Abstract
Background
Extensive surgical resection has been found to be associated with longer survival in patients with gliomas, but the interactive prognostic value of molecular pathology of the surgical resection is unclear. This study evaluated the impact of molecular pathology and clinical characteristics on the surgical benefit in WHO grade 3 IDH-mutant gliomas.
Methods
Clinical and pathological information of 246 patients with WHO grade 3 IDH-mutant gliomas were collected from the Chinese Glioma Genome Atlas database (2006–2020). The role of the extent of resection on overall survival, stratified by molecular pathology and clinical characteristics, was investigated. We then assessed prognostic factors using a univariate log-rank test and multivariate Cox proportional hazards model in the subgroups.
Results
The extent of resection was an independent prognostic factor in the entire cohort, even when adjusted for molecular pathology. Gross total resection was found to be associated with longer survival in all patients and in the astrocytoma group but not in the oligodendroglioma group. Compared with subtotal resections, gross total resections resulted in a longer survival time for astrocytoma patients aged ≤ 45 years. However, there was no survival benefit from total resection in patients with astrocytoma aged > 45 years.
Conclusions
Extensive resection benefits only a proportion of patients with WHO grade 3 IDH-mutant gliomas. Younger patients with astrocytomas had survival benefits from extensive resection. In addition to clinical characteristics (especially age), molecular pathology impacted prognosis in patients with gliomas. Our findings provide guiding information to neurosurgeons while planning surgeries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffuse gliomas are the most common primary intracranial malignant tumor in adults, which display infiltrative growth and a high rate of recurrence [1]. Currently, surgical resection is still the first choice for treatment of gliomas. However, it was always a dilemma for neurosurgeons whether to expand the extent of resection (EOR) or preserve the neurological function. The default premise of this problem is that increasing the EOR brings survival benefits for all cases. However, this hypothesis has been demonstrated to be untrue when considering the molecular pathological type of gliomas [2,3,4,5]. If expanding the EOR cannot prolong survival time and yet will increase the risk of neurological damage for certain genetic types of gliomas, resection should be done conservatively for these cases.
The prognostic value of surgical resection for different molecular pathological types of gliomas is still unclear. Patients with isocitrate dehydrogenase (IDH) wild-type gliomas have a poorer prognosis than those with IDH mutant gliomas [6, 7]. Additionally, IDH mutant astrocytomas benefit from complete resection [8]. Thus, a more extensive resection is suggested for IDH wild-type gliomas [9]. The 1p/19q co-deletion is a prognostic biomarker in glioma and was found to be associated with a longer survival time [10]. Gross total resection (GTR) does not prolong overall survival (OS) for lower-grade gliomas with 1p/19q co-deletion [4]. The current evidence suggests that the prognostic value of EOR could vary between gliomas with different molecular pathologies. Nevertheless, this issue is unclear since most previous studies lack analyses of the interactive effect between surgical resection, clinical characteristics, and genetic characteristics.
In the past, histopathological and molecular pathological information could only be obtained by postoperative assessment of tumor samples, and thus, the information cannot be used for guiding surgical resection. Recently, an artificial intelligence model based on radiomics features has enabled highly precise predictions of the molecular biomarker status of gliomas prior to surgery [11,12,13,14,15,16]. Additionally, the development of intraoperative rapid pathological assessment, such as Raman spectroscopy technology, has realized real-time tumor diagnosis [9, 17]. In the future, surgical planning considering molecular pathological information may be applied in practice.
To test our hypothesis that certain subtypes of World Health Organization (WHO) grade 3 gliomas would not benefit from extensive surgical resection, WHO grade 3 IDH-mutant gliomas were collected from the Chinese Glioma Genome Atlas (CGGA) database. According to the 2021 WHO Classification of Tumors of the Central Nervous System [18], WHO grade 3 gliomas encompass astrocytoma with IDH-mutant and oligodendroglioma with IDH-mutant and 1p/19q co-deletion. We analyzed the role of extensive EOR in prolonging OS for certain molecular subtypes of WHO grade 3 gliomas. We further explored whether the identified surgical benefit can be influenced by clinical factors, such as age at diagnosis. The knowledge we acquired from these meticulous analyses will be instrumental in guiding customized surgical resection for WHO grade 3 IDH-mutant gliomas.
Materials and methods
Patients
We conducted a retrospective analysis of a consecutive series of patients (n = 476) who were newly diagnosed with WHO grade 3 IDH-mutant gliomas and who underwent surgical resection from March 2006 to August 2020. All surgeries were carried out by highly experienced senior physicians. Clinical characteristics, MR images, histopathology, molecular pathology, adjuvant therapy after surgical resection, and follow-up were obtained from the CGGA database (http://www.cgga.org.cn) [19]. This study was approved by the ethics committee of Beijing Tiantan Hospital and was conducted in accordance with the principles of the Declaration of Helsinki (2008). Written informed consent was not required due to the retrospective design of the study.
The inclusion criteria for the cohort were as follows: (1) aged 18 years or older, (2) pathologically confirmed grade 3 gliomas based on the 2021 WHO classification, (3) availability of IDH and 1p/19q statuses, (4) pre- and postoperative MR scans, and (5) no previous adjuvant treatment.
Degree of tumor resection and volumetric analysis
The EOR was assessed by comparing postoperative MR images with preoperative MR images [20], and the tumor volume was performed using the free-access software, MRIcro (http://www.mccauslandcenter.sc.edu/mricro/). If the tumor was enhanced on preoperative MR images, GTR of the tumor was defined as resection with no residual enhanced tumor. In the case that the tumor was not enhanced or partially enhanced on preoperative MR images, the extent of resection was assessed based on the residual high intensity region on T2/fluid-attenuated inversion recovery MR images. EOR was calculated as (preoperative volume – postoperative volume) / preoperative volume. A gross total resection (GTR) was defined as the removal of more than 100% of tumor regions, indicating the absence of any remaining contrast-enhanced tissue or T2/FLAIR high signal area on postoperative MR images. A subtotal resection (STR) was defined as a resection of higher than 90% tumor regions. A partial resection (PR) was defined as a resection of less than 90% [21,22,23].
Molecular analysis
Glioma molecular pathologies were obtained from the CGGA database. Tumor tissue obtained from surgery were assessed to detect IDH mutations, 1p/19q co-deletion, and methylguanine-DNA methyltransferase (MGMT) promoter methylation. The IDH mutation and the methylation status of the MGMT promoter were tested using DNA pyrosequencing [24, 25]. The 1p/19q co-deletion was detected using fluorescence in situ hybridization, as previously reported [26].
All patients in this study were classified into two subgroups according to the 2021 WHO classification of a Central Nervous System tumor: (1) astrocytoma (WHO grade 3), IDH mutant, and 1p/19q non-co-deleted; or (2) oligodendroglioma (WHO grade 3), IDH mutant, and 1p/19q co-deleted.
Statistical analyses
Categorical variables were demonstrated with frequencies and percentages, and continuous variables with medians and interquartile range. We used the unpaired t-test and chi-squared tests to identify differences in clinical characteristics between the cohorts. The Fisher’s exact test was applied when the assumptions of the chi-squared tests were violated. OS was defined as the duration between the primary surgery and death or the last follow-up. Progression-free survival time was defined as the time from primary surgery to the first clinical or radiological progression as indicated by the clinicians. The Kaplan–Meier method and the log-rank test was used to assess survival differences between the groups. A univariate survival analysis was applied to assess the prognostic significance of the variables. Multivariate survival analyses of certain characteristics (sex, age, preoperative Karnofsky Performance Score [KPS], molecular subtype, MGMT methylation status, EOR, and adjuvant therapy after surgery) were performed using a Cox proportional hazards model. A p < 0.05 was considered statistically significant. Statistical analyses were performed using R-software (v 4.1.0 The R Foundation, Vienna, Austria) and GraphPad Prism 8.3. (GraphPad Software, La Jolla CA, United States).
Results
Patient characteristics
The records of 476 patients with WHO grade 3 IDH-mutant gliomas were retrieved from the CGGA database. Of them, 230 patients were excluded from the study cohort (3 were < 18 years old, 149 had recurrent gliomas, 12 lacked follow-up information, and 66 did not have molecular pathology data). A total of 246 patients were finally enrolled in this study (Fig. 1). Clinical characteristics of all patients are summarized in Table 1.
There were 127 (51.6%) males and 119 (48.4%) females; the median age at diagnosis was 42 (interquartile range [IQR], 36–50) years. The median preoperative KPS was 90 (IQR, 90–90). There were 131 (53.3%) patients with astrocytoma (IDH mutant and 1p/19q non-co-deletion) and 115 (46.7%) patients with oligodendroglioma (IDH mutant and 1p/19q co-deletion). The major preoperative symptoms of patients were epilepsy (48.0%) and headache (43.9%). Patients with astrocytomas were younger than those with oligodendrogliomas (P < 0.0001). Compared with patients who underwent GTR, patients with STR and PR were more likely to have a tumor involving deep brain structures, such as the thalamus, basal ganglia, and corpus callosum (P < 0.0001) (Table 2).
Patient outcome per molecular subtype and extent of resection
The median follow-up time for all patients was 70 months, with oligodendroglioma and astrocytoma subgroups having median follow-up times of 58.4 months and 81.5 months, respectively. The entire cohort had a median overall survival (OS) of 112 months, while the median follow-up time of the 169 (68.7%) patients still alive upon data collection was 56 months. No operation-related mortalities were reported. The OS significantly differed between the astrocytoma and oligodendroglioma groups (hazard ratio [HR]: 2.6; 95% confidence interval [CI]: 1.7–4.1; P = 0.0001), with median OS of 79.7 months and 120.9 months for the astrocytoma and oligodendroglioma groups, respectively.
We analyzed the impact of EOR on the prognosis for WHO grade 3 IDH-mutant gliomas. Patients who underwent GTR (OS: undefined) showed a longer OS compared with those who underwent subtotal resection (OS: 99 months) or partial resection (OS: 26 months) (P < 0.0001). The value of GTR remained for IDH-mutant astrocytoma after adjusting for the molecular subgroups (P < 0.0001) (Fig. 2). However, those with oligodendroglioma did not benefit more from GTR than from subtotal resection (P = 0.1701) (Fig. 2). Additionally, the similar results were also found in PFS.
Kaplan–Meier curves of overall survival (OS) and progression-free survival (PFS) stratified by extent of resection. (A) and (D) All IDH-mutant gliomas; (B) and (E) astrocytomas; (C) and (F) oligodendrogliomas. *Comparison between GTR and STR; GTR, gross total resection; STR, subtotal resection; PR, partial resection
In the univariate survival analysis of all patients, the EOR (non-GTR vs. GTR; HR, 3.416; P < 0.0001), molecular subtype (astrocytoma vs. oligodendroglioma; HR, 2.619; P = 0.0001), MGMT (unmethylated vs. methylated; HR, 1.795; P = 0.0141) and adjuvant therapy (no vs. yes; HR, 2.109; P = 0.0094) were significant prognostic factors for OS (Table S1). Similarly, the multivariate analysis revealed that sex (female vs. male, HR, 1.922; P = 0.011), preoperative KPS (≤ 80 vs. > 80, HR, 2.074; P = 0.008), EOR (Non-GTR vs. GTR, HR, 4.144; P < 0.0001), MGMT (unmethylated vs. methylated, HR, 1.876; P = 0.011), adjuvant therapy (no vs. yes, HR, 2.834; P = 0.002), and molecular subtype (astrocytoma vs. oligodendroglioma, HR, 3.151; P < 0.0001) were identified to be significant prognostic factors for the OS of patients (Table S2).
A multivariate analysis of the astrocytoma group demonstrated that EOR (non-GTR vs. GTR; HR, 3.955; P < 0.0001), preoperative KPS (≤ 80 vs. > 80; HR, 2.390; P = 0.006), MGMT (unmethylated vs. methylated; HR, 2.154; P = 0.008), and adjuvant therapy (no vs. yes; HR, 2.447; P = 0.023) were significant prognostic factors for OS. Non-GTR (HR, 5.180; P = 0.012) led to worse OS for oligodendrogliomas (Table 3).
Impact of EOR in different age groups
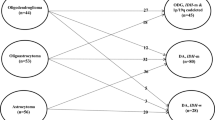
The role of EOR on survival was assessed in subgroups of WHO grade 3 IDH-mutant gliomas (Fig. 3) according to their clinical characteristics and molecular pathology. A cut-off point of 45 years was chosen as it revealed the greatest survival difference between groups. In the astrocytoma group (IDH mutant and 1p/19q non-co-deletion), patients with age ≤ 45 years had a higher survival benefit from GTR than from subtotal resection (P = 0.0133). In contrast, there was no difference in the OS between subtotal resection and GTR in the subgroup of patients with astrocytoma aged > 45 years (P = 0.2810). In the oligodendroglioma group, there was no difference in the OS between GTR and subtotal resection regarding age (age ≤ 45 years, P = 0.0558; age > 45 years, P = 0.9785).
Survival analysis of patients with WHO grade 3 IDH-mutant gliomas stratified by clinical characteristics and molecular pathology. GTR was identified to benefit patients aged ≤ 45 years with astrocytoma. No benefit of GTR was found for patients aged > 45 years with astrocytomas. Patients with oligodendrogliomas do not have a survival benefit from GTR regardless of age. GTR, gross total resection; STR, subtotal resection
Discussion
Currently, surgical resection is the preferred treatment for diffuse gliomas. Nevertheless, the impact of surgical resection to survival should be reevaluated in this era of molecular pathology, concerning the diagnosis and treatment of gliomas. Prior studies suggested that the prognostic value of EOR should be different for specific molecular subtypes of gliomas [2, 4, 27, 28], although the conclusion is not clear. This study aimed to investigate the impact of the EOR in patients with WHO grade 3 gliomas with IDH-mutant, while considering the molecular pathology and clinical characteristics. We found that the value of the EOR depended on molecular subtypes and clinical characteristics. Notably, EOR holds greater significance in astrocytomas compared to oligodendrogliomas, particularly for young patients. For younger patients diagnosed with astrocytoma, advocating more active surgical strategies and performing more extensive resections is recommended. However, for other groups, including older patients with astrocytoma and patients with oligodendroglioma, complete resection did not result in a prolonged OS. Consequently, relatively conservative surgical methods can be adopted in these cases, with a primary focus on preserving neurological function. This discovery can enable the strategy of pathology-guided surgery to be applied. Combined with molecular pathology considerations, the idea of maximum safe resection would be promoted for effective safe resection because extensive resections are not always beneficial to the survival time of patients with glioma.
Several studies have demonstrated that extensive surgical resection has a positive impact on the survival of patients with glioma. A greater EOR was associated with longer OS [20, 29,30,31,32]. This study revealed that the EOR is an independent prognostic factor for WHO grade 3 gliomas, even after adjusting for the molecular type. Specifically, this study identified a particularly strong positive prognostic value for GTR in astrocytomas. A previous study found that even a small postoperative residual tumor has a negative impact on OS in IDH-mutant astrocytoma, which advocates for a secondary operation in this subtype to remove minor residues if safe [27]. However, for oligodendrogliomas with 1p/19q co-deleted, increasing the EOR does not ensure prolonging the OS [4, 27]. This study further confirms the inconsistency of prognostic values for extensive surgical resection regarding the subtypes of gliomas.
Clinical characteristics (such as age at diagnosis, KPS) are also considered as significant prognostic factors for patients with gliomas [33,34,35,36]. A few studies focused on the interaction between molecular pathology and surgical resection. Notably, this study investigated the interaction effect between clinical characteristics, surgical resection, and molecular pathology. The molecular pathology was applied as the first-level classifier and the clinical characteristic (age) as the second-level classifier, and our analysis revealed that the prognostic benefits from surgical resection varied between specific clinicopathological subgroups in this study.
For patients with astrocytomas aged ≤ 45 years, GTR was associated with a longer OS and is therefore encouraged. However, for patients with astrocytoma aged > 45 years, a higher survival benefit was not identified with GTR than with subtotal resection. Considering the fact that extensive resection greatly increases the risk of postoperative neurological dysfunction, pursuing complete resection is not necessarily optimal for these cases, as preservation of neurological function should be primarily considered. For oligodendrogliomas, no prognostic value for GTR was found to prolong OS, even after age stratification. The absence of a strong association between GTR and OS in oligodendrogliomas might be explained by the indolent natural course of these tumors [37] and their sensitivity to radiotherapy and chemotherapy [38]. Patients with oligodendrogliomas therefore still have a long survival time even if complete resection is not achieved. Based on these results, there is no need to pursue the complete resection for all WHO grade 3 gliomas. Both clinical features and molecular pathology characteristics are important considerations in customized surgical planning.
To date, molecular pathology information can only be obtained days after surgery, limiting its value in surgical planning. However, artificial intelligence (AI) technology has made it possible to accurately predict the molecular biomarker status of gliomas before surgery using radiomics features [11,12,13,14,15,16]. Additionally, Raman spectroscopy technology obtains a highly accurate diagnosis for glioma intraoperatively [17]. This study proposes a possible new surgical strategy, based on evidence, that molecular pathology could be considered preoperatively to guide surgery. This study provides a practical solution for the dilemma regarding choosing between maximum resection and function preservation by updating the concept of maximum safe resection to the strategy of effective safe resection.
There are limitations to this study. The study is retrospective and it was collected from a database with limited clinical details. The lack of the status of CDKN2A/B homozygous deletion in this study cannot exclude some grade 4 astrocytomas. And due to the retrospective design, the sample sizes of each subgroup were unbalanced. A prospective clinical trial is therefore highly encouraged. Although molecular pathology information is still obtained after surgery, technologies for preoperative prediction and those for intraoperative detection are advancing. The results of this study may possibly contribute to realizing pathology-guided neurosurgery in the near future. Additionally, as information on the extent of resection was not provided in The Cancer Genome Atlas and other open accessed glioma databases, independent validation was not available in this study. Further testing of our findings in an independent dataset is needed in future studies.
Conclusions
This study found that younger (≤ 45 years old) patients with WHO grade 3 astrocytoma can benefit from extensive surgical resection, while others may not. Age and molecular pathology are critical factors influencing the surgical resection prognosis. Therefore, these factors should be thoroughly considered in the surgical planning for WHO grade 3 gliomas. Our findings provide guiding information to neurosurgeons while planning surgeries.
References
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY (2012) Primary brain tumours in adults. Lancet 379:1984–1996. https://doi.org/10.1016/s0140-6736(11)61346-9
Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, Shonka N, Gilbert MR, Sawaya R, Prabhu SS, Weinberg J, Lang FF, Aldape KD, Sulman EP, Rao G, McCutcheon IE, Cahill DP (2014) IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol 16:81–91. https://doi.org/10.1093/neuonc/not159
Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, Zhang Y, Phillips JJ, Shai A, Lafontaine M, Crane J, Chandra A, Flanigan P, Jahangiri A, Cioffi G, Ostrom Q, Anderson JE, Badve C, Barnholtz-Sloan J, Sloan AE, Erickson BJ, Decker PA, Kosel ML, LaChance D, Eckel-Passow J, Jenkins R, Villanueva-Meyer J, Rice T, Wrensch M, Wiencke JK, Oberheim Bush NA, Taylor J, Butowski N, Prados M, Clarke J, Chang S, Chang E, Aghi M, Theodosopoulos P, McDermott M, Berger MS (2020) Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced Tumor with Survival within Molecular Subgroups of patients with newly diagnosed Glioblastoma. JAMA Oncol 6:495–503. https://doi.org/10.1001/jamaoncol.2019.6143
Ding X, Wang Z, Chen D, Wang Y, Zhao Z, Sun C, Chen D, Tang C, Xiong J, Chen L, Yao Z, Liu Y, Wang X, Cahill DP, de Groot JF, Jiang T, Yao Y, Zhou L (2018) The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: a multicenter retrospective study. J Neurooncol 140:591–603. https://doi.org/10.1007/s11060-018-2985-3
Patel T, Bander ED, Venn RA, Powell T, Cederquist GY, Schaefer PM, Puchi LA, Akhmerov A, Ogilvie S, Reiner AS, Moussazadeh N, Tabar V (2018) The role of extent of Resection in IDH1 Wild-Type or Mutant Low-Grade Gliomas. Neurosurgery 82:808–814. https://doi.org/10.1093/neuros/nyx265
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Delev D, Heiland DH, Franco P, Reinacher P, Mader I, Staszewski O, Lassmann S, Grau S, Schnell O (2019) Surgical management of lower-grade glioma in the spotlight of the 2016 WHO classification system. J Neurooncol 141:223–233. https://doi.org/10.1007/s11060-018-03030-w
Koriyama S, Nitta M, Kobayashi T, Muragaki Y, Suzuki A, Maruyama T, Komori T, Masui K, Saito T, Yasuda T, Hosono J, Okamoto S, Shioyama T, Yamatani H, Kawamata T (2018) A surgical strategy for lower grade gliomas using intraoperative molecular diagnosis. Brain Tumor Pathol 35:159–167. https://doi.org/10.1007/s10014-018-0324-1
Ricard D, Kaloshi G, Amiel-Benouaich A, Lejeune J, Marie Y, Mandonnet E, Kujas M, Mokhtari K, Taillibert S, Laigle-Donadey F, Carpentier AF, Omuro A, Capelle L, Duffau H, Cornu P, Guillevin R, Sanson M, Hoang-Xuan K, Delattre JY (2007) Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol 61:484–490. https://doi.org/10.1002/ana.21125
Han Y, Xie Z, Zang Y, Zhang S, Gu D, Zhou M, Gevaert O, Wei J, Li C, Chen H, Du J, Liu Z, Dong D, Tian J, Zhou D (2018) Non-invasive genotype prediction of chromosome 1p/19q co-deletion by development and validation of an MRI-based radiomics signature in lower-grade gliomas. J Neurooncol 140:297–306. https://doi.org/10.1007/s11060-018-2953-y
Li Y, Liu X, Qian Z, Sun Z, Xu K, Wang K, Fan X, Zhang Z, Li S, Wang Y, Jiang T (2018) Genotype prediction of ATRX mutation in lower-grade gliomas using an MRI radiomics signature. Eur Radiol 28:2960–2968. https://doi.org/10.1007/s00330-017-5267-0
Li Y, Qian Z, Xu K, Wang K, Fan X, Li S, Jiang T, Liu X, Wang Y (2018) MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. Neuroimage Clin 17:306–311. https://doi.org/10.1016/j.nicl.2017.10.030
Kim M, Jung SY, Park JE, Jo Y, Park SY, Nam SJ, Kim JH, Kim HS (2020) Diffusion- and perfusion-weighted MRI radiomics model may predict isocitrate dehydrogenase (IDH) mutation and tumor aggressiveness in diffuse lower grade glioma. Eur Radiol 30:2142–2151. https://doi.org/10.1007/s00330-019-06548-3
Li ZC, Bai H, Sun Q, Li Q, Liu L, Zou Y, Chen Y, Liang C, Zheng H (2018) Multiregional radiomics features from multiparametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: a multicentre study. Eur Radiol 28:3640–3650. https://doi.org/10.1007/s00330-017-5302-1
Qian J, Herman MG, Brinkmann DH, Laack NN, Kemp BJ, Hunt CH, Lowe V, Pafundi DH (2020) Prediction of MGMT Status for Glioblastoma Patients using Radiomics feature extraction from (18)F-DOPA-PET imaging. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2020.06.073
Livermore LJ, Isabelle M, Bell IM, Scott C, Walsby-Tickle J, Gannon J, Plaha P, Vallance C, Ansorge O (2019) Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neurooncol Adv 1:vdz008. https://doi.org/10.1093/noajnl/vdz008
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neuro Oncol. https://doi.org/10.1093/neuonc/noab106
Zhao Z, Zhang KN, Wang Q, Li G, Zeng F, Zhang Y, Wu F, Chai R, Wang Z, Zhang C, Zhang W, Bao Z, Jiang T (2021) Chinese glioma genome Atlas (CGGA): a Comprehensive Resource with functional genomic data from chinese gliomas. Genomics Proteom Bioinf. https://doi.org/10.1016/j.gpb.2020.10.005
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Salvati M, Pichierri A, Piccirilli M, Floriana Brunetto GM, D’Elia A, Artizzu S, Santoro F, Arcella A, Giangaspero F, Frati A, Simione L, Santoro A (2012) Extent of tumor removal and molecular markers in cerebral glioblastoma: a combined prognostic factors study in a surgical series of 105 patients. J Neurosurg 117:204–211. https://doi.org/10.3171/2012.4.JNS101702
Choi J, Kim SH, Ahn SS, Choi HJ, Yoon HI, Cho JH, Roh TH, Kang SG, Chang JH, Suh CO (2020) Extent of resection and molecular pathologic subtype are potent prognostic factors of adult WHO grade II glioma. Sci Rep 10:2086. https://doi.org/10.1038/s41598-020-59089-x
Motomura K, Chalise L, Ohka F, Aoki K, Tanahashi K, Hirano M, Nishikawa T, Yamaguchi J, Shimizu H, Wakabayashi T, Saito R (2021) Impact of the extent of resection on the survival of patients with grade II and III gliomas using awake brain mapping. J Neurooncol. https://doi.org/10.1007/s11060-021-03776-w
Wei Y, Wei Z, Gan Y, Zhaoshi B, Yongzhi W, Yanwei L, Chunsheng K, Yongping Y, Lei W, Tao J (2012) Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PLoS ONE 7:e30339. https://doi.org/10.1371/journal.pone.0030339
Hu H, Wang Z, Liu Y, Zhang C, Li M, Zhang W, Wang K, Cai J, Cheng W, Huang H, Jiang T (2015) Genome-wide transcriptional analyses of chinese patients reveal cell migration is attenuated in IDH1-mutant glioblastomas. Cancer Lett 357:566–574. https://doi.org/10.1016/j.canlet.2014.12.018
Wang K, Wang Y, Fan X, Li Y, Liu X, Wang J, Ai L, Dai J, Jiang T (2018) Regional specificity of 1p/19q co-deletion combined with radiological features for predicting the survival outcomes of anaplastic oligodendroglial tumor patients. J Neurooncol 136:523–531. https://doi.org/10.1007/s11060-017-2673-8
Wijnenga MMJ, French PJ, Dubbink HJ, Dinjens WNM, Atmodimedjo PN, Kros JM, Smits M, Gahrmann R, Rutten G-J, Verheul JB, Fleischeuer R, Dirven CMF, Vincent AJPE, van den Bent MJ (2018) The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neurooncology 20:103–112. https://doi.org/10.1093/neuonc/nox176
Kawaguchi T, Sonoda Y, Shibahara I, Saito R, Kanamori M, Kumabe T, Tominaga T (2016) Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co-deletion. J Neurooncol 129:505–514. https://doi.org/10.1007/s11060-016-2201-2
Sankar T, Moore NZ, Johnson J, Ashby LS, Scheck AC, Shapiro WR, Smith KA, Spetzler RF, Preul MC (2012) Magnetic resonance imaging volumetric assessment of the extent of contrast enhancement and resection in oligodendroglial tumors. J Neurosurg 116:1172–1181. https://doi.org/10.3171/2012.2.JNS102032
Pessina F, Navarria P, Cozzi L, Ascolese AM, Simonelli M, Santoro A, Tomatis S, Riva M, Fava E, Scorsetti M, Bello L (2016) Value of Surgical Resection in patients with newly diagnosed Grade III glioma treated in a Multimodal Approach: surgery, chemotherapy and Radiotherapy. Ann Surg Oncol 23:3040–3046. https://doi.org/10.1245/s10434-016-5222-3
Almenawer SA, Badhiwala JH, Alhazzani W, Greenspoon J, Farrokhyar F, Yarascavitch B, Algird A, Kachur E, Cenic A, Sharieff W, Klurfan P, Gunnarsson T, Ajani O, Reddy K, Singh SK, Murty NK (2015) Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro Oncol 17:868–881. https://doi.org/10.1093/neuonc/nou349
Snyder LA, Wolf AB, Oppenlander ME, Bina R, Wilson JR, Ashby L, Brachman D, Coons SW, Spetzler RF, Sanai N (2014) The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J Neurosurg 120:309–314. https://doi.org/10.3171/2013.10.JNS13368
Garton ALA, Kinslow CJ, Rae AI, Mehta A, Pannullo SC, Magge RS, Ramakrishna R, McKhann GM, Sisti MB, Bruce JN, Canoll P, Cheng SK, Sonabend AM, Wang TJC (2020) Extent of resection, molecular signature, and survival in 1p19q-codeleted gliomas. J Neurosurg 1–11. https://doi.org/10.3171/2020.2.JNS192767
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Gorlia T, Delattre JY, Brandes AA, Kros JM, Taphoorn MJ, Kouwenhoven MC, Bernsen HJ, Frenay M, Tijssen CC, Lacombe D, van den Bent MJ (2013) New clinical, pathological and molecular prognostic models and calculators in patients with locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma. A prognostic factor analysis of european Organisation for Research and Treatment of Cancer Brain Tumour Group Study 26951. Eur J Cancer 49:3477–3485. https://doi.org/10.1016/j.ejca.2013.06.039
Hong JB, Roh TH, Kang SG, Kim SH, Moon JH, Kim EH, Ahn SS, Choi HJ, Cho J, Suh CO, Chang JH (2020) Survival, prognostic factors, and volumetric analysis of extent of resection for anaplastic gliomas. Cancer Res Treat 52:1041–1049. https://doi.org/10.4143/crt.2020.057
Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O'Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T, Jr., Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Yang L, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG, Jr., Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, McLendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ, Zhang J (2015) Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med 372: 2481–2498. https://doi.org/10.1056/NEJMoa1402121
Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, Buckner JC, Fink KL, Souhami L, Laperriere NJ, Huse JT, Mehta MP, Curran WJ (2014) Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin oncology: official J Am Soc Clin Oncol 32:783–790. https://doi.org/10.1200/jco.2013.49.3726
Acknowledgements
We would like to acknowledge Dr. Guanzhang Li and Dr. Zheng Zhao for their expert assistance in data acquisition and management.
Funding
National Natural Science Foundation of China (grant number: 82072786); Beijing Medical Development Project (grant number: JYY2019-5).
Author information
Authors and Affiliations
Contributions
Conception and design: T.J. and Y.W. Data collection, analysis, and interpretation: Z.H., H.J., K.Z., Z.Y., X.L. and S.F. Manuscript writing: Z.H. and Y.W. Final approval of manuscript: Z.H., K.Z., X.L., S.F., T.J. and Y.W. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors indicated no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ziming Hou and Jie Hu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, Z., Hu, J., Liu, X. et al. Decision system for extent of resection in WHO grade 3 gliomas: a Chinese Glioma Genome Atlas database analysis. J Neurooncol 164, 461–471 (2023). https://doi.org/10.1007/s11060-023-04420-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04420-5