Abstract
Aim
This study aimed to evaluate the clinical benefits of systemic therapy (ST) combined with stereotactic radiosurgery (SRS) for brain metastases (BM).
Methods
The patient data were extracted from the institutional disease database from 2016 to 2021. Surgical and whole-brain radiotherapy cases and poor Karnofsky performance status (KPS < 70) were excluded. The eligible patients were divided into monotherapy (SRS alone or ST alone) and combined therapy (SRS and ST, combined within a month). Univariate and multivariate Cox proportional hazards analyses were used to examine factors associated with increased risk of death and intracranial progression. The propensity score for selecting treatment was calculated based on existing prognostic covariates. Two groups were matched 1:1 and compared for intracranial progression-free survival (PFS) and overall survival (OS).
Results
We identified 1605 patients and analyzed 928 (monotherapy: n = 494, combined therapy: n = 434). In a multivariable model, the combined therapy was independently associated with improved PFS and OS relative to the monotherapy. At the median follow-up of 383 days in the matched dataset, the combined therapy group showed significantly longer PFS (median, 7.4 vs. 5.0 months, P < 0.001) and OS (median, 23.1 vs. 17.2 months, P = 0.036) than the monotherapy group. The overall intracranial progression and mortality risk was reduced in the combined therapy group, with an estimated HR of 0.70 and 0.78.
Conclusions
Combined therapy exhibited longer PFS and OS than monotherapy in BM patients. The results support the recent trend toward combining systemic and local therapies, encouraging future clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy (RT) and surgery have been established as effective local therapies in adults with brain metastases (BM), but the benefit of systemic therapy (ST) to the central nervous system (CNS) is limited. The ASCO-SNO-ASTRO guideline recommends that symptomatic BM patients should be offered local therapy regardless of systemic regimens (chemotherapy, immunotherapy, and targeted therapy) used for the systemic disease [1]. Local therapy may be delayed or skipped in unsuitable patients (e.g., Karnofsky Performance Status [KPS] < 70 and no ST options or KPS ≤ 50) or patients with a chance to receive an established ST regimen [1,2,3]. BM from EGFR-, ALK-mutant non-small-cell lung cancer (NSCLC), HER2-positive breast cancer, or melanoma may be offered specific targeted agents such as Osimertinib, Alectinib, the combination of Tucatinib, Trastuzumab, and Capecitabin, or Ipilimumab plus Nivolumab [1,2,3,4,5,6,7,8,9,10,11,12]. A new generation of CNS-active targeted therapy (CNS-Tx) or immune checkpoint inhibitors (ICI) has emerged in multiple clinical trials, including BM patients [1,2,3,4,5,6,7,8, 13,14,15,16,17,18,19]. Unlike the older generation of targeted therapy or chemotherapy, it has produced many promising results.
No clear answer is whether ST should be combined with local therapy when considering treatment options for BM. However, most traditional chemotherapeutic drugs have limited CNS activity and are usually combined with local therapy [20, 21]. In two small randomized controlled trials (78 patients and 55 patients), the combination of whole-brain RT (WBRT) with temozolomide improved progression-free survival (PFS) in a population of BM patients not specific to a single cancer type [22, 23]. In Gamboa-Vignolle, et al., overall survival (OS) was also significantly improved [23]. Regardless of its CNS activity and evidence, various systemic regimens for systemic diseases may be used before or after local therapy in clinical practice. Several recent reports have shown improved intracranial control and overall survival when SRS is combined with targeted therapy and immunotherapy [4, 24, 25]. Unfortunately, such reports are limited to retrospective observation with a small sample size, and differences in the patient background may have affected the results. In addition, although there has been much interest in the optimal sequencing of SRS and ST, the benefit of such a sequential or concurrent ST combination, including the optimal sequence, is still unclear [26, 27]. This study aims to evaluate the clinical benefits of SRS and ST combination therapy compared to SRS or ST monotherapy in BM patients. Also, a secondary analysis was performed to explore whether the benefits differ in the order of ST and RT treatment (upfront RT or upfront ST) in the combined therapy.
Methods
Study design, patient selection, and endpoint
This study is a retrospective, single-institutional study. Our institutional review board approved this study, and informed consent was obtained using the opt-out form that participants are included in this study unless they give their express decision to be excluded. All patient data for “brain metastases” or “leptomeningeal disease,” including suspicion, were extracted from the institutional disease database from 2016 to 2021. All research was performed following the relevant guidelines and regulations. Patients met the following eligibility and exclusion criteria analyzed in this study. The eligibility criteria are: (1) diagnosed with computed tomography (CT) or magnetic resonance imaging (MRI), not only physical examination or just suspicion, and (2) treated the intracranial disease in 2016–2021. The exclusion criteria are: (1) treated with surgery or WBRT in the past or at this time, (2) KPS < 70 at BM diagnosis. The patient follow-up for this study ended on May 31, 2022, and patients who were alive or lost follow-up at this time were censored.
The following information was collected from eligible patients; age at BM diagnosis, gender, type of cancer, KPS at BM diagnosis, number of BM lesions, and extracranial metastases (ECM). In addition, some driver mutations (EGFR, ALK, and others) and programmed death-ligand 1 (PD-L1) status of lung adenocarcinoma, HER2 status of breast cancer, and BRAF status of melanoma were also collected. PD-L1 expression ≥ 1% was considered positive (1–49% and 50–100%). BM progression was distinguished from adverse radiation effects (pseudoprogression) following the Response Assessment in Neuro-Oncology Brain Metastases criteria [28]. Intracranial PFS was measured from the BM diagnosis to death or BM progression. OS was calculated from the BM diagnosis to death.
This study compared combined therapy as an experimental and monotherapy as the standard of care. The primary endpoint was defined as PFS in the propensity score matching (PSM) dataset, and OS was the secondary endpoint. In addition, treatment timing between ST and RT was also specified and compared as a subgroup analysis: ST followed by RT (STRT, upfront ST) or RT followed by ST within a month (RTST, upfront RT).
Systemic regimens classification
We collected information about the ST regimen initiated after the BM diagnosis to treat the BM. According to CNS efficacy, the regimens were classified into the following four types: ICI, CNS-Tx, non-CNS targeted therapy (non-CNS-Tx), and conventional non-CNS chemotherapy (non-CNS-Cx). The details of the regimens are described in Table S1. The CNS-Tx includes new-generation anti-EGFR/ALK/HER2 regimens from previous randomized controlled trials and guidelines (Osimertinib and other third-generation TKIs, anti-ALK therapy, and the following anti-HER2 therapies: Lapatinib, Neratinib, Trastuzumab emtansine, Trastuzumab deruxtecan, Tucatinib) [1,2,3,4,5,6,7,8, 13,14,15,16,17,18,19].
Statistical analysis
We used multivariable logistic regression to estimate the propensity score for selecting BM treatment based on the following covariates: the disease-specific graded prognostic assessment (GPA) score, age, KPS, the number of BM, extracranial metastases (ECM), cancer type, HER2 in breast cancer, EGFR/ALK and PDL1 in lung adenocarcinoma (NSCLC-Ad). Types of ST regimens were considered as a covariate in subgroup analyses. PSM was performed in 1:1 matching without replacement, using calipers of a width equal to 0.2 of the standard deviation of the logit of the propensity score [29, 30]. The kernel density estimation curves were constructed to compare the propensity scores’ homogeneity.
GPA is an established tool for each cancer type to estimate the survival time from BM diagnosis to death [31,32,33]. GPA has been covered with lung (NSCLC-Ad, NSCLC-non-Ad, SCLC), breast, gastrointestinal (GI), kidney, and melanoma, but not all cancer types. The basic structure of the GPA consists of age, the number of BM, KPS, and ECM. The GPA score is 0–4.0 in 0.5-point increments, with 4.0 representing the group with the best prognosis. EGFR/ALK and PDL1 status in NSCLC-Ad were considered in the latest version [29]. In addition, HER2 status in breast cancer and BRAF status in melanoma are also considered. The cancer types in this study are classified based on GPA, but renal cell carcinoma (n = 6) and melanoma (n = 13) are rare in the raw dataset and are grouped as uncommon diseases at this time.
We compared the patient characteristics in both groups with the chi-squared test for categorical variables and analysis of variance for continuous variables. Survival analysis was performed using the Log-rank test, and survival curves were constructed using the Kaplan–Meier method. The assumption of the Cox proportional hazards between the groups (monotherapy versus combined therapy) was assessed visually using survival curves and Schoenfeld residuals plot, which did not indicate any significant violation. Univariate and multivariate Cox proportional hazards analyses were used to examine factors associated with increased risk of death and BM progression. We used R software version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria) for all statistical analyses. Statistical significance was set as P < 0.05, and all tests were 2-tailed.
Results
Dataset
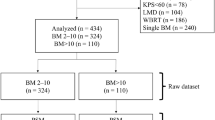
We identified 1605 patients from the institutional disease database from 2016 to 2021, and 928 met the eligibility criteria: 494 in the monotherapy group and 434 in the combined therapy group (Fig. 1). A total of 677 were excluded (Surgery in 238, WBRT in 198, KPS < 70 in 241). Table 1 shows the patient characteristics in the raw and matched datasets. Between the two groups in the raw dataset, the monotherapy group tends to have a worse GPA score (P = 0.007), worse KPS (P < 0.001), more likely LMD (P < 0.001), more BM numbers (P = 0.024), less lung cancer (P < 0.001), and less PD-L1 expression (P = 0.007). Newly diagnosed BM included 235 patients in the monotherapy group and 198 in the combined therapy group (P = 0.60). SRS was used in 625 cases (67.3%) and ST in 737 patients (79.4%).
Based on the propensity score, 710 patients were 1:1 matched between the monotherapy and combined therapy groups (355 in each group). The propensity score distributions between the two groups in the raw and matched datasets are shown in Figure S1. The kernel density estimation shows an initial dissimilarity across the two groups. After PSM, the propensity score distributions were relatively homogenous and better balanced. As shown in Table 1, covariates were well-balanced, with no significant differences in the groups.
Survival analysis in the matched dataset
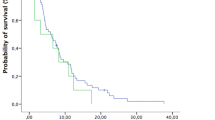
At the end of the last follow-up date, 511 death and 758 disease progression were observed. The median follow-up period for OS was 383 days in the matched dataset. After controlling for significant covariables in a multivariable model, including sex, GPA, KPS, age, ECM, number of BM, recurrent status, cancer type, HER2, and EGFR/ALK mutation, the combined therapy was independently associated with better PFS relative to the monotherapy (adjusted hazard ratio, 0.70; 95% CI, 0.59 to 0.83; P < 0.001; Table 2). Similarly, combined therapy was independently associated with better OS versus monotherapy (adjusted hazard ratio, 0.78; 95% CI, 0.60 to 0.92; P = 0.0019; Table 3). Figure 2 shows PFS and OS estimated by the Kaplan–Meier method. PFS for patients in the combined therapy group was significantly longer than those in the monotherapy group (median PFS, 7.4 months vs. 5.0 months, P < 0.001, Fig. 2A). OS for patients in the combined therapy group was also significantly longer than those in the monotherapy group (median OS, 23.1 months vs. 17.2 months, P = 0.036, Fig. 2B). Similarly, in the raw dataset, the combined therapy group showed better PFS (7.2 months vs. 5.0 months, P < 0.001) and OS (24.3 months vs. 16.1 months, P < 0.001) than the monotherapy group.
Subgroup analysis
Two groups exist in the combination therapy group: upfront RT (RTST) and upfront ST (STRT). Therefore, we compared newly-matched STRT and RTST groups to explore the optimal treatment order. The new propensity score calculation included ST regimens regarding CNS activity. Table S2 shows the patient backgrounds for STRT and RTST in the matched dataset. Based on the propensity score, 434 patients were newly matched between the RTST and STRT groups (134 in each group, Table S2). Figure S2 shows PFS and OS estimated by the Kaplan–Meier method. PFS for patients in the RTST group was significantly longer than those in the STRT group (median PFS, 8.3 months vs. 6.3 months, P = 0.038, Figure S2A). However, OS for patients in both groups was not significantly different (median OS, 23.6 months vs. 24.8 months, P = 0.35, Figure S2B).
Discussion
This study compared RT or ST alone with combined therapy in BM patients, aiming to evaluate combined therapy’s clinical benefits. The strength of this study is that it examined a large BM population of 928 patients. Surgical cases were excluded because the indications for treatment are limited compared to radiation therapy as a local therapy (e.g., number and location of BM, the possibility of general anesthesia), which could complicate the considerations. Furthermore, patients with KPS < 70 were excluded because they frequently had difficulty undergoing local therapy or ST [1,2,3]. However, it was not limited to any cancer type not to lose external validity in the clinical setting. Also, the study was limited to BM cases diagnosed in the recent five years, considering driver mutations and PDL1 status, which are included in the latest predictive models [31, 32]. In addition, the study compared 355 patients in each group who were adjusted by propensity score matching for background factors related to clinical importance and prognosis. Based on the previous literature review, CNS-active agents were limited; Temozolomide having known to be effective [22, 23], not specific to cancer type, and some immunotherapy or targeted agents for NSCLC-ad, breast cancer, and melanoma [1, 4,5,6,7,8,9,10,11,12]. The proportion of BM patients treated with ST has been unclear. However, in the present study population, 80% of patients were treated with ST alone or in combination with RT. Although the lack of adequate evidence for ST alone or in combination with BM patients, this study demonstrated the use of ST is not uncommon in clinical practice.
The results of this study show that PFS and OS in the combination therapy group were significantly better than those in the monotherapy group in both the raw and matched datasets. In a multivariable model, the combined therapy was independently associated with better PFS and OS (Table 2–3). Although the MST is higher than previously reported, we believe this is not surprising since our cohort includes NSCLC-ad in 65% of the data set. For example, in the latest report on GPA in lung cancer, the MST for NSCLC-ad was 17 months and 30–52 months on a better GPA population (GPA > 2.0). The propensity scores of the two groups were calculated based on multiple prognostic factors; as shown in Figure S1, PSM successfully homogenized the patient characteristics in both groups. In the combined therapy group, ST was initiated no later than a month after RT and was not limited to those CNS-activity. ECM was detected in 90% of the raw data set, suggesting that ST may have been mainly aimed at treating systemic disease. It is considered that prompt ST from BM diagnosis may reduce intracranial progression and death. Randomized controlled trials are desirable to determine the benefit of combination therapy. Still, given the lack of evidence for ST in BM patients, this study may contribute to the selection of BM therapy. Larger ongoing clinical trials compared with ST alone and ST plus SRS will assist in further addressing these endpoints. Osimertinib, Nivolumab, and other ICI in NSCLC-ad (NCT03497767, NCT03769103, NCT02978404), Ipilimumab and Nivolumab in melanoma (ABC-X: NCT03340129), and Pembrolizumab, Abemaciclib, and T-DM1 in breast cancer (NCT03449238, NCT04923542, NCT03190967) are ongoing clinical trials compared to SRS combined therapy in BM patients.
A new matching between the RTST and STRT groups was performed as a subgroup analysis in the combination therapy group, which had better PFS and OS in the primary analysis. Results showed that PFS was better in the RTST group, but no significant difference in OS existed. Because both groups included drugs with differential CNS activity as ST content, ST regimens were also included as an adjustment factor; as shown in Table S1, 40% of the analyzed population was treated with ICIs or CNS-targeted therapies. Even though PSM could adjust patient background between the two groups, how patients were selected for any treatment modality was not random. For patients with EGFR/ALK-positive NSCLC-Ad and HER2-positive breast cancer, there are options to delay local therapy with new generation EGFR-TKIs and Anit-HER2 therapy, and the content of ST is considered to have a significant impact on treatment choice. The optimal sequence of combining targeted therapies or immune checkpoint inhibitors (ICI) with RT is still unknown, with conflicting published results [20]. Upfront ST has sometimes been preferred to avoid delays in ST for systemic disease or by the expectation of its high CNS activity enough to control intracranial lesion progression [4,5,6,7]. Conceptually, however, upfront SRS can disrupt the blood–brain barrier and improve the CNS penetration of targeted drugs [34], or impaired tumor cells generate new antigens to evoke the immune response, causing an abscopal effect outside the irradiated field [35, 36]. Magnuson et al. compared upfront RT, upfront erlotinib, and erlotinib alone for NSCLC-Ad. Upfront RT showed the most prolonged OS in this study [4]. In addition, one study reported that in melanoma BM patients, upfront ipilimumab had a higher partial response rate than upfront RT [37]. However, the results from another report conflicted that upfront RT had better survival and intracranial control rates than upfront ICI [38]. In their systematic review, Yang et al. found that the ICI’s combination timing is defined within two weeks or one month of SRS in most previous studies [39]. The combinational approach of SRS plus ICI was supported by many retrospective data [20, 21, 38, 40]. But the timing and sequencing of ST and RT should be evaluated urgently through ongoing or future prospective studies. A randomized trial of SRS timing with ICI in patients with untreated BM from NSCLC (NCT04650490) is ongoing and will assist in further addressing the optimal sequence.
The limitations of this study are as follows: This study included a patient population from a single institution, which may have low external validity due to inter-institutional imbalances in patient backgrounds. We identified all the institutional patients in this study to reduce potential patient selection bias. Next, the result of this study may be confounded by other unobserved variables. Although we used a strict statistical method to adjust for baseline characteristics between the groups with PSM, such unobserved confounders could be unbalanced across the groups, possibly affecting the survival difference. Therefore, our results should be interpreted carefully, especially the details of RT and ST and their selection process (e.g., WBRT or SRS/SRT, what drug was used, and the dose of each treatment) were not considered. Even though PSM could adjust patient background between the two groups, how patients were selected for any treatment modality was not random. There may be an immortal time bias that monotherapy patients were given the option to delay ST. It is unclear whether the monotherapy patients initially selected it or whether the combination was originally planned but was withdrawn. In addition, the analysis did not consider the potential toxicity and quality of life impacts of BM treatment. Furthermore, a new treatment is added to each BM diagnosis in case of repeated disease progression, and repeated cases are counted as duplicates.
In conclusion, RT and ST combined therapy for BM demonstrated the potential for PFS and OS improvement compared to RT or ST alone on our propensity score-matching dataset. The results support the recent trend toward combining systemic and local therapies and encourage future randomized controlled trials to investigate the optimal RT and treatment for each cancer type.
Data availability
Research data are stored in an institutional repository and anonymized numerical data will be shared upon request to the corresponding author. Research image data are not available at this time.
References
Vogelbaum MA, Brown PD, Messersmith H et al (2021) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. https://doi.org/10.1200/JCO.21.02314
Gondi V, Bauman G, Bradfield L et al (2022) Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. https://doi.org/10.1016/j.prro.2022.02.003
Ramakrishna N, Anders CK, Lin NU et al (2022) Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol 40:2636–2655. https://doi.org/10.1200/JCO.22.00520
Magnuson WJ, Lester-Coll NH, Wu AJ et al (2017) Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 35:1070–1077. https://doi.org/10.1200/JCO.2016.69.7144
Dai L, Luo C-Y, Hu G-X et al (2020) Comparative analysis of first-line treatment regimens for advanced EGFR-mutant non-small cell lung cancer patients with stable brain metastases. Ann Palliat Med 9:2062–2071. https://doi.org/10.21037/apm-20-1136
Reungwetwattana T, Nakagawa K, Cho BC et al (2018) CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2018.78.3118
Zhang Z, Guo H, Lu Y et al (2019) Anaplastic lymphoma kinase inhibitors in non-small cell lung cancer patients with brain metastases a meta-analysis. J Thorac Dis 11:1397–1409. https://doi.org/10.21037/jtd.2019.03.76
Murthy RK, Loi S, Okines A et al (2020) Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 382:597–609. https://doi.org/10.1056/NEJMoa1914609
Gori S, Puglisi F, Moroso S et al (2019) The HERBA study: a retrospective multi-institutional Italian study on patients with brain metastases from HER2-positive breast cancer. Clin Breast Cancer 19:e501–e510. https://doi.org/10.1016/j.clbc.2019.05.006
Lin NU, Pegram M, Sahebjam S et al (2021) Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J Clin Oncol 39:2667–2675. https://doi.org/10.1200/JCO.20.02822
Bailleux C, Eberst L, Bachelot T (2021) Treatment strategies for breast cancer brain metastases. Br J Cancer 124:142–155. https://doi.org/10.1038/s41416-020-01175-y
Long GV, Atkinson V, Lo S et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 5877:579. https://doi.org/10.1016/S1470-2045(18)30139-6
Zhou Y, Wang B, Qu J et al (2020) Survival outcomes and symptomatic central nervous system (CNS) metastasis in EGFR-mutant advanced non-small cell lung cancer without baseline CNS metastasis: osimertinib vs. first-generation EGFR tyrosine kinase inhibitors. Lung Cancer 150:178–185. https://doi.org/10.1016/j.lungcan.2020.10.018
Petrelli F, Ghidini M, Lonati V et al (2017) The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer 84:141–148. https://doi.org/10.1016/j.ejca.2017.07.024
Bachelot T, Romieu G, Campone M et al (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14:64–71. https://doi.org/10.1016/S1470-2045(12)70432-1
Saura C, Oliveira M, Feng Y-H et al (2020) Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol 38:3138–3149. https://doi.org/10.1200/JCO.20.00147
Lin NU, Borges V, Anders C et al (2020) Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol 38:2610–2619. https://doi.org/10.1200/JCO.20.00775
Krop IE, Lin NU, Blackwell K et al (2015) Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 26:113–119. https://doi.org/10.1093/annonc/mdu486
Modi S, Saura C, Yamashita T et al (2020) Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610–621. https://doi.org/10.1056/NEJMoa1914510
Tonse R, Tom MC, Mehta MP et al (2021) Integration of systemic therapy and stereotactic radiosurgery for brain metastases. Cancers. https://doi.org/10.3390/cancers13153682
Suh JH, Kotecha R, Chao ST et al (2020) Current approaches to the management of brain metastases. Nat Rev Clin Oncol 17:279–299. https://doi.org/10.1038/s41571-019-0320-3
Gamboa-Vignolle C, Ferrari-Carballo T, Arrieta Ó, Mohar A (2012) Whole-brain irradiation with concomitant daily fixed-dose temozolomide for brain metastases treatment: a randomised phase II trial. Radiother Oncol 102:187–191. https://doi.org/10.1016/j.radonc.2011.12.004
Liu H-P, Zheng K-B, Wang J-W (2017) Efficacy and safety of temozolomide plus whole-brain radiotherapy in the treatment of intracranial metastases. J Cancer Res Ther 13:785. https://doi.org/10.4103/jcrt.JCRT_323_17
Schapira E, Hubbeling H, Yeap BY et al (2018) Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiati Oncol*Biol*Phys 101:624–629. https://doi.org/10.1016/j.ijrobp.2018.02.175
Borius P-Y, Régis J, Carpentier A et al (2021) Safety of radiosurgery concurrent with systemic therapy (chemotherapy, targeted therapy, and/or immunotherapy) in brain metastases: a systematic review. Cancer Metastasis Rev 40:341–354. https://doi.org/10.1007/s10555-020-09949-9
Ramakrishna R, Formenti S (2019) Radiosurgery and immunotherapy in the treatment of brain metastases. World Neurosurg 130:615–622. https://doi.org/10.1016/j.wneu.2019.04.032
Kraft J, van Timmeren JE, Frei S et al (2022) Comprehensive summary and retrospective evaluation of prognostic scores for patients with newly diagnosed brain metastases treated with upfront radiosurgery in a modern patient collective. Radiother Oncol. https://doi.org/10.1016/j.radonc.2022.04.024
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Zhu J, Sharma DB, Gray SW et al (2012) Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA 307:1593–1601. https://doi.org/10.1001/jama.2012.454
Takekuma M, Takahashi F, Mabuchi S et al (2020) Propensity score-matched analysis of systemic chemotherapy versus salvage hysterectomy for persistent cervical cancer after definitive radiotherapy/concurrent chemoradiotherapy. BMC Cancer 20:1169. https://doi.org/10.1186/s12885-020-07672-w
Sperduto PW, De B, Li J et al (2022) Graded prognostic assessment (GPA) for patients with lung cancer and brain metastases: initial report of the small cell lung cancer GPA and update of the non-small cell lung cancer GPA including the effect of programmed death ligand 1 and other prognostic factors. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2022.03.020
Sperduto PW, Mesko S, Li J et al (2020) Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of THE eligibility quotient. J Clin Oncol 38:3773–3784. https://doi.org/10.1200/JCO.20.01255
Patrikidou A, Chaigneau L, Isambert N et al (2020) Development of a disease-specific graded prognostic assessment index for the management of sarcoma patients with brain metastases (Sarcoma-GPA). BMC Cancer 20:117. https://doi.org/10.1186/s12885-020-6548-6
Geraud A, Xu HP, Beuzeboc P, Kirova YM (2017) Preliminary experience of the concurrent use of radiosurgery and T-DM1 for brain metastases in HER2-positive metastatic breast cancer. J Neurooncol 131:69–72. https://doi.org/10.1007/s11060-016-2265-z
Hatten SJ Jr, Lehrer EJ, Liao J et al (2022) A patient-level data meta-analysis of the abscopal effect. Adv Radiat Oncol 7:100909. https://doi.org/10.1016/j.adro.2022.100909
Lin X, Lu T, Xie Z et al (2019) Extracranial abscopal effect induced by combining immunotherapy with brain radiotherapy in a patient with lung adenocarcinoma: a case report and literature review. Thorac Cancer 10:1272–1275. https://doi.org/10.1111/1759-7714.13048
Silk AW, Bassetti MF, West BT et al (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2:899–906. https://doi.org/10.1002/cam4.140
Kotecha R, Kim JM, Miller JA et al (2019) The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol 21:1060–1068. https://doi.org/10.1093/neuonc/noz046
Yang Y, Deng L, Yang Y et al (2022) Efficacy and safety of combined brain radiotherapy and immunotherapy in non-small-cell lung cancer with brain metastases: a systematic review and meta-analysis. Clin Lung Cancer 23:95–107. https://doi.org/10.1016/j.cllc.2021.06.009
Chen L, Douglass J, Kleinberg L et al (2018) Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys 100:916–925. https://doi.org/10.1016/j.ijrobp.2017.11.041
Acknowledgements
The authors thank all the patients, investigators, and institutions involved in this study.
Funding
YK has received research funding from JSPS (Grant Number 20K16402), Aichi Cancer Research Foundation, and The Hori Science and Arts Foundation.
Author information
Authors and Affiliations
Contributions
YK collected the data and wrote the main manuscript. NN, RM, TK, TA, HS, HT, TK collected the data and supervised the study. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
YK has received research funding from JSPS, Aichi Cancer Research Foundation, and The Hori Science and Arts Foundation. TK has speaker bureau from Hitachi Co., Bristle Myers Squibb., Accuray Co., Elekta Co., Ono Pharmaceutical Co., AstraZeneca Co., Taiho Pharmaceutical Co., Canon Co., and Janssen Pharmaceutical Co.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koide, Y., Nagai, N., Miyauchi, R. et al. Radiotherapy or systemic therapy versus combined therapy in patients with brain metastases: a propensity-score matched study. J Neurooncol 160, 191–200 (2022). https://doi.org/10.1007/s11060-022-04132-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04132-2