro BCA protein assay kits using bovine serum albumin as the standard (Pierce Chemical, USA). Aliquots containing 50 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue, and 30% glycerol. Aliquots were then loaded onto 15% polyacrylamide gels. After electrophoresis, gels were transferred to nitrocellulose membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min, and then incubated with rabbit anti-AVP (1:2,000), peroxidase-conjugated goat anti-rabbit IgG (Sigma, USA) and an ECL kit (Pierce Chemical, Rockford, IL).
Quantification of data and statistical analysis
All measurements were performed in order to ensure objectivity under blind conditions, by two observers per experiment, who performed analyses using identical conditions.
In order to quantitatively analyze AVP immunoreactivity, corresponding the PVN and SON areas were measured using in total 10 sections per animal. Images of all AVP immunoreactive structures were taken using a BX51 light microscope (Olympus, Japan) equipped with a digital camera (DP71, Olympus) connected to a PC monitor. The number of AVP immunoreactive neurons was measured using an image analyzing system equipped with a computer-based CCD camera (software: Optimas 6.5, CyberMetrics, USA) in all groups. The number of AVP immunoreactive neurons was counted in 500 μm2 of PVN in 25 sections/each animal. The number of cresyl violet positive neurons was compared to that of the 4-week-old ZLC group. Western blots were scanned and quantified using Scion Image software (Scion Corp., USA).
The data shown represent experimental means ± SEM for each experimental area. One-way analysis of variance (ANOVA) was used to compare differences among groups, and two-way ANOVA for repeated measure, followed by Tukey and Duncan post hoc test, to compare the values. Differences were considered significant if P < 0.05.
Results
Genotyping of the fa gene
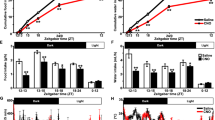
We confirmed the genotype of the fa gene in order to identify homo and others. The PCR amplified product of 1.8-kb was digested with Msp I to generate a 1.1-kb band for the homozygote wild type (+/+), a 1.0-kb band for the homozygotic fa gene (fa/fa), and 1.1- and 1.0-kb bands for the heterozygote (fa/+) (Fig. 1)
Blood glucose levels
In the 4-week-old ZDF group, mean blood glucose level was 166.2 ± 8.65 mg/dl (mean ± SEM) and in the 4-week-old ZLC group this was 156.2 ± 13.83 mg/dl (Fig. 2a). In the 12-week-old ZDF group mean blood glucose was 395.2 ± 12.91 mg/dl and in the 12-week-old ZLC group it was 166.6 ± 5.09 mg/dl (Fig. 2a). There were significant divergences between 12-week-old ZLC and ZDF and between 4-week-old ZLC, ZDF and 12-week-old ZDF (P < 0.0001).
Blood glucose levels (a) and water consumptions (b) presented as bar graphs. Blood glucose levels in the 4-week old ZDF and ZLC groups are similar, but levels differ in the corresponding of 12-week-old groups. Moreover, significant differences are observed between 4-week-old ZLC, ZDF and 12-week-old ZDF (P < 0.0001). Water consumptions are recorded for 72 h in 4-week-, 12-week-old ZDF and ZLC. Moreover, significant differences are observed between 4-week-old ZLC, ZDF and 12-week-old ZDF (P < 0.0001)
Water consumption
The water consumptions of each group were described in Fig. 2b. In comparative analysis of differences cross the groups, significant differences were observed between 12-week-old ZLC and ZDF and between 4-week-old ZLC, ZDF and 12-week-old ZDF (P < 0.0001).
Cresyl violet staining
All cresyl violet positive neurons in PVN and SON appeared structurally normal (Figs. 3 and 4). High density large spindle-shaped cresyl violet positive neurons were observed in the lateral magnocellular area of the PVN and a lower density of cresyl violet positive middle size neurons were observed in the medial magnocellular area of the PVN. In the medial parvocellular area of the PVN, small neurons were stained by cresyl violet at low density. The distributions and number of cresyl violet positive neurons in SON and PVN were similar between 4-week and 12-week ZLC and ZDF rats (Table 1).
Immunohistochemistry
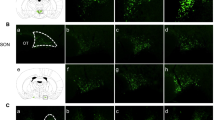
In PVN, mean numbers of AVP-positive neurons were 135.4 ± 2.79 and 129.8 ± 3.37 in 4-week old ZDF and ZLC rats, respectively (Figs. 5a, b and e). No significant difference was observed between AVP positive cell numbers in the 4-week ZDF and ZLC groups in the PVN. However, in 12-week-old rat, mean numbers of AVP-positive neurons were 164.0 ± 3.10 and 140.0 ± 3.48 in the ZDF and ZLC, respectively (Figs. 5c–e). Numbers of AVP-positive neurons were significantly more frequent the 12-week-old ZDF group than that in age-matched ZLC group and 4-week-old ZLC and ZDF groups. In addition, in two-way ANOVA considered interaction analysis cross the groups, there were significant differences with age and genotypes (P < 0.005).
Microphotographs of arginine vasopression (AVP) immunoreactivity in the paraventricular nucleus in ZLC (a and c) and ZDF (b and d) rats at 4 (a and b) and 12 weeks (c and d) of age. Note that AVP immunoreactivity is significantly elevated in the parvocellular and magnocellular regions of the hypothalamus in 12-week-old ZDF rats. Bar = 100 μm. Numbers of AVP-positive neurons are presented as bar graphs. The bars indicate means ± SEM
In SON, mean numbers of AVP-positive neurons were 55.2 ± 2.18 and 53.2 ± 2.62 in 4-week-old ZDF and ZLC rats, respectively (Figs. 6a, b and e), but no significant differences were observed between the 4-week-old ZDF and ZLC groups. However, in 12-week-old-rats, mean numbers of AVP-positive neurons were 91.0 ± 2.39 and 61.6 ± 3.80 for ZDF and ZLC rats, respectively (Fig. 6c–e). Increases in AVP-positive neurons were significantly higher in 12-week-old ZDF rats than in 12-week-old ZLC rats. Numbers of AVP-positive neurons were significantly more frequent the 12-week-old ZDF group than that in age-matched ZLC group and 4-week-old ZLC and ZDF groups. In addition, in two-way ANOVA considered interaction analysis cross the groups, there were significant differences with age and genotypes (P < 0.005).
Microphotographs of arginine vasopressin (AVP) immunoreactivity in the supraoptic nucleus in ZLC (a and c) and ZDF (b and d) rats at 4 (a and b) and 12 weeks (c and d) of age. Note that the AVP immunoreactivity is significantly elevated in both sides of the SON in 12-week-old ZDF rats. Bar = 100 μm. Numbers of AVP-positive neurons are presented as bar graphs. Bars indicate means ± SEM
Change in AVP levels in 4- and 12-week ZDF and ZLC rats
AVP levels in hypothalamus of the 4-week-old and 12-week-old group are shown in Fig. 7. AVP levels corresponded to immunohistochemically determined changes. AVP expression levels were similar in the 4-week-old ZDF and ZLC group. However, in 12-week-old rats, AVP expression levels were much higher in ZDF rats than in ZLC rats. No significant differences were observed between AVP levels in 4-week-old ZDF and ZLC rats, but a significant difference was observed between AVP levels between 12-week-old ZDF and ZLC rats and between 4-week-old ZLC, ZDF and 12-week-old ZDF. In addition, in two-way ANOVA considered interaction analysis cross the groups, there were significant differences with age and genotypes (P < 0.005).
Discussion
The present study demonstrates the expression of AVP in ZDF rats represent type 2 diabetes mellitus. A number of studies have demonstrated AVP expressional changes in type 1 diabetes mellitus using STZ-induced diabetic rats [1, 8, 13, 14, 21, 22]. However, few studies have been conducted on AVP in animal models of type 2 diabetes.
Morita et al. (2001) reported the mRNA expression levels of AVP and vasopressin V1a receptor in type 2 diabetic db/db mice, but not their protein levels. A significant increase in brain AVP mRNA levels was observed 10 week-old db/db mice verses control mice (+/+). However, it remained unclear whether AVP protein expression levels in brain were up-regulated in type 2 diabetes animal models versus normal controls since mRNA upregulation does not imply concomitant protein up-regulation. Therefore, the present study may be the first to report elevated AVP peptide expression in the brain by immunohistochemistry and western blotting in a genetically established type 2 diabetic animal model. In addition, body weights, blood sugar levels, liver weights, and plasma osmolality were significant different in 10-week-old db/db mice versus control (+/+) mice [7]. Blood sugar levels were coincided with the present study with ZDF rat models. As hyperglycemia can result in hyperosmolality, which is known to be the physiological impetus for AVP secretion, plasma osmolality may be higher in ZDF than in ZLC rats [23].
In the STZ-induced diabetic rat model, similar results to those mentioned above have been reported in brain. AVP and oxytocin-immunoreactive neurons were found to be elevated in the PVN and SON of the hypothalamus [8, 13, 21, 22]. In particularly, dysfunction of the HPA axis was induced during the acute and chronic stress of both diabetes mellitus types. Elevated levels of AVP stimulate the secretion of ACTH, which stimulates the synthesis and release of glucocorticoids [24]. Moreover, chronic increases in plasma glucocorticoid levels aggravate the symptoms by elevating blood glucose levels and enhancing insulin resistance. The effects of HPA axis hyperactivation in diabetes are not clear, however, AVP is presumed to have a key role during acute or chronic stress [1, 22].
There were no significant differences in water consumption between ZLC and ZDF of 4-week-old groups, however, there were between those of 12-week-old groups. There were also significant differences between 4-week-old ZLC, ZDF and 12-week-old ZDF groups. It is considered that osmotic demands in response to AVP climbing are increased in diabetic stress. In lithium (Li)-induced diabetes insipidus (DI) rats, it has been found that the plasma concentration of sodium is significantly increased compared with that of controls. Thus, it could be explained that stimulation of sodium receptors or osmoreceptors in the brain or in the hepato/portal circulation may contribute to the secretion of AVP and upregulation of AVP gene expression in the PVN and SON in Li-induced DI rats [25]. Gillard et al. (2007) reported that stimulation of local AVP release within SON in response to osmotic challenge is dependent on stimulation of magnocellular neuron cell AVP autoreceptors by locally released AVP, signaling mediated nitric oxide, and ionotropic glutamate receptor activity [26].
In ZDF rats, the pre-diabetic condition (4-week-old) was selected since the diabetic condition appears from 6 weeks. Conversely the typical diabetes condition (12-week-old) was selected since the obese diabetic condition started from 10 weeks. Moreover, cresyl violet stained positive cells were not different statistically in 4-week-old ZDF and ZLC rats in the PVN and SON. Immunostaining and western blotting findings were also similar in these two groups. However, cresyl violet stained positive cells were not different in every group in the PVN and SON, immunostaining and western immunoblot findings were significantly different between 4-week-old ZLC, ZDF and 12-week-ZDF groups in PVN and SON.
AVP has a high affinity for the oxytocin receptor and three vasopressin receptor types, V1a, V1b and V2 receptor [27]. Several reports have shown that plasma AVP level was increased in patients with type 1 and type 2 diabetes [28, 29]. In addition, the antidiuretic or hepatic function of AVP was decreased in human and rodent subjects with type 1 and type 2 diabetes [30–32]. From these findings proposed that the altered glucose homeostasis could occasionally result in or from AVP resistance, which is, in part, mediated via the V1a receptor [30–32]. The phenotype of V1a receptor knocked out (V1aR-/-) mice was consistent with clinical data from a type 2 diabetes patient [33], who showed impaired glucose tolerance and intact insulin secretion [34]. Moreover, because the V1aR-/- mice exhibited high hepatic glucose production, V1aR could contribute to developing glucose intolerance. V1b receptor is known to a receptor via which AVP-induced insulin and glucagons secretion is mediated [35–39]. Fujiwara et al. [27] recently reported that the effect of AVP on regulating blood glucose levels in V1b receptor-deficient mice. When V1bR was deficient, it showed lower plasma glucose levels were recorded by decreased plasma insulin levels and glucagons secretion under the fasting condition. They also indicated the possibilities of reduced plasma glucose levels by affecting glucocorticoid hormone regulation. The reduced plasma glucose levels in V1bR-/- mice could have been discriminated by enhanced insulin sensitivity, reduced glucagons, and/or reduced corticosterone levels [27].
The glucose levels of 4-week-old ZLC, ZDF and 12-week-old ZLC is seemed to be slightly higher than previously published levels (<150 mg/dl) [18]. The causes of increased blood glucose levels could be diverse, however, we couldn’t get to the exact answers. Since the values were under the constant range, however, it is considered that there are not severe problems in this study and the reason must be further confirmed.
In conclusion, AVP immunoreactivity and protein levels were significantly increased in the PVN and the SON of ZDF rats compared to those of ZLC rats. In addition, The AVP levels above mentioned were higher in diabetic rats (12-week-old) than that in pre-diabetic rats (4-week-old) and in that of control (12-week-old). These results suggest that type 2 diabetes mellitus may be crossly related with water conservation and homeostasis by AVP regulation in the brain.
References
Zelena D, Filaretova L, Mergl Z, Barna I, Toth ZE, Makara GB (2006) Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am J Physiol Endocrinol Metab 290:243–250
Franco-Bourland RE (1998) Vasopressinergic, oxytocinergic, and somatostatinergic neuronal activity after adrenalectomy and immobilization stress. Neurochem Res 23:695–701
Medeiros Mdos S, Turner AJ (1996) Metabolism and function of neuropeptide Y. Neurochem Res 21:1125–1132
Antoni FA (1999) Vasopressinergic control pituitary adrenocorticotropin secretion comes of age. Clin Sci (Lond) 96:513–523
Scatt LV, Dinan TG (1998) Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci 62:1985–1998
Van Vugt DA, Lujan ME, Froats M, Krzemien A, Couceyro PR, Reid RL (2006) Effect of fasting on cocaine-amphetamine-regulated transcript, neuropeptide Y, and leptin receptor expression in the non-human primate hypothalamus. Neuroendocrinology 84:83–93
Morita M, Kita Y, Morikawa N, Iwami M, Notsu Y (2001) Expression of arginine vasopressin and vasopressin V1a receptor mRNA in diabetic (db/db) mice. Exp Clin Endocrinol Diabetes 109:261–266
Saravia FE, Gonzalez SL, Roig P, Alves V, Homo-Delarche F, De Nicola AF (2001) Diabetes increases the expression of hypothalamic neuropeptides in a spontaneous model of type I diabetes, the nonobese diabetic (NOD) mouse. Cell Mol Neurobiol 21:15–27
Hayashi M, Arima H, Goto M et al (2006) Vasopressin gene transcription increases in response to decrease in plasma volume, but not to increase in plasma osmolality, in chronically dehydrated rats. Am J Physiol Endocrinol Metab 290:E213–E217
Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M (1994) Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol 6:369–373
Neumann I, Landgraf R, Bauce L, Pittman QJ (1995) Osmotic responsiveness and cross talk involving oxytocin, but not vasopressin or amino acids, between the supraoptic nuclei in virgin and lactating rats. J Neurosci 15:3408–3417
Bankir L, Bardoux P, Ahloulay M (2001) Vasopressin and diabetes mellitus. Nephron 87:8–18
Luo Y, Kaur C, Ling EA (2002) Neuronal and glial response in the rat hypothalamus-neurohypophysis complex with streptozotocin-induced diabetes. Brain Res 925:42–54
Yibchok-anun S, Abu-Basha EA, Yao CY, Panichkriangkrai W, Hsu WH (2004) The role of arginine vasopressin in diabetes-associated increase in glucagons secretion. Regul Pept 122:157–162
Kim MJ, Hong SJ, Yang J, Kim HK (2007) Silkworm (Bombyx mori L.) reduces vasopressin expression in the hypothalamus of streptozotocin-induced diabetic mice. Neurol Res 29:S72–S77
Chua SC Jr, Chung WK, Wu-Peng XS et al (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271:994–996
Lugarini F, Hrupka BJ, Schwartz GJ, Plata-Salaman CR, Langhans W (2005) Acute and chronic administration of immunomodulator induces anorexia in Zucker rats. Physiol Behav 84:165–173
Peterson RG (2001) The zucker diabetic fatty (ZDF) rat. In: Sima AAF, Shafrir E (eds) Animal models of diabetes a primer. Harwood academic publishers, Amsterdam, pp 109–128
Harmons JS, Gleason CE, Tanaka Y, Poitout V, Robertson RP (2001) Antecedent hyperglycemia, not hyperlipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes 50:2481–2486
Hwang IK, Yi SS, Kim YN et al (2007) Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res doi. 10.1007/s11064-007-9940-8
Dheen ST, Tay SS, Wong WC (1994) Arginine vasopressin- and oxytocin-like immunoreactive neurons in the hypothalamic paraventricular and supraoptic nuclei of streptozotocin-induced diabetic rats. Arch Histol Cytol 57:461–472
Zelena D, Mergl Z, Makara GB (2006) The role of vasopressin in diabetes mellitus-induced hypothalamo-pituitary-adrenal axis activation: studies in Barttleboro rats. Brain Res Bull 69:48–56
Vokes TP, Aycinena PR, Rebertson GL (1987) Effect of insulin on osmoregulation of vasopressin. Am J Physiol 255:E538–E548
Chan O, Inouye K, Riddell MC, Vranic M, Matthews SG (2003) Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol 28:87–102
Anai H, Ueta Y, Serino R et al (1997) Upregulation of the expression of vasopressin gene in the paraventricular and supraoptic nuclei of the lithium-induced diabetes insipidus rat. Brain Res 772:161–166
Gillard ER, Coburn CG, de Leon A et al (2007) Vasopressin autoreceptors and nitric oxide-dependent glutamate release are required for somatodendritic vasopressin release from rat magnocellular neuroendocrine cells responding to osmotic stimuli. Endocrinology 148:479–489
Fujiwara Y, Hiroyama M, Sanbe A et al (2007) Insulin hypersensitivity in mice lacking the V1b vasopressin receptor. J Physiol doi:10.1113/jphysiol.2007.136481
Walsh CH, Baylis PH, Malins JM (1979) Plasma arginine vasopressin in diabetic ketoacidosis. Diabetologia 16:93–96
Zerbe RL, Vinicor F, Robertson GL (1979) Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes 28:503–508
Hems DA, Ma GY (1976) Resistance to hepatic action of vasopressin in genetically obese (ob/ob) mice. Biochem J 160:23–28
McKenna K, Morris AD, Ryan M et al (2000) Renal resistance to vasopressin in poorly controlled type 1 diabetes mellitus. Am J Physiol Endocrinol Metab 279:E155–E160
Agha A, Smith D, Finucane F et al (2004) Attenuation of vasopressin-induced antidiuresis in poorly controlled type 2 diabetes. Am J Physiol Endocrinol Metab 287:E1100–E1106
Waldhausl W, Bratusch-Marrain P, Gasic S, Korn A, Nowotny P (1982) Insulin production rate, hepatic insulin retention, and splanchnic carbohydrate metabolism after oral glucose ingestion in hyperinsulinemic type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 23:6–15
Aoyagi T, Birumachi J, Hiroyama M et al (2007) Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 148:2075–2084
Lee B, Yang C, Chen TH, al-Azawi N, Hsu WH (1995) Effect of AVP and oxytocin on insulin release: involvement of V1b receptor. Am J Physiol 269:E1095–E1100
Richardson SB, Laya T, VanOoy M (1995) Similarities between hamster pancreatic islet beta (HIT) cell vasopressin receptors and V1b receptors. J Endocrinol 147:59–65
Yibchok-anun S, Cheng H, Heine PA, Hsu WH (1999) Characterization of receptors mediating AVP- and OT- induced glucagons release from the rat pancreas. Am J Physiol 277:E56–E62
Yibchok-anun S, Hsu WH (1998) Effects of arginine vasopressin and oxytocin on glucagons release from clonal alpha-cell line In-R1-G9: involvement of V1b receptors. Life Sci 63:1871–1878
Folny V, Raufaste D, Likovic L et al (2003) Pancreatic vasopressin V1b receptors: characterization in In-R1-G9 cells and localization in human pancreas. Am J Physiol Endocrinol Metab 285:E566–E576
Acknowledgements
This work was supported by the Grants of MRC for Chronic Metabolic Syndrome from the Ministry of Health and Welfare in Korea to J. K. Seong and Research Institute for Veterinary Science, Seoul National University to Y. S. Yoon.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Special issue article in honor of George Fink.
Rights and permissions
About this article
Cite this article
Yi, S.S., Hwang, I.K., Kim, Y.N. et al. Enhanced Expressions of Arginine Vasopressin (Avp) in the Hypothalamic Paraventricular and Supraoptic Nuclei of Type 2 Diabetic Rats. Neurochem Res 33, 833–841 (2008). https://doi.org/10.1007/s11064-007-9519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9519-2