Abstract
Purpose
Given recently suggested utility of hypofractionated stereotactic radiosurgery (SRS) in treating large brain metastases (BMs) > 3 cm, we sought to prospectively control tumor size variable to investigate the efficacy and safety of hypofractionated SRS for medium-sized BMs (2.5 to 3 cm) compared with single-fraction SRS.
Methods
Between 2011 and 2015, a total of 100 patients with newly diagnosed BMs (n = 105) of 2.5 to 3 cm had been treated with either single-fraction (n = 67; median dose 20 Gy) or hypofractionated SRS (n = 38; median cumulative dose 35 Gy in 5 daily fractions). No patients received any prior or upfront whole brain radiotherapy. In each patient, treatment outcome was measured by local tumor control (LTC), overall and progression-free survival (OS and PFS), and the occurrence of radiation necrosis (RN).
Results
With a median follow-up of 14 months, significant differences were observed between the single-fraction versus hypofractionated SRS groups in the incidence of RN (29.9% vs. 5.3%, P < 0.001) and LTC (1-year LTC rates 66.6% vs. 92.4%, P = 0.028). There were no differences in PFS (median 6 months vs. 6 months, P = 0.381) and OS (median 13 months vs. 18 months, P = 0.239). Treatment-related adverse events ( ≥ grade 2 toxicity by CTCAE ver. 4.0) occurred more frequently in single-fraction group, although the difference did not reach statistical significance (56.3% vs. 36.1%, P = 0.084).
Conclusions
Our results suggest a better safety and efficacy profile of hypofractionated SRS for medium-sized BMs compared with single-fraction SRS. Further prospective studies are needed to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) are drawing greater attention in the field of neuro-oncology in terms of patient quality of life as well as survival with their growing incidence and advanced cancer therapeutics. Treatment options include radiation treatment such as stereotactic radiosurgery (SRS) or whole brain radiotherapy (WBRT), surgery, and pharmacotherapy such as targeted agents. SRS has been expanding its role in the treatment of BMs over the past decades [1], which employs highly conformal dose of radiation to the target typically delivered in a single fraction. It is usually indicated for tumors less than 2 to 3 cm in diameter, because the risk of radiation toxicity such as radiation necrosis (RN) substantially increases as the tumor size increases thereover [1,2,3,4]. For larger tumors, radiation dose to tumor should be reduced to maintain the risk of radiation toxicity, which may compromise tumor control probability conversely. As a matter of fact, the incidence of RN has been reported as high as 17.2% to 38.8% in recent studies employing single-fraction SRS with reduced dose approach for the treatment of large BMs, while local tumor control (LTC) rates at 1 year were 58% to 84.6% [5,6,7,8,9].
In recent years, hypofractionated SRS (2 to 5 fractions) has been used for the treatment of large BMs based on the theoretical advantages of fractionation radiobiology, and the data in the literature suggest a promise of this approach in terms of both efficacy and safety compared with single-fraction SRS [6, 8, 10,11,12,13,14,15]. However, previous studies on this topic are all retrospective in nature with a huge heterogeneity in study design, SRS modality and technique, and dose/fractionation protocol, especially in tumor size the major confounding variable affecting the treatment outcomes. Here we sought to prospectively control tumor size to investigate the efficacy and safety of hypofractionated SRS specifically for medium-sized BMs (2.5 to 3 cm) at the border zone of tumor size for treatment with conventional single-fraction SRS and compared its outcomes with those of single-fraction SRS.
Materials and methods
Eligibility
This retrospective study with prospectively managed clinical data was approved by our institutional review board. Between January 2011 and December 2015, a total of 100 patients with 105 BMs of 2.5 to 3.0 cm were entered into the study according to the inclusion and exclusion criteria below.
Inclusion criteria
-
(1)
Age of ≥ 18 years, with histologically proven solid cancer and fewer than 10 BMs, any of which is 2.5 to 3 cm in maximum diameter on brain magnetic resonance images (MRI)
-
(2)
Patients who had been treated with either single-fraction SRS using the Gamma Knife (GK; Elekta AB, Stockholm, Sweden) or hypofractionated (3 to 5 fractions) SRS using the Cyberknife (CK; Accuray Inc., Sunnyvale, CA) for their medium-sized BMs
Exclusion criteria.
-
(1)
Previous history of cranial irradiation including WBRT and SRS
-
(2)
Prior surgical resection of the targeted lesions
-
(3)
Absence of follow-up data at least 3 months after treatment
Demographic data and tumor variables
Baseline characteristics of the patients and tumors are summarized in Table 1. A total of 105 BMs had been treated with either single-fraction (n = 67) or hypofractionated SRS (n = 38). There were no differences between the two groups in gender, age, number of lesions, tumor volume (median 9.7 cc and 11.0 cc, respectively), location, origin and status of primary cancers, patient performance, the Radiation Therapy Oncology Group-recursive partitioning analysis (RTOG-RPA) class and the diagnosis-specific graded prognostic assessment (DS-GPA) score except for extracranial metastases.
Stereotactic radiosurgery and dosimetric parameters
The Leksell Gamma Knife Perfexion System was used for single-fraction SRS and the Cyberknife Robotic Radiosurgery System Version 9.0 was used for hypofractionated SRS. All GK plans were generated using the Elekta GammaPlan system (version 9.0) based on gadolinium-enhanced axial three-dimensional T1-magnetization-prepared rapid acquisition gradient echo (3D-T1-MPRAGE) MRI (1.5 mm slice thickness) fused with computed tomography (CT) images (1.25 mm slice). The optimal plan was produced by adjustment of the sectors and collimators such that optimal dose coverage of the target while minimizing dose to surrounding normal tissues was achieved. The prescription isodose percentage was applied to 50% of the maximum dose and the median prescription dose was 20 Gy (range 18–22 Gy). For CK plans, planning CT images were fused with gadolinium-enhanced 3D-T1-MPRAGE images in the Accuray MultiPlan system (version 4.5) to facilitate delineation of the gross tumor volume (GTV; equal to the planning target volume). The prescription isodose percentage was applied to around 80% with planning objectives of GTV coverage > 99% and the conformity index (CI) < 1.2. The median prescription dose was 35 Gy (range 27–41 Gy). Doses were administered in 3 or 5 daily fractions.
Tumor coverage, the homogeneity index (HI), CI, and the gradient inde× (GI) were calculated in each plan to compare dosimetric quality between the two groups. HI was measured as the ratio of the maximum dose over the prescription dose. CI was defined as the ratio of prescription isodose volume (PIV) to the volume of tumor receiving the prescription dose or more. GI was the ratio of the isodose volume receiving 50% of the prescription dose to PIV. Table 2 summarizes comparison of dosimetric parameters.
Follow-up, outcome measures, and statistics
Follow-up clinical examinations and MRIs were performed at 3 month intervals after treatment. LTC was defined as complete or partial response and stable disease using the criteria of MacDonald et al. [16]. Significant increase of tumor size ( > 25%) on interval MRIs was defined as local failure. Progression was defined as local failure and/or development of a new lesion. RN was assessed objectively using MRI or confirmed pathologically after surgical resection. The following criteria were considered for RN: (1) increased T1 contrast enhancement in treated volume with central hypointensity and increased peripheral edema [17] (2) substantial regression or stability (for at least 3 months) of enhancing areas on serial follow-up MRIs without additional treatment [18], or (3) absence of perfusion within the contrast-enhancing lesion on dynamic susceptibility contrast perfusion MRI [19]. Treatment-related clinical toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Event (version 4.0) (CTCAE ver. 4.0).
Differences in baseline patient characteristics were compared using the Student’s t-test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. Dosimetric parameters were compared using the Student’s t-test. LTC, the overall and progression-free survival (OS and PFS), and RN were estimated using the Kaplan–Meier method. The Cox proportional hazards model was used to adjust for the baseline imbalances in gender, age ( ≥ 65 years vs. < 65), number of tumors (single vs. multiple), location, primary cancer type, status of primary cancer, presence of extracranial metastases, KPS score ( ≥ 70 vs. < 70), RTOG-RPA class, and DS-GPA score. Multivariate analysis was performed by backward elimination with candidate variables of P < 0.15 on univariate analysis. The cumulative incidences of local failure, progression, and RN were compared using the Gray’s test. The Fine and Gray method was used on modeling the hazard of sub-distribution accounting for death as a competing risk. All statistical tests were conducted using the SPSS Version 21.0 (SPSS Inc., Chicago, IL) and the R software Version 3.1.1. Statistical significance was set at P < 0.05.
Results
Local tumor control
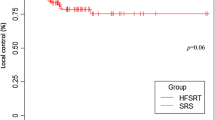
With a median follow-up of 14 months (3–59 months), the estimated LTC rates at 6 months, 1 year, and 1.5 years were 92.9%, 66.6%, and 57.2% in single-fraction SRS group, and 100%, 92.4%, and 88.2% in hypofractionated SRS group, respectively (P = 0.028, Fig. 1a). In multivariate Cox regression analysis (Table 3), positive predictive factors for LTC included hypofractionation (P = 0.022), primary cancers of non-gastrointestinal origin (P = 0.003), and newly diagnosed primary cancers (P = 0.038). In the Fine and Gray analysis accounting for death as a competing risk, the statistical significance of treatment modality appeared to decline (P = 0.093).
Overall and progression-free survival
The Kaplan–Meier curves for OS are shown in Fig. 1b. There were no differences in OS between the single-fraction versus hypofractionated SRS groups (the estimated OS rates at 6 months, 1 year, and 1.5 years of 92.2%, 53.1%, and 32.8% vs. 86.1%, 69.4%, and 52.8%, respectively; P = 0.239). Primary cancer of gastrointestinal origin was the only independent predictor for OS (hazard ratio, 2.699; 95% confidence interval, 1.489–4.891; P = 0.001; Supplementary Table 1). Along with the OS, PFS did not differ between the two groups with the estimated PFS rates at 6 months, 1 year, and 1.5 years of 60.2%, 27.3%, and 15.9% in single-fraction group versus 58.0%, 40.9%, and 33.5% in hypofractionated group, respectively (P = 0.381, Fig. 1c). Multiple BMs were associated with increased risks of progression (hazard ratio, 1.692; 95% confidence interval, 1.057–2.709; P = 0.028; Supplementary Table 2).
Radiation necrosis and treatment-related toxicity
The incidence of RN was significantly lower in hypofractionated group than in single-fraction group: the estimated RN rates at 6 months, 1 year, and 1.5 years were 0%, 0%, and 9.3% in hypofractionated group compared with 15.1%, 39.8%, and 43.6% in single-fraction group, respectively (P < 0.001, Fig. 1d). The hazard ratio for RN was 8.479 in single-fraction SRS group (95% confidence interval, 1.966–36.570; P = 0.004; Table 4) and no other factors were associated with the occurrence of RN. Treatment-related clinical toxicity of ≥ grade 2 by CTCAE ver. 4.0 was seen in 36 patients (56.3%) in single-fraction group versus in 13 patients (36.1%) in hypofractionated group (P = 0.084; Table 5).
Discussion
Our current study shows the clinical advantages of hypofractionated SRS approach compared with single-fraction SRS in treating medium-sized BMs (2.5 to 3 cm) at the border zone of tumor size for conventional single-fraction SRS treatment in terms of both efficacy and safety. The key clinical relevance is focused on the superior safety profile of hypofractionation approach without compromised tumor control as shown by significantly reduced risks of RN along with clinical radiation toxicity. Moreover, a certain benefit in LTC is also suggested with this approach, although statistical significance appears to be marginal.
These observations are well in line with the theoretical advantages of fractionation radiobiology. Fractionated administration of radiation dose potentially reduces toxicity to late-responding normal tissues with a low α/β ratio compared with a single acute dose of radiation for a given level of tumor damage [3, 20]. In addition, reoxygenation and redistribution of the cell cycle between fractions render hypoxic tumor cells and cells in less responding cell cycles more radiosensitive [21, 22]. In this theoretical context, it is reasonable to assume that fractionation delivery of SRS would potentially mitigate the risks of radiation toxicity and enhance tumor control probability compared with single-fraction SRS.
Currently, few studies are available on direct comparison of the efficacy and safety between single-fraction versus hypofractionated SRS for large BMs. Minniti et al. [6] reported on 289 patients with BMs > 2 cm who had been treated with either single-fraction SRS (n = 151; median dose 18 Gy) or hypofractionated SRS (n = 138; 27 Gy in 3 fractions) and compared LTC and the risk of RN between the groups. In their series, LTC rates at 1 year were 77% in single-fraction group and 91% in hypofractionated group, while the incidences of RN were 20% and 8%, respectively. Similarly, Feuvret et al. [8], in their small series of BMs > 3 cm, reported a superior tumor control rate (LTC in 100% at 1 year) in hypofractionated group (n = 12; 23.1 Gy in 3 fractions) compared with single-fraction group (n = 24; 14 Gy; LTC in 58% at 1 year), although RN was not observed in both groups with relatively lower prescription doses used. Our study adds to these observations with a merit of prospective control of tumor size in a narrow range of 2.5 to 3 cm, which enables us to interpret the data more intuitively and clearly. One recent international meta-analysis including 15 studies on SRS for large BMs > 2 cm mostly of a single-arm treatment design, either single-fraction or hypofractionated SRS (2 comparative studies described above included) concluded hypofractionated SRS may offer a reduced risk of RN while maintaining or enhancing 1-year LTC as compared with single-fraction SRS [23], which is almost consistent with our current results. Although certain limitations do exist including the retrospective nature of all studies included, unstandardized terms and definitions, and, most of all, a vast heterogeneity in histology, SRS modality and technique, prescription dose, and fractionation protocol, the results of these studies along with ours provide a reasonable rationale for implementing prospective controlled clinical trials to move forward into a better standard of care treatment for large BMs.
Conclusions
Our results suggest a better safety and efficacy profile of hypofractionated SRS for medium-sized BMs of 2.5 to 3 cm compared with single-fraction SRS. Further prospective controlled studies are needed to confirm these results.
References
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Kalkanis SN (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:45–68. https://doi.org/10.1007/s11060-009-0073-4
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 47:291–298
Giubilei C, Ingrosso G, D'Andrea M, Benassi M, Santoni R (2009) Hypofractionated stereotactic radiotherapy in combination with whole brain radiotherapy for brain metastases. J Neurooncol 91:207–212. https://doi.org/10.1007/s11060-008-9700-8
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001. https://doi.org/10.1016/j.ijrobp.2009.06.006
Prabhu RS, Press RH, Patel KR, Boselli DM, Symanowski JT, Lankford SP, McCammon RJ, Moeller BJ, Heinzerling JH, Fasola CE, Asher AL, Sumrall AL, Buchwald ZS, Curran WJ Jr, Shu HG, Crocker I, Burri SH (2017) Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: a multi-institutional analysis. Int J Radiat Oncol Biol Phys 99:459–467. https://doi.org/10.1016/j.ijrobp.2017.04.006
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, Osti M, Enrici RM, Esposito V (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (%3e2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Han JH, Kim DG, Chung HT, Paek SH, Park CK, Jung HW (2012) Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 83:113–120. https://doi.org/10.1016/j.ijrobp.2011.06.1965
Feuvret L, Vinchon S, Martin V, Lamproglou I, Halley A, Calugaru V, Chea M, Valery CA, Simon JM, Mazeron JJ (2014) Stereotactic radiotherapy for large solitary brain metastases. Cancer Radiother 18:97–106. https://doi.org/10.1016/j.canrad.2013.12.003
Zimmerman AL, Murphy ES, Suh JH, Vogelbaum MA, Barnett GH, Angelov L, Ahluwalia M, Reddy CA, Chao ST (2016) Treatment of large brain metastases with stereotactic radiosurgery. Technol Cancer Res Treat 15:186–195. https://doi.org/10.1177/1533034614568097
Kim JW, Park HR, Lee JM, Kim JW, Chung HT, Kim DG, Jung HW, Paek SH (2016) Fractionated stereotactic gamma knife radiosurgery for large brain metastases: a retrospective, single center study. PLoS ONE 11:e0163304. https://doi.org/10.1371/journal.pone.0163304
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH (2015) Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc 58:217–224. https://doi.org/10.3340/jkns.2015.58.3.217
Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A, Franzese C, Franceschini D, Tozzi A, D'Agostino G, Comito T, Iftode C, Maggi G, Reggiori G, Bello L, Scorsetti M (2016) Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 11:76. https://doi.org/10.1186/s13014-016-0653-3
Wegner RE, Leeman JE, Kabolizadeh P, Rwigema JC, Mintz AH, Burton SA, Heron DE (2015) Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol 38:135–139. https://doi.org/10.1097/COC.0b013e31828aadac
Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J, Noda SE, Nakano T (2014) Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res 55:334–342. https://doi.org/10.1093/jrr/rrt127
Murai T, Ogino H, Manabe Y, Iwabuchi M, Okumura T, Matsushita Y, Tsuji Y, Suzuki H, Shibamoto Y (2014) Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol 26:151–158. https://doi.org/10.1016/j.clon.2013.11.027
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/jco.2009.26.3541
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD (2010) T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 66:486–491. https://doi.org/10.1227/01.Neu.0000360391.35749.A5 (discussion 491-482)
Parvez K, Parvez A, Zadeh G (2014) The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15:11832–11846. https://doi.org/10.3390/ijms150711832
Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE (2013) Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 55:361–369. https://doi.org/10.1007/s00234-012-1127-4
Tome WA (2009) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy: in regard to Parks et al. (Int J Radiat Oncol Biol Phys 2008;72:1620-1621). Int J Radiat Oncol Biol Phys 73:1286. https://doi.org/10.1016/j.ijrobp.2008.12.001
Hall EJ, Brenner DJ (1993) The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys 25:381–385
Ling CC, Lo YC, Larson DA (1995) Radiobiophysical aspects of stereotaxic radiation treatment of central nervous system diseases. Semin Radiat Oncol 5:192–196. https://doi.org/10.1054/srao00500192
Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, Chao ST, Sheehan JP, Trifiletti DM (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103:618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chon, H., Yoon, K., Lee, D. et al. Single-fraction versus hypofractionated stereotactic radiosurgery for medium-sized brain metastases of 2.5 to 3 cm. J Neurooncol 145, 49–56 (2019). https://doi.org/10.1007/s11060-019-03265-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03265-1